POLYMERS POLYMER Poly many Mer units Polymers are







- Slides: 15

POLYMERS

POLYMER Poly = many Mer = units • Polymers are large molecules that has many units bonded together • The individual units made up the polymer are called monomers

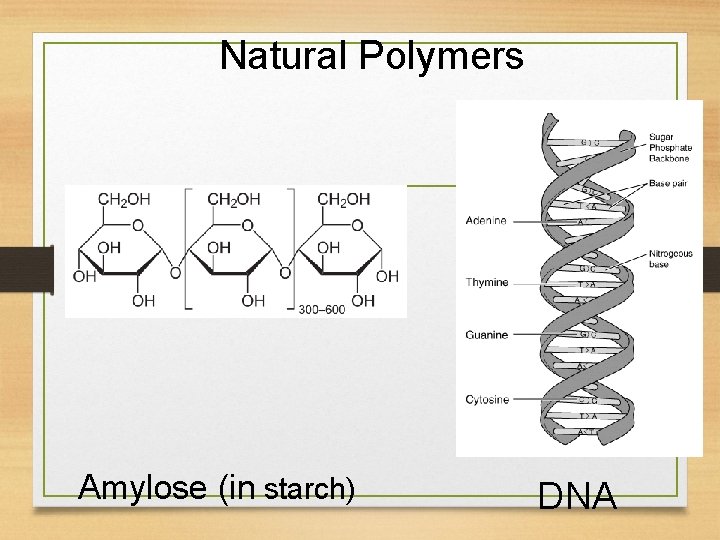
Natural Polymers Amylose (in starch) DNA

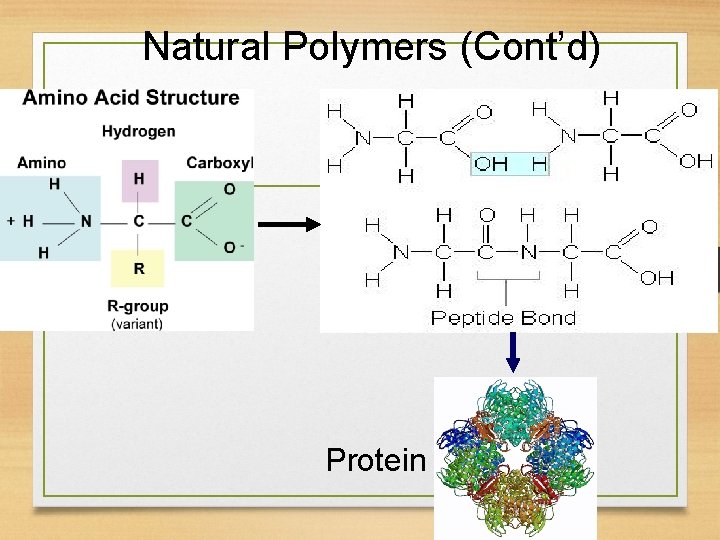
Natural Polymers (Cont’d) Protein

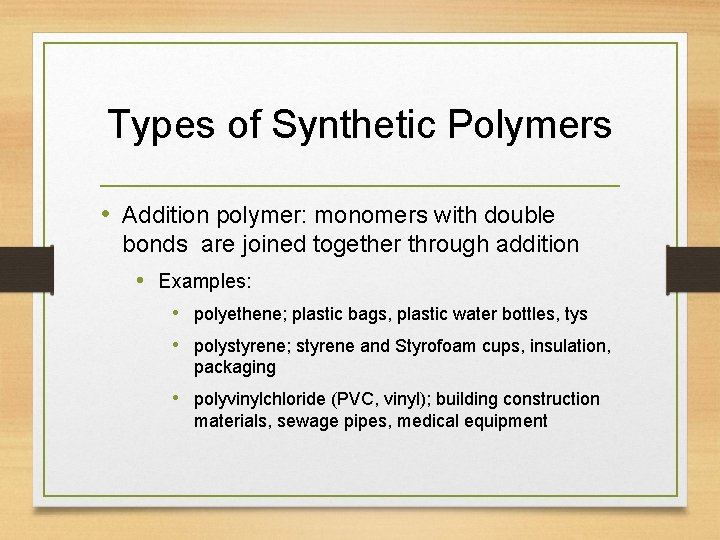
Types of Synthetic Polymers • Addition polymer: monomers with double bonds are joined together through addition • Examples: • polyethene; plastic bags, plastic water bottles, tys • polystyrene; styrene and Styrofoam cups, insulation, packaging • polyvinylchloride (PVC, vinyl); building construction materials, sewage pipes, medical equipment

• Condensation polymer: monomers are joined by formation of esters or amide • Need a carboxylic acid or a diamide • Examples: • Nylon – tires, synthetic fibres used to make rope and clothing • Polyester (Dacron. TM) – fibres used to make fabric for clothing and surgery

Type 1: Addition polymers • Made by addition reaction of monomers that have double bonds or triple bonds

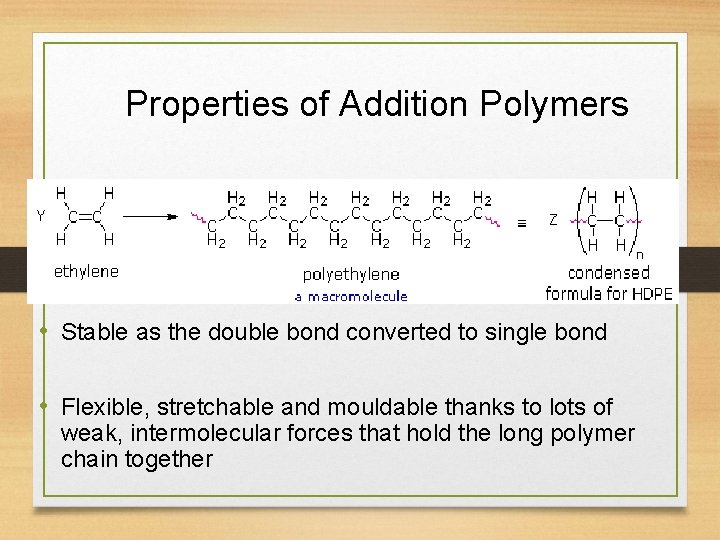
Properties of Addition Polymers • Stable as the double bond converted to single bond • Flexible, stretchable and mouldable thanks to lots of weak, intermolecular forces that hold the long polymer chain together

Plastic bag- How it’s made? ? ? • Made from a polymer • Polyethylene • Take 1 million years to break down/ degrade a plastic bag

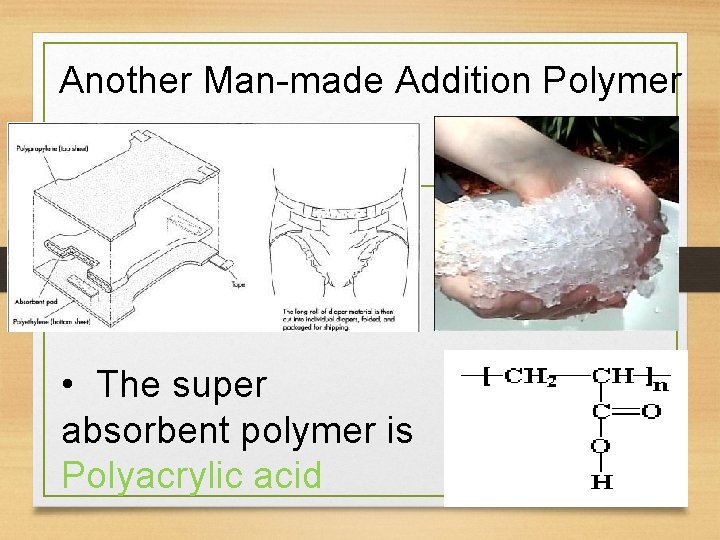
Another Man-made Addition Polymer • The super absorbent polymer is Polyacrylic acid

Super absorbent Polymer (Cont) • Absorb up to 1, 000 g of water per gram of polymer

Applications of Nylon • Invented by Dupont • Nylon stands for New York & London • Tear resistant • Water-proof Used in making: • parachutes • stocking • sleeping bags • tent, sail • cooking utensils

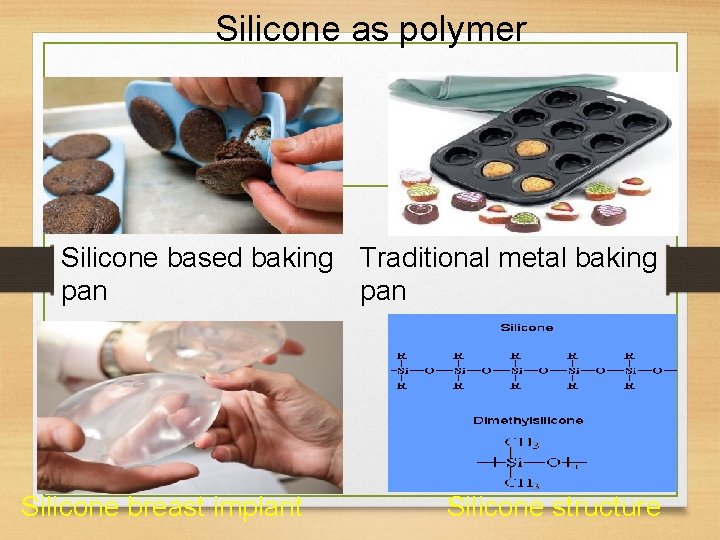
Silicone as polymer Silicone based baking Traditional metal baking pan Silicone breast implant Silicone structure

POLYMER TIME! Making slime • Pour Elmer Glue to the mark in a plastic cup • Add equal volume of water to dissolve the glue • Add desire food colorant • Stir • Add prepared Borax solution DROPWISE until the slime is somewhat thicken • Don’t add too much Borax
