Organic Polymers Organic Polymer Chemistry Polymer from the


- Slides: 15

Organic Polymers

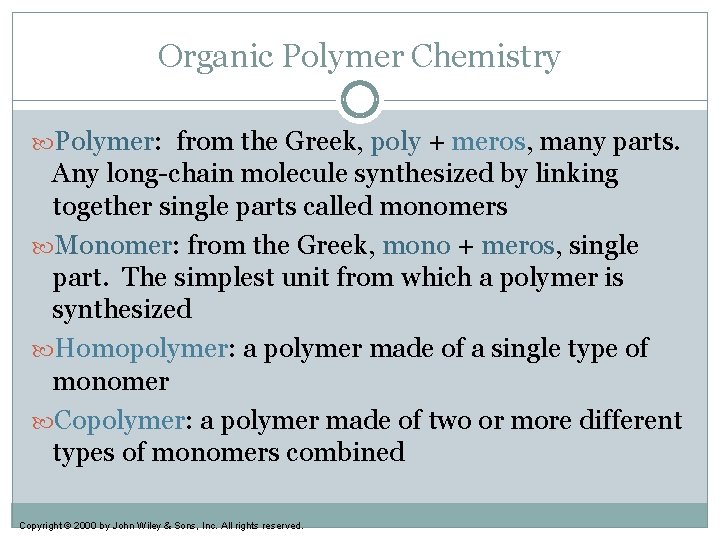
Organic Polymer Chemistry Polymer: from the Greek, poly + meros, many parts. Any long-chain molecule synthesized by linking together single parts called monomers Monomer: from the Greek, mono + meros, single part. The simplest unit from which a polymer is synthesized Homopolymer: a polymer made of a single type of monomer Copolymer: a polymer made of two or more different types of monomers combined Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Notation & Nomenclature Show the structure by placing square brackets around the repeat unit n = average degree of polymerization To name a polymer, prefix poly to the name of the monomer from which the polymer is derived Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

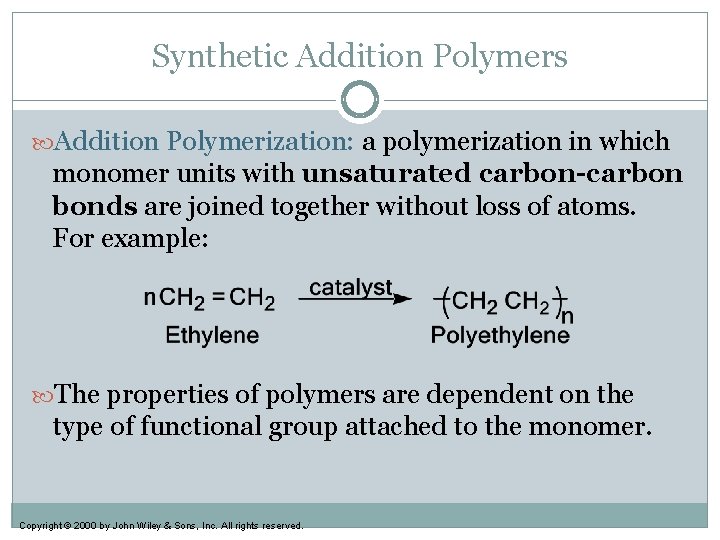
Synthetic Addition Polymers Addition Polymerization: a polymerization in which monomer units with unsaturated carbon-carbon bonds are joined together without loss of atoms. For example: The properties of polymers are dependent on the type of functional group attached to the monomer. Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Polyethylenes Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Polyethylenes Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Cross-Linking Some monomer functional groups have the ability to form cross-links. Cross-linking is bonding of adjacent polymer strands As a general rule, the more cross-linking present the more rigid and inflexible the polymer becomes. Dienes (monomers with 2 unsaturated bonds) form strong cross-links Sulphur is also used to create cross-links due to the fact it is able to form two covalent bonds. (vulcanization) Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Homework Please read section 2. 1 and 2. 2 Page 87 #1 -3 Page 93 #1, 2, 4 Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Synthetic Condensation Polymers Condensation polymerization: a polymerization in which chain growth occurs by a condensation reaction (removal of water) between difunctional monomers Polyesters: A polymer formed by a condensation reaction that results in ester linkages between monomers. Polyamides: a polymer formed by condensation reactions resulting in amide linkages between monomers. Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Polyesters Poly(ethylene terephthalate) (PET) is fabricated into plastic beverage containers Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Polyamides Nylon 66 (from two six-carbon monomers) during fabrication, nylon fibers are cold-drawn to about 4 times their original length, which increases crystallinity, tensile strength, and stiffness Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

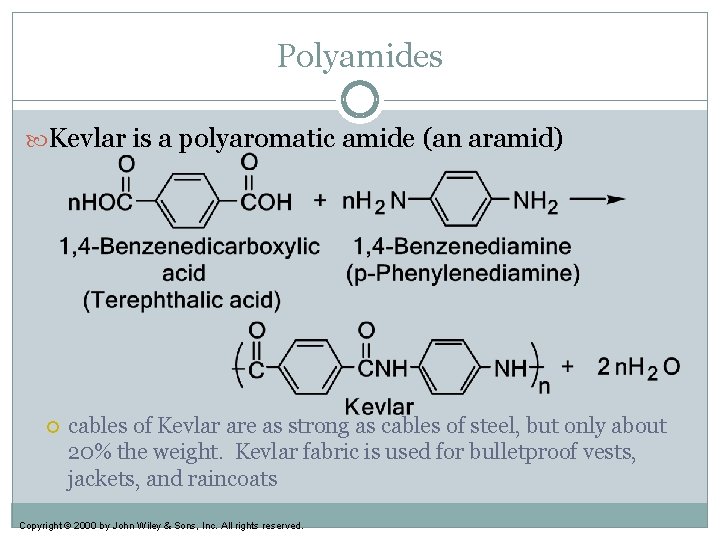
Polyamides Kevlar is a polyaromatic amide (an aramid) cables of Kevlar are as strong as cables of steel, but only about 20% the weight. Kevlar fabric is used for bulletproof vests, jackets, and raincoats Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Recycling Codes Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Recycling Codes Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.

Homework Please read section 2. 4 Page 98 #1 Page 99 #1 -6 Copyright © 2000 by John Wiley & Sons, Inc. All rights reserved.
 Synthetic organic polymers
Synthetic organic polymers Poly cyclopentene
Poly cyclopentene Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry What is organic polymer
What is organic polymer What is the proper procedure for applying one color monomer
What is the proper procedure for applying one color monomer Entane
Entane Number of organic compounds
Number of organic compounds Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Organic chemistry third edition david klein
Organic chemistry third edition david klein Chemistry
Chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition What is organic chemistry like
What is organic chemistry like Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry