Chapter 4 Structures of Polymers polymers ceramics metals

- Slides: 17

Chapter 4 Structures of Polymers

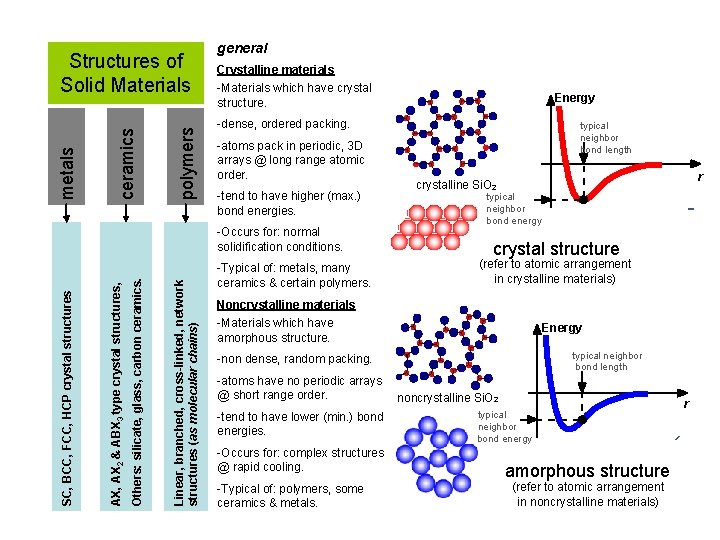
polymers ceramics metals Structures of Solid Materials general Crystalline materials -Materials which have crystal structure. -dense, ordered packing. -atoms pack in periodic, 3 D arrays @ long range atomic order. -tend to have higher (max. ) bond energies. Linear, branched, cross-linked, network structures (as molecular chains) Others: silicate, glass, carbon ceramics. AX, AX 2 & ABX 3 type crystal structures, -Occurs for: normal solidification conditions. SC, BCC, FCC, HCP crystal structures Energy -Typical of: metals, many ceramics & certain polymers. typical neighbor bond length r crystalline Si. O 2 typical neighbor bond energy crystal structure (refer to atomic arrangement in crystalline materials) Noncrystalline materials -Materials which have amorphous structure. Energy typical neighbor bond length -non dense, random packing. -atoms have no periodic arrays @ short range order. -tend to have lower (min. ) bond energies. -Occurs for: complex structures @ rapid cooling. -Typical of: polymers, some ceramics & metals. noncrystalline Si. O 2 typical neighbor bond energy amorphous structure (refer to atomic arrangement in noncrystalline materials) r

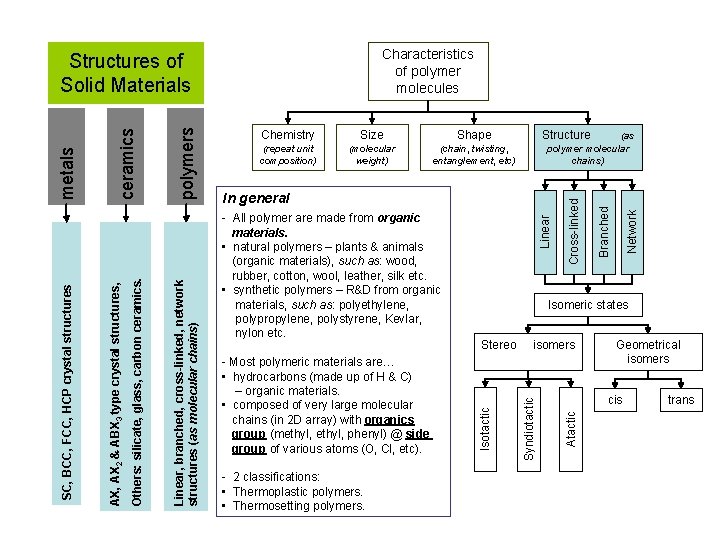
Characteristics of polymer molecules (chain, twisting, entanglement, etc) Structure (as polymer molecular chains) - 2 classifications: • Thermoplastic polymers. • Thermosetting polymers. Stereo isomers Geometrical isomers cis Atactic - Most polymeric materials are… • hydrocarbons (made up of H & C) – organic materials. • composed of very large molecular chains (in 2 D array) with organics group (methyl, phenyl) @ side group of various atoms (O, Cl, etc). Isomeric states Syndiotactic - All polymer are made from organic materials. • natural polymers – plants & animals (organic materials), such as: wood, rubber, cotton, wool, leather, silk etc. • synthetic polymers – R&D from organic materials, such as: polyethylene, polypropylene, polystyrene, Kevlar, nylon etc. Linear In general Network Shape (molecular weight) Branched Size (repeat unit composition) Cross-linked Chemistry Isotactic polymers Linear, branched, cross-linked, network structures (as molecular chains) ceramics Others: silicate, glass, carbon ceramics. AX, AX 2 & ABX 3 type crystal structures, SC, BCC, FCC, HCP crystal structures metals Structures of Solid Materials trans

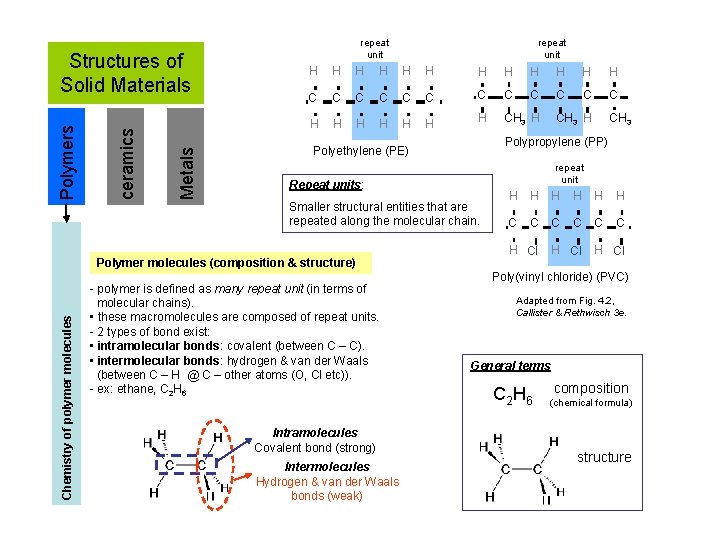
Metals ceramics Polymers Structures of Solid Materials repeat unit H H H C C C H H H H CH 3 H Smaller structural entities that are repeated along the molecular chain. Intramolecules Covalent bond (strong) Intermolecules Hydrogen & van der Waals bonds (weak) CH 3 repeat unit Repeat units: - polymer is defined as many repeat unit (in terms of molecular chains). • these macromolecules are composed of repeat units. - 2 types of bond exist: • intramolecular bonds: covalent (between C – C). • intermolecular bonds: hydrogen & van der Waals (between C – H @ C – other atoms (O, Cl etc)). - ex: ethane, C 2 H 6 CH 3 H Polypropylene (PP) Polyethylene (PE) Polymer molecules (composition & structure) Chemistry of polymer molecules repeat unit H H H C C C H Cl Poly(vinyl chloride) (PVC) Adapted from Fig. 4. 2, Callister & Rethwisch 3 e. General terms C 2 H 6 composition (chemical formula) structure

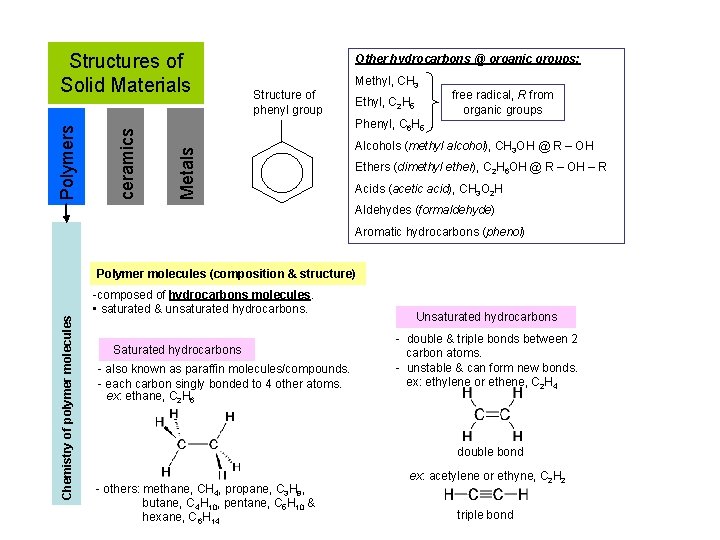
Other hydrocarbons @ organic groups: Structure of phenyl group Methyl, CH 3 Ethyl, C 2 H 5 free radical, R from organic groups Phenyl, C 6 H 5 Metals ceramics Polymers Structures of Solid Materials Alcohols (methyl alcohol), CH 3 OH @ R – OH Ethers (dimethyl ether), C 2 H 6 OH @ R – OH – R Acids (acetic acid), CH 3 O 2 H Aldehydes (formaldehyde) Aromatic hydrocarbons (phenol) Chemistry of polymer molecules Polymer molecules (composition & structure) -composed of hydrocarbons molecules. • saturated & unsaturated hydrocarbons. Saturated hydrocarbons - also known as paraffin molecules/compounds. - each carbon singly bonded to 4 other atoms. ex: ethane, C 2 H 6 Unsaturated hydrocarbons - double & triple bonds between 2 carbon atoms. - unstable & can form new bonds. ex: ethylene or ethene, C 2 H 4 double bond - others: methane, CH 4, propane, C 3 H 8, butane, C 4 H 10, pentane, C 5 H 10 & hexane, C 6 H 14 ex: acetylene or ethyne, C 2 H 2 triple bond

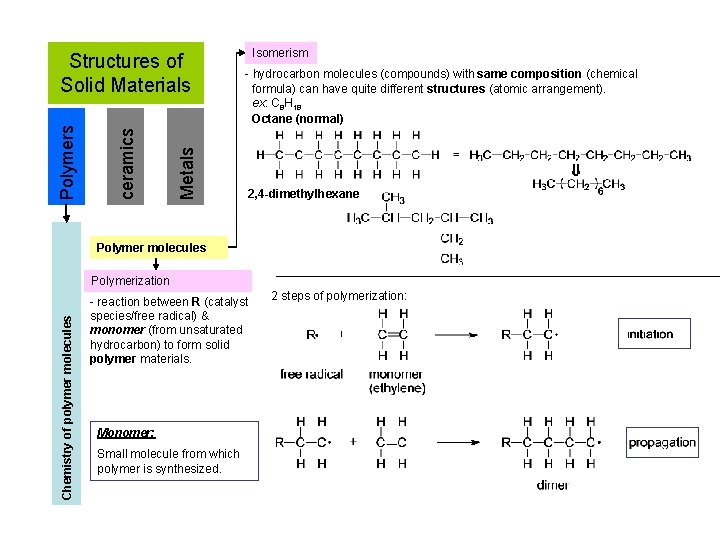
Metals ceramics Polymers Structures of Solid Materials Isomerism - hydrocarbon molecules (compounds) with same composition (chemical formula) can have quite different structures (atomic arrangement). ex: C 8 H 18 Octane (normal) 2, 4 -dimethylhexane Polymer molecules Chemistry of polymer molecules Polymerization - reaction between R (catalyst species/free radical) & monomer (from unsaturated hydrocarbon) to form solid polymer materials. Monomer: Small molecule from which polymer is synthesized. 2 steps of polymerization:

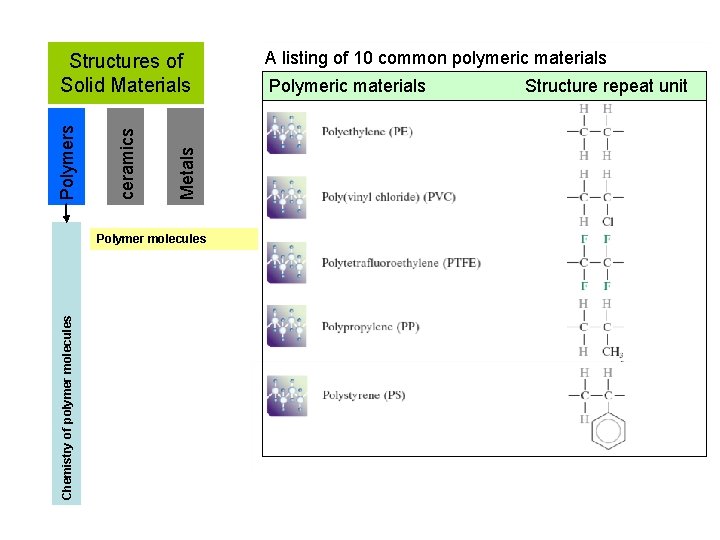
Metals ceramics Polymers Structures of Solid Materials Chemistry of polymer molecules Polymer molecules A listing of 10 common polymeric materials Polymeric materials Structure repeat unit

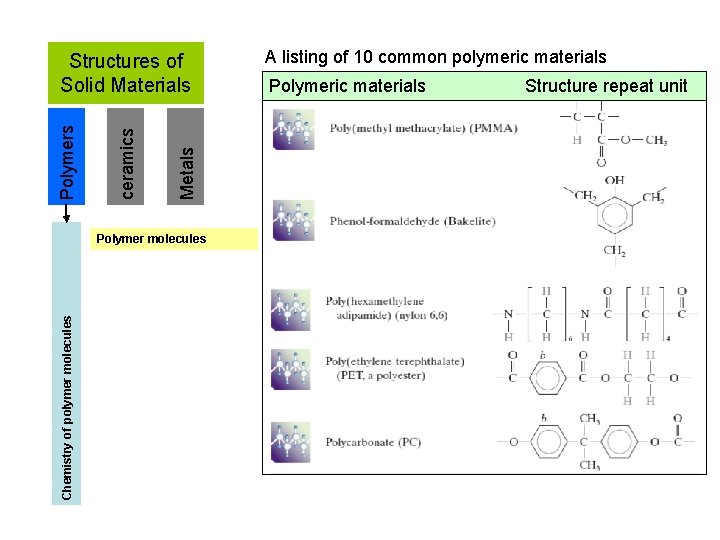
Metals ceramics Polymers Structures of Solid Materials Chemistry of polymer molecules Polymer molecules A listing of 10 common polymeric materials Polymeric materials Structure repeat unit

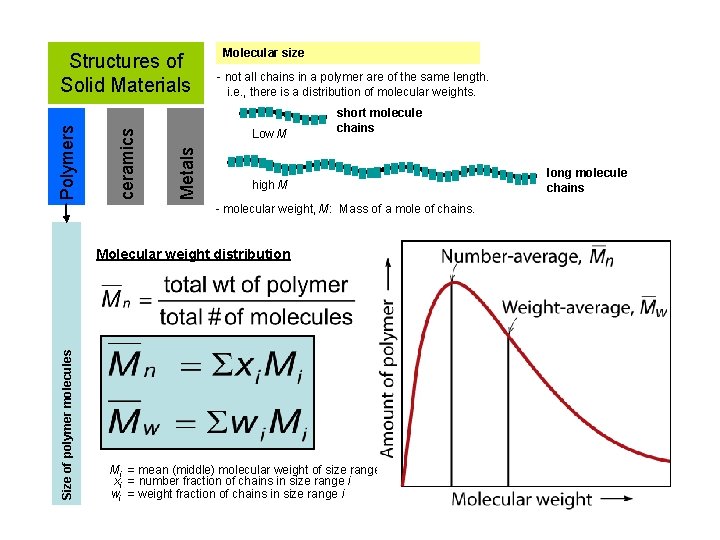
Molecular size - not all chains in a polymer are of the same length. i. e. , there is a distribution of molecular weights. Low M Metals ceramics Polymers Structures of Solid Materials short molecule chains high M - molecular weight, M: Mass of a mole of chains. Size of polymer molecules Molecular weight distribution Mi = mean (middle) molecular weight of size range i xi = number fraction of chains in size range i wi = weight fraction of chains in size range i long molecule chains

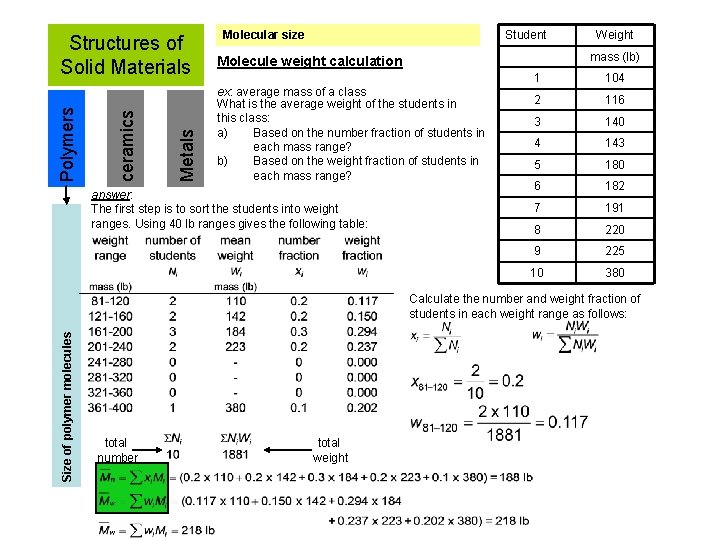
Metals ceramics Polymers Structures of Solid Materials Student Molecular size mass (lb) Molecule weight calculation ex: average mass of a class What is the average weight of the students in this class: a) Based on the number fraction of students in each mass range? b) Based on the weight fraction of students in each mass range? answer: The first step is to sort the students into weight ranges. Using 40 lb ranges gives the following table: Weight 1 104 2 116 3 140 4 143 5 180 6 182 7 191 8 220 9 225 10 380 Size of polymer molecules Calculate the number and weight fraction of students in each weight range as follows: total number total weight

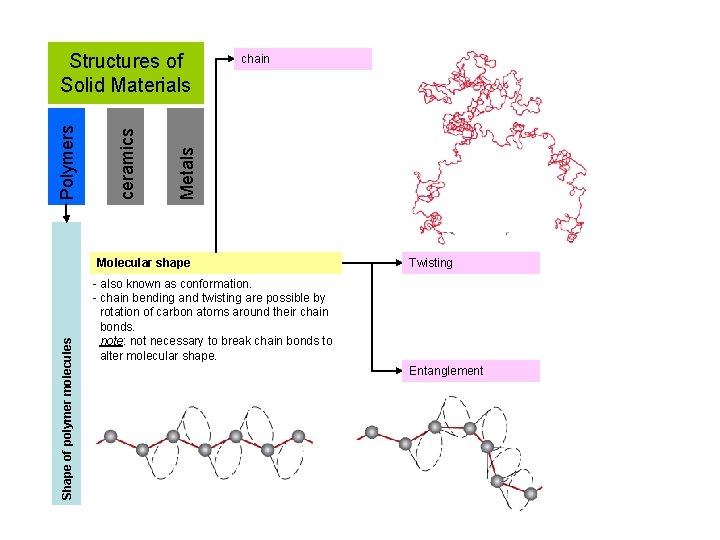
chain Metals ceramics Polymers Structures of Solid Materials Shape of polymer molecules Molecular shape Twisting - also known as conformation. - chain bending and twisting are possible by rotation of carbon atoms around their chain bonds. note: not necessary to break chain bonds to alter molecular shape. Entanglement

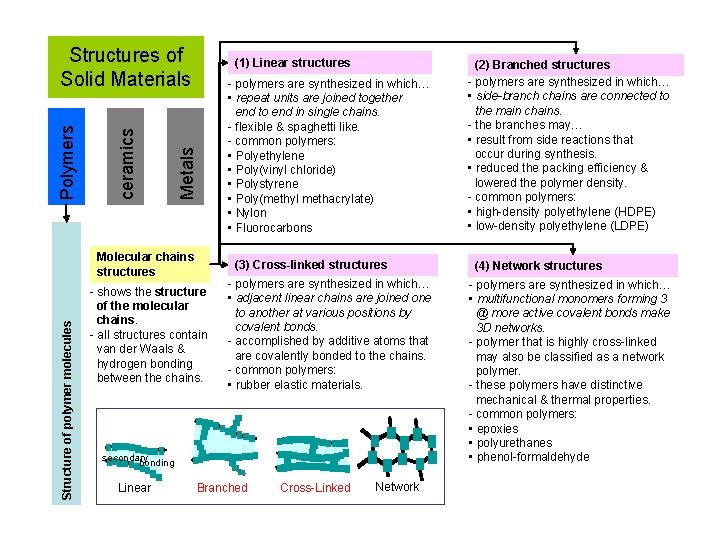
(1) Linear structures - polymers are synthesized in which… • repeat units are joined together end to end in single chains. - flexible & spaghetti like. - common polymers: • Polyethylene • Poly(vinyl chloride) • Polystyrene • Poly(methyl methacrylate) • Nylon • Fluorocarbons Metals ceramics Polymers Structures of Solid Materials Structure of polymer molecules Molecular chains structures (3) Cross-linked structures - shows the structure of the molecular chains. - all structures contain van der Waals & hydrogen bonding between the chains. - polymers are synthesized in which… • adjacent linear chains are joined one to another at various positions by covalent bonds. - accomplished by additive atoms that are covalently bonded to the chains. - common polymers: • rubber elastic materials. secondary bonding Linear Branched Cross-Linked Network (2) Branched structures - polymers are synthesized in which… • side-branch chains are connected to the main chains. - the branches may… • result from side reactions that occur during synthesis. • reduced the packing efficiency & lowered the polymer density. - common polymers: • high-density polyethylene (HDPE) • low-density polyethylene (LDPE) (4) Network structures - polymers are synthesized in which… • multifunctional monomers forming 3 @ more active covalent bonds make 3 D networks. - polymer that is highly cross-linked may also be classified as a network polymer. - these polymers have distinctive mechanical & thermal properties. - common polymers: • epoxies • polyurethanes • phenol-formaldehyde

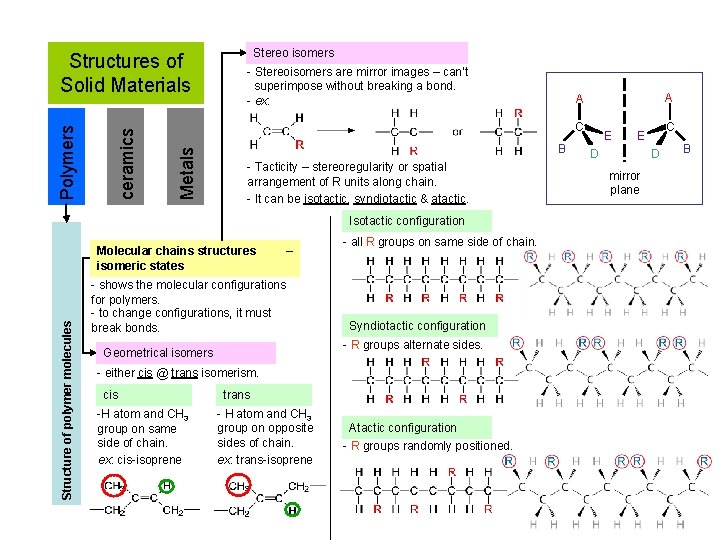
Stereo isomers - Stereoisomers are mirror images – can’t superimpose without breaking a bond. - ex: B - Tacticity – stereoregularity or spatial arrangement of R units along chain. - It can be isotactic, syndiotactic & atactic. Isotactic configuration Molecular chains structures isomeric states Structure of polymer molecules A A C Metals ceramics Polymers Structures of Solid Materials – - shows the molecular configurations for polymers. - to change configurations, it must break bonds. - all R groups on same side of chain. Syndiotactic configuration - R groups alternate sides. Geometrical isomers - either cis @ trans isomerism. cis -H atom and CH 3 group on same side of chain. ex: cis-isoprene trans - H atom and CH 3 group on opposite sides of chain. ex: trans-isoprene Atactic configuration - R groups randomly positioned. E C E D D mirror plane B

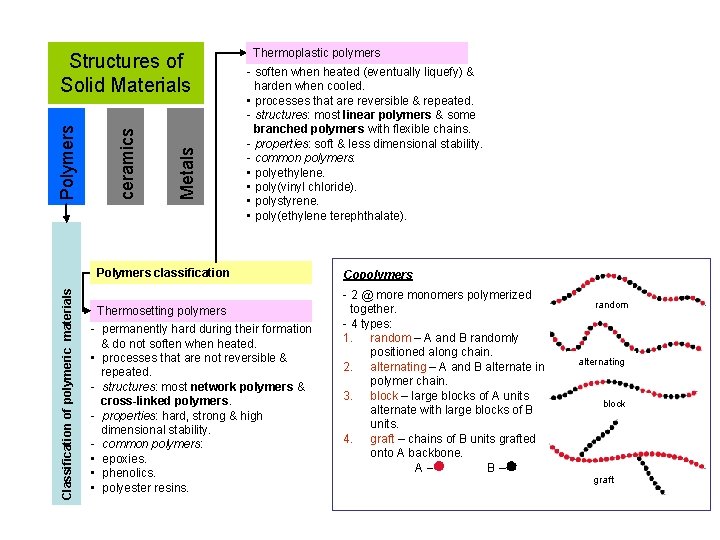
Metals ceramics Polymers Structures of Solid Materials Thermoplastic polymers - soften when heated (eventually liquefy) & harden when cooled. • processes that are reversible & repeated. - structures: most linear polymers & some branched polymers with flexible chains. - properties: soft & less dimensional stability. - common polymers: • polyethylene. • poly(vinyl chloride). • polystyrene. • poly(ethylene terephthalate). Classification of polymeric materials Polymers classification Thermosetting polymers - permanently hard during their formation & do not soften when heated. • processes that are not reversible & repeated. - structures: most network polymers & cross-linked polymers. - properties: hard, strong & high dimensional stability. - common polymers: • epoxies. • phenolics. • polyester resins. Copolymers - 2 @ more monomers polymerized together. - 4 types: 1. random – A and B randomly positioned along chain. 2. alternating – A and B alternate in polymer chain. 3. block – large blocks of A units alternate with large blocks of B units. 4. graft – chains of B units grafted onto A backbone. A– B– random alternating block graft

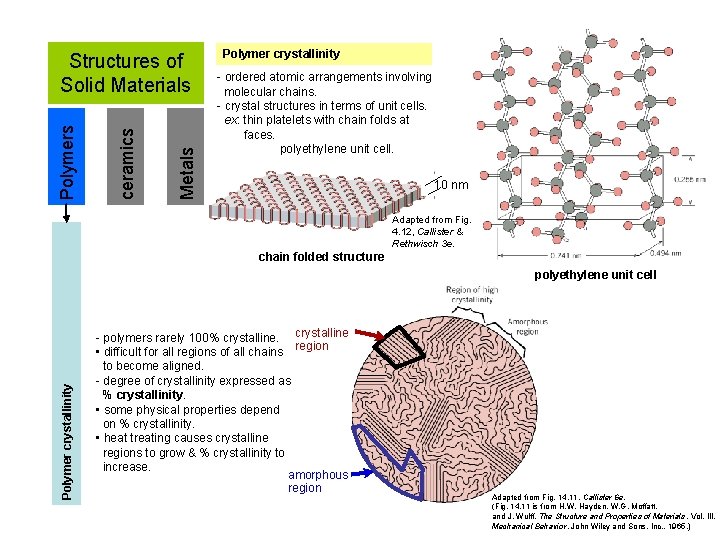
Metals ceramics Polymers Structures of Solid Materials Polymer crystallinity - ordered atomic arrangements involving molecular chains. - crystal structures in terms of unit cells. ex: thin platelets with chain folds at faces. polyethylene unit cell. 10 nm Adapted from Fig. 4. 12, Callister & Rethwisch 3 e. chain folded structure Polymer crystallinity polyethylene unit cell - polymers rarely 100% crystalline • difficult for all regions of all chains region to become aligned. - degree of crystallinity expressed as % crystallinity. • some physical properties depend on % crystallinity. • heat treating causes crystalline regions to grow & % crystallinity to increase. amorphous region Adapted from Fig. 14. 11, Callister 6 e. (Fig. 14. 11 is from H. W. Hayden, W. G. Moffatt, and J. Wulff, The Structure and Properties of Materials , Vol. III, Mechanical Behavior, John Wiley and Sons, Inc. , 1965. )

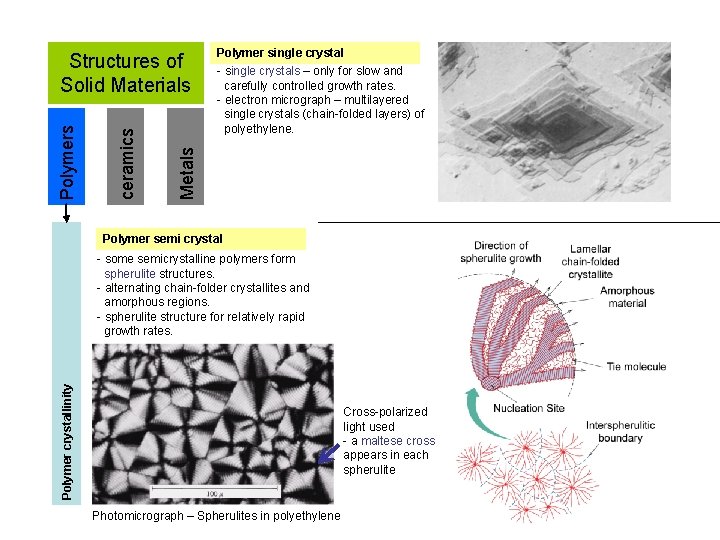
Polymer single crystal - single crystals – only for slow and carefully controlled growth rates. - electron micrograph – multilayered single crystals (chain-folded layers) of polyethylene. Metals ceramics Polymers Structures of Solid Materials Polymer semi crystal Polymer crystallinity - some semicrystalline polymers form spherulite structures. - alternating chain-folder crystallites and amorphous regions. - spherulite structure for relatively rapid growth rates. Cross-polarized light used - a maltese cross appears in each spherulite Photomicrograph – Spherulites in polyethylene

End of Chapter 4