DisclosureDisclaimer All material is intended for your medical



- Slides: 27

Disclosure/Disclaimer • All material is intended for your medical education purposes only 1

Exenatide Once Weekly PK, Efficacy, Safety, and Tolerability Amylin Pharmaceuticals, Inc. , San Diego, CA, USA Eli Lilly and Company, Indianapolis, IN, USA 2

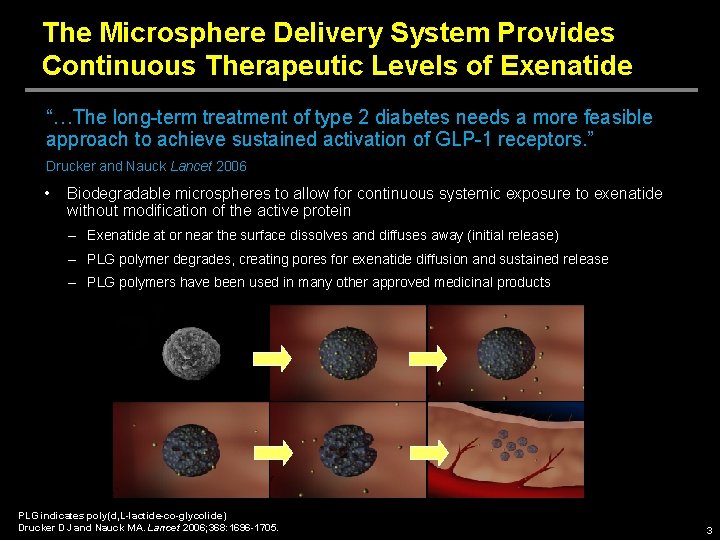
The Microsphere Delivery System Provides Continuous Therapeutic Levels of Exenatide “…The long-term treatment of type 2 diabetes needs a more feasible approach to achieve sustained activation of GLP-1 receptors. ” Drucker and Nauck Lancet 2006 • Biodegradable microspheres to allow for continuous systemic exposure to exenatide without modification of the active protein – Exenatide at or near the surface dissolves and diffuses away (initial release) – PLG polymer degrades, creating pores for exenatide diffusion and sustained release – PLG polymers have been used in many other approved medicinal products PLG indicates poly(d, L-lactide-co-glycolide) Drucker DJ and Nauck MA. Lancet 2006; 368: 1696 -1705. 3

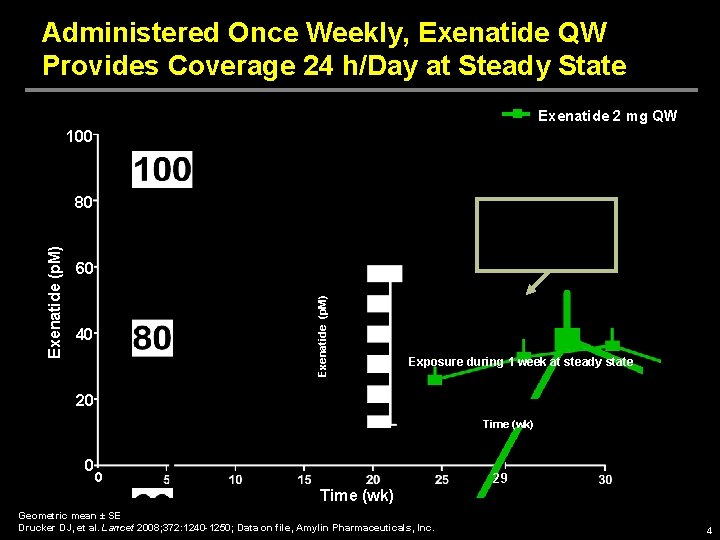
Administered Once Weekly, Exenatide QW Provides Coverage 24 h/Day at Steady State Exenatide 2 mg QW 100 60 Exenatide (p. M) 80 40 Exposure during 1 week at steady state 20 Time (wk) 0 0 29 Time (wk) Geometric mean ± SE Drucker DJ, et al. Lancet 2008; 372: 1240 -1250; Data on file, Amylin Pharmaceuticals, Inc. 4

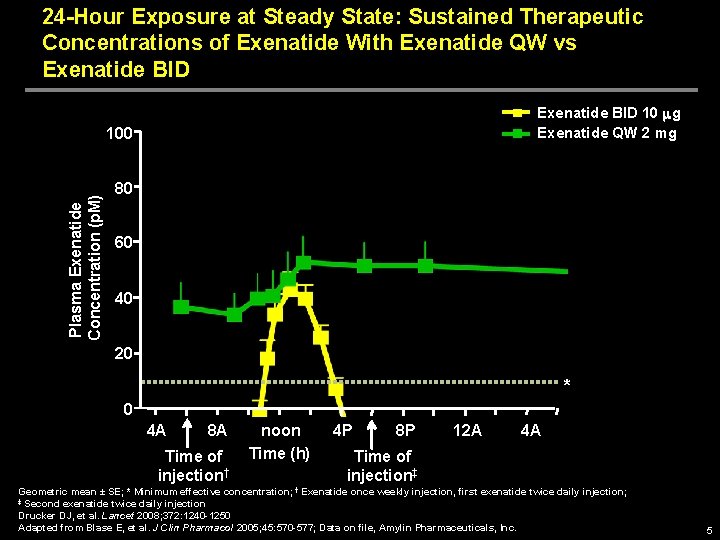
24 -Hour Exposure at Steady State: Sustained Therapeutic Concentrations of Exenatide With Exenatide QW vs Exenatide BID 10 mg Exenatide QW 2 mg Plasma Exenatide Concentration (p. M) 100 80 60 40 20 * 0 4 A 8 A Time of injection† noon Time (h) 4 P 8 P 12 A 4 A Time of injection‡ Geometric mean ± SE; * Minimum effective concentration; † Exenatide once weekly injection, first exenatide twice daily injection; ‡ Second exenatide twice daily injection Drucker DJ, et al. Lancet 2008; 372: 1240 -1250 Adapted from Blase E, et al. J Clin Pharmacol 2005; 45: 570 -577; Data on file, Amylin Pharmaceuticals, Inc. 5

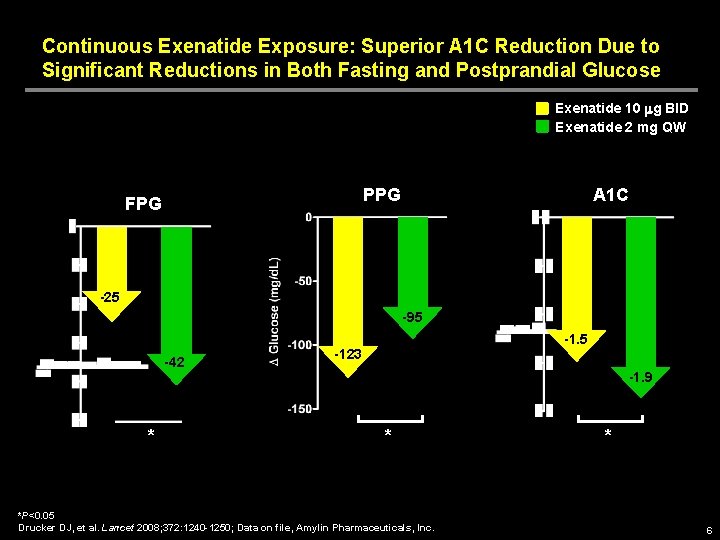
Continuous Exenatide Exposure: Superior A 1 C Reduction Due to Significant Reductions in Both Fasting and Postprandial Glucose Exenatide 10 mg BID Exenatide 2 mg QW PPG FPG A 1 C -25 -95 -42 * -1. 5 -123 -1. 9 * *P<0. 05 Drucker DJ, et al. Lancet 2008; 372: 1240 -1250; Data on file, Amylin Pharmaceuticals, Inc. * 6

Administered Once Weekly, the Microsphere Delivery System Provides Continuous Exposure to Therapeutic Levels of Exenatide 7

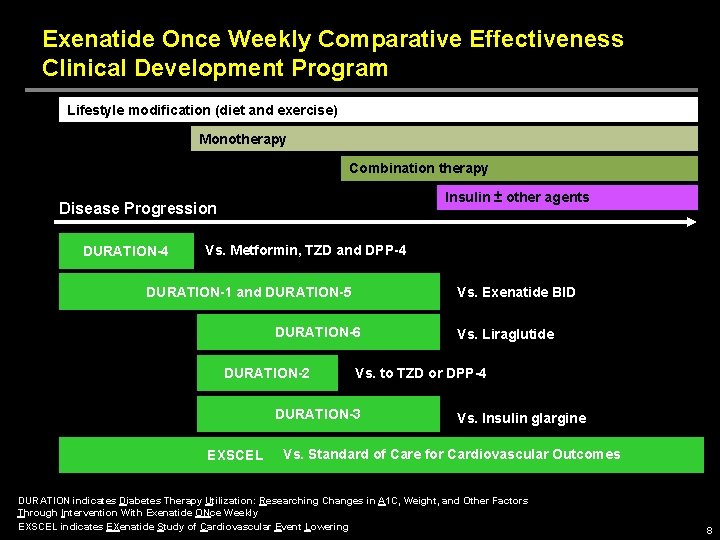
Exenatide Once Weekly Comparative Effectiveness Clinical Development Program Lifestyle modification (diet and exercise) Monotherapy Combination therapy Insulin ± other agents Disease Progression DURATION-4 Vs. Metformin, TZD and DPP-4 Vs. Exenatide BID DURATION-1 and DURATION-5 DURATION-6 DURATION-2 Vs. to TZD or DPP-4 DURATION-3 EXSCEL Vs. Liraglutide Vs. Insulin glargine Vs. Standard of Care for Cardiovascular Outcomes DURATION indicates Diabetes Therapy Utilization: Researching Changes in A 1 C, Weight, and Other Factors Through Intervention With Exenatide ONce Weekly EXSCEL indicates EXenatide Study of Cardiovascular Event Lowering 8

DURATION Superiority Program: Core Trial Design • Randomized, controlled superiority design – Enrolled type 2 patients not achieving targets (A 1 C 7 -11%) – Mimics real world setting (no wash out period, established background treatment) § Powered to assess superiority versus: § Exenatide BID and liraglutide § Oral agents (maximally approved doses pioglitazone, sitagliptin) § Basal insulin (insulin glargine, titrated to target) 2 mg Exenatide Once Weekly Comparator Agent(s) Long-term extension* 24 -30 weeks * DURATION-1, DURATION-2, DURATION-3 9

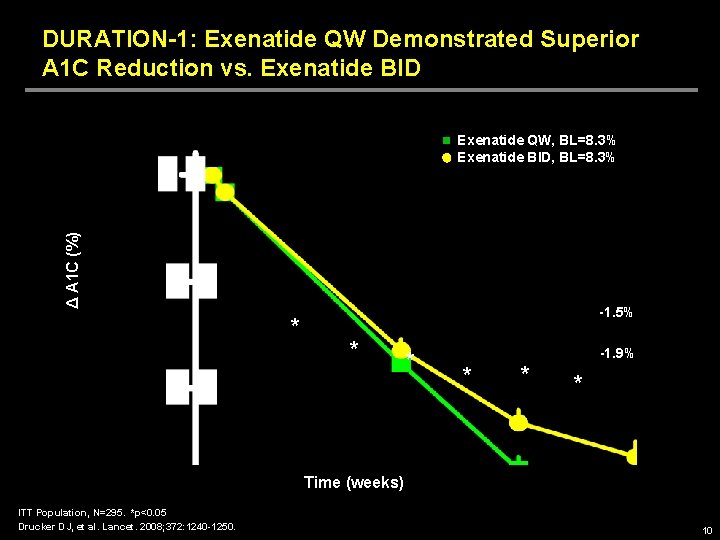
DURATION-1: Exenatide QW Demonstrated Superior A 1 C Reduction vs. Exenatide BID Δ A 1 C (%) Exenatide QW, BL=8. 3% Exenatide BID, BL=8. 3% * -1. 5% * * -1. 9% * * * Time (weeks) ITT Population, N=295. *p<0. 05 Drucker DJ, et al. Lancet. 2008; 372: 1240 -1250. 10

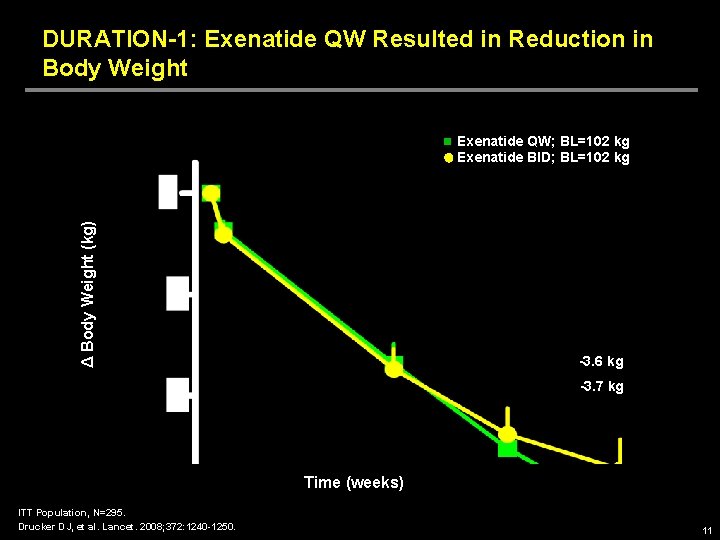
DURATION-1: Exenatide QW Resulted in Reduction in Body Weight Δ Body Weight (kg) Exenatide QW; BL=102 kg Exenatide BID; BL=102 kg -3. 6 kg -3. 7 kg Time (weeks) ITT Population, N=295. Drucker DJ, et al. Lancet. 2008; 372: 1240 -1250. 11

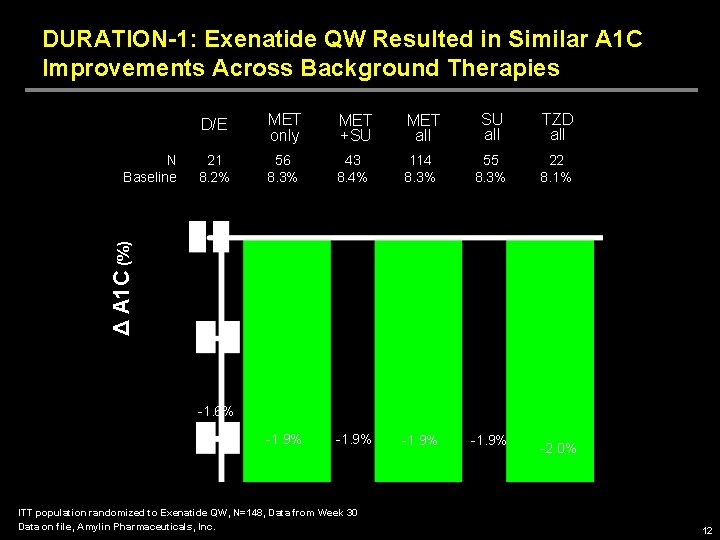
DURATION-1: Exenatide QW Resulted in Similar A 1 C Improvements Across Background Therapies MET only MET +SU MET all SU all TZD all 21 8. 2% 56 8. 3% 43 8. 4% 114 8. 3% 55 8. 3% 22 8. 1% -1. 9% Δ A 1 C (%) N Baseline D/E -1. 6% ITT population randomized to Exenatide QW, N=148, Data from Week 30 Data on file, Amylin Pharmaceuticals, Inc. -2. 0% 12

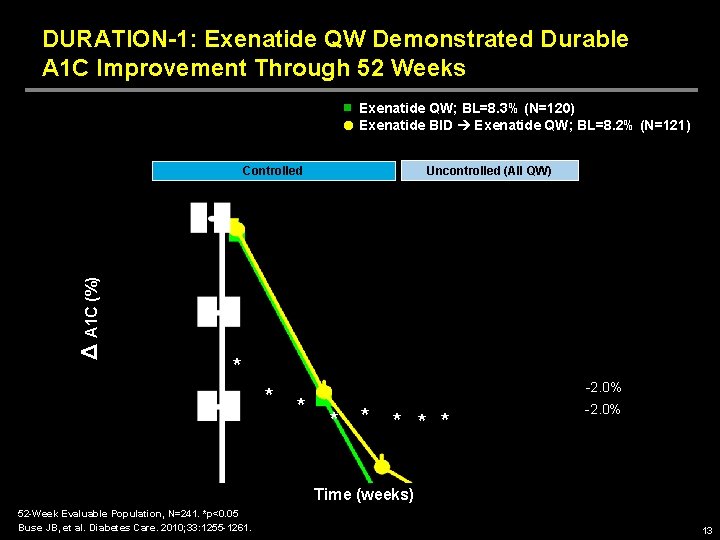
DURATION-1: Exenatide QW Demonstrated Durable A 1 C Improvement Through 52 Weeks Exenatide QW; BL=8. 3% (N=120) Exenatide BID Exenatide QW; BL=8. 2% (N=121) Uncontrolled (All QW) Δ A 1 C (%) Controlled * * * -2. 0% Time (weeks) 52 -Week Evaluable Population, N=241. *p<0. 05 Buse JB, et al. Diabetes Care. 2010; 33: 1255 -1261. 13

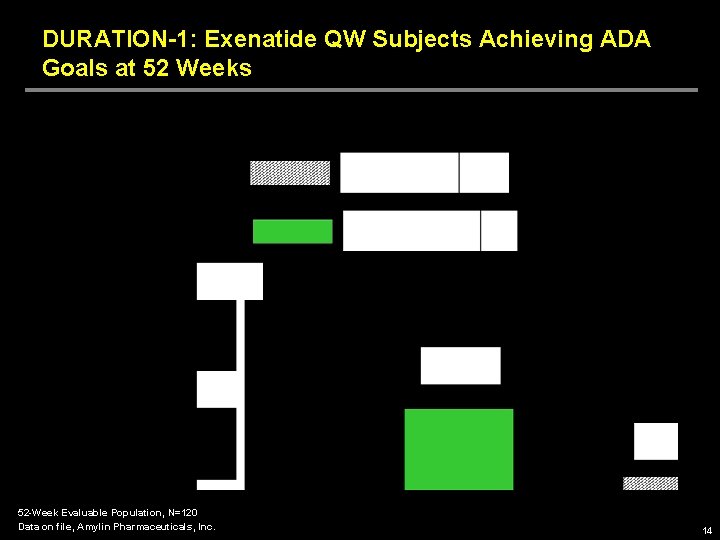
DURATION-1: Exenatide QW Subjects Achieving ADA Goals at 52 Weeks 52 -Week Evaluable Population, N=120 Data on file, Amylin Pharmaceuticals, Inc. 14

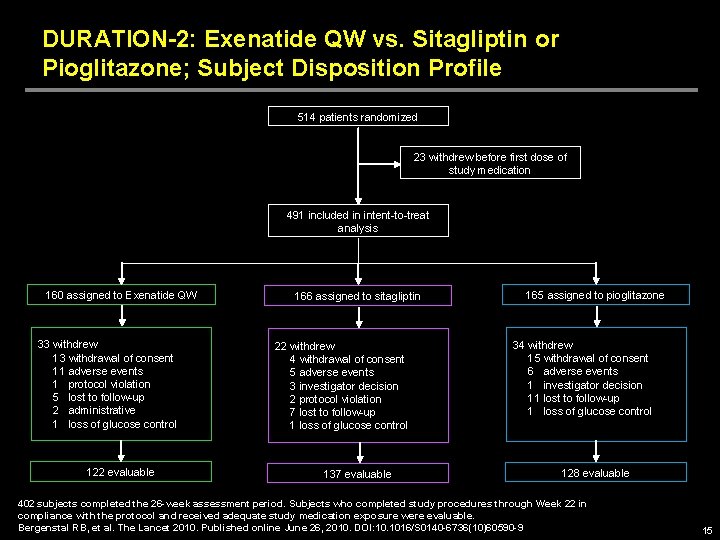
DURATION-2: Exenatide QW vs. Sitagliptin or Pioglitazone; Subject Disposition Profile 514 patients randomized 23 withdrew before first dose of study medication 491 included in intent-to-treat analysis 160 assigned to Exenatide QW 33 withdrew 13 withdrawal of consent 11 adverse events 1 protocol violation 5 lost to follow-up 2 administrative 1 loss of glucose control 122 evaluable 166 assigned to sitagliptin 22 withdrew 4 withdrawal of consent 5 adverse events 3 investigator decision 2 protocol violation 7 lost to follow-up 1 loss of glucose control 137 evaluable 165 assigned to pioglitazone 34 withdrew 15 withdrawal of consent 6 adverse events 1 investigator decision 11 lost to follow-up 1 loss of glucose control 128 evaluable 402 subjects completed the 26 -week assessment period. Subjects who completed study procedures through Week 22 in compliance with the protocol and received adequate study medication exposure were evaluable. Bergenstal RB, et al. The Lancet 2010. Published online June 26, 2010. DOI: 10. 1016/S 0140 -6736(10)60590 -9 15

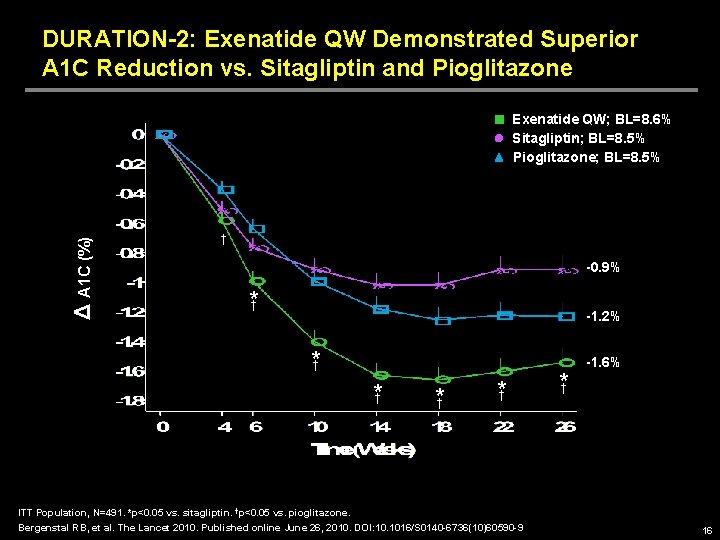
DURATION-2: Exenatide QW Demonstrated Superior A 1 C Reduction vs. Sitagliptin and Pioglitazone Δ A 1 C (%) Exenatide QW; BL=8. 6% Sitagliptin; BL=8. 5% Pioglitazone; BL=8. 5% † -0. 9% * † -1. 2% *† *† ITT Population, N=491. *p<0. 05 vs. sitagliptin. †p<0. 05 vs. pioglitazone. Bergenstal RB, et al. The Lancet 2010. Published online June 26, 2010. DOI: 10. 1016/S 0140 -6736(10)60590 -9 *† -1. 6% 16

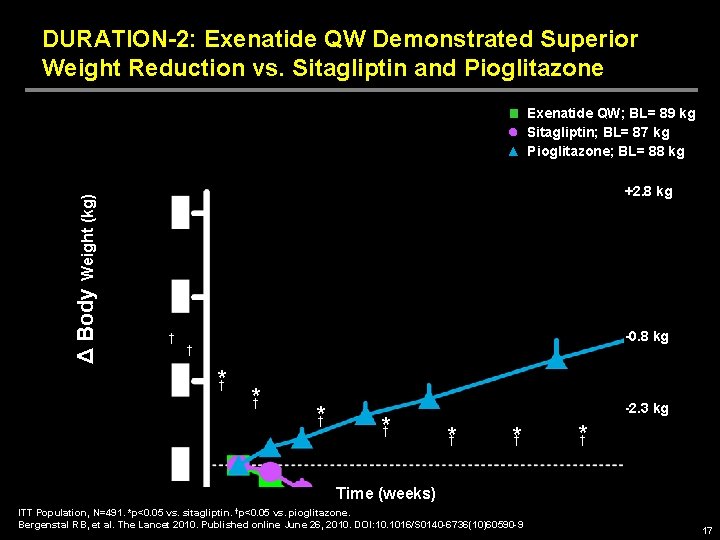
DURATION-2: Exenatide QW Demonstrated Superior Weight Reduction vs. Sitagliptin and Pioglitazone Δ Body Weight (kg) Exenatide QW; BL= 89 kg Sitagliptin; BL= 87 kg Pioglitazone; BL= 88 kg +2. 8 kg † -0. 8 kg † *† *† -2. 3 kg *† *† *† Time (weeks) ITT Population, N=491. *p<0. 05 vs. sitagliptin. †p<0. 05 vs. pioglitazone. Bergenstal RB, et al. The Lancet 2010. Published online June 26, 2010. DOI: 10. 1016/S 0140 -6736(10)60590 -9 17

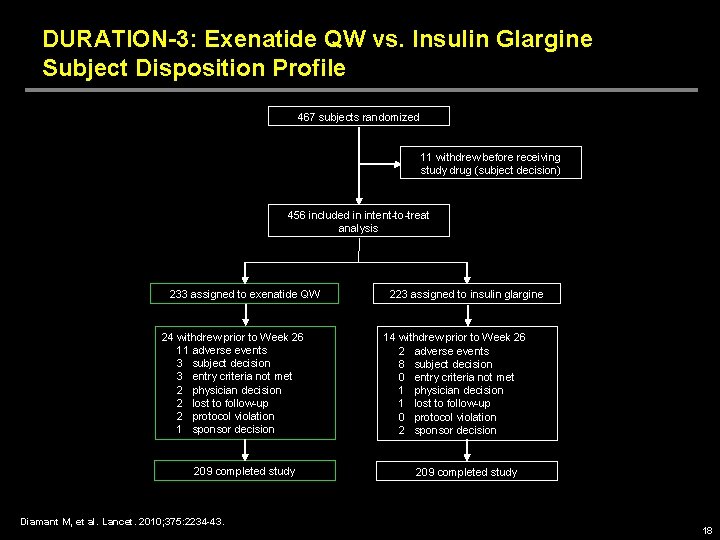
DURATION-3: Exenatide QW vs. Insulin Glargine Subject Disposition Profile 467 subjects randomized 11 withdrew before receiving study drug (subject decision) 456 included in intent-to-treat analysis 233 assigned to exenatide QW 24 withdrew prior to Week 26 11 adverse events 3 subject decision 3 entry criteria not met 2 physician decision 2 lost to follow-up 2 protocol violation 1 sponsor decision 209 completed study Diamant M, et al. Lancet. 2010; 375: 2234 -43. 223 assigned to insulin glargine 14 withdrew prior to Week 26 2 adverse events 8 subject decision 0 entry criteria not met 1 physician decision 1 lost to follow-up 0 protocol violation 2 sponsor decision 209 completed study 18

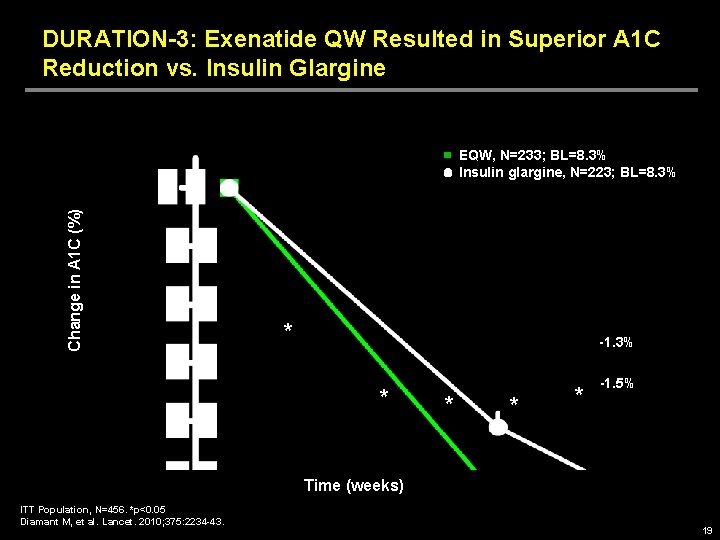
DURATION-3: Exenatide QW Resulted in Superior A 1 C Reduction vs. Insulin Glargine Change in A 1 C (%) EQW, N=233; BL=8. 3% Insulin glargine, N=223; BL=8. 3% * -1. 3% * * -1. 5% Time (weeks) ITT Population, N=456. *p<0. 05 Diamant M, et al. Lancet. 2010; 375: 2234 -43. 19

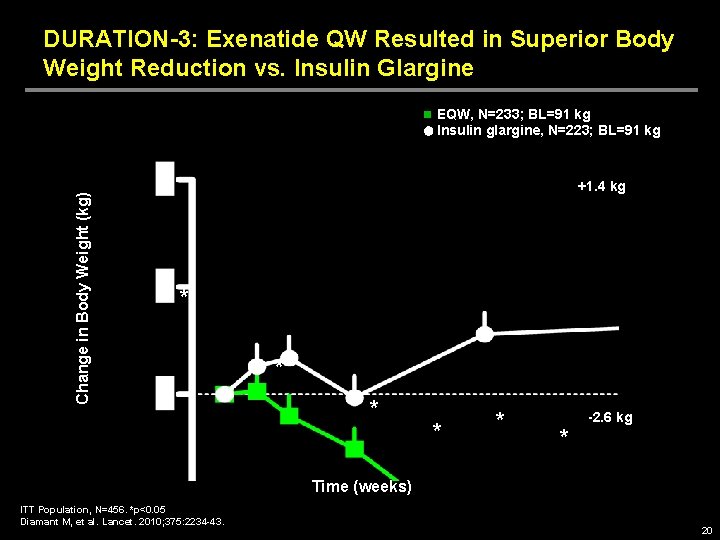
DURATION-3: Exenatide QW Resulted in Superior Body Weight Reduction vs. Insulin Glargine Change in Body Weight (kg) EQW, N=233; BL=91 kg Insulin glargine, N=223; BL=91 kg +1. 4 kg * * * -2. 6 kg * Time (weeks) ITT Population, N=456. *p<0. 05 Diamant M, et al. Lancet. 2010; 375: 2234 -43. 20

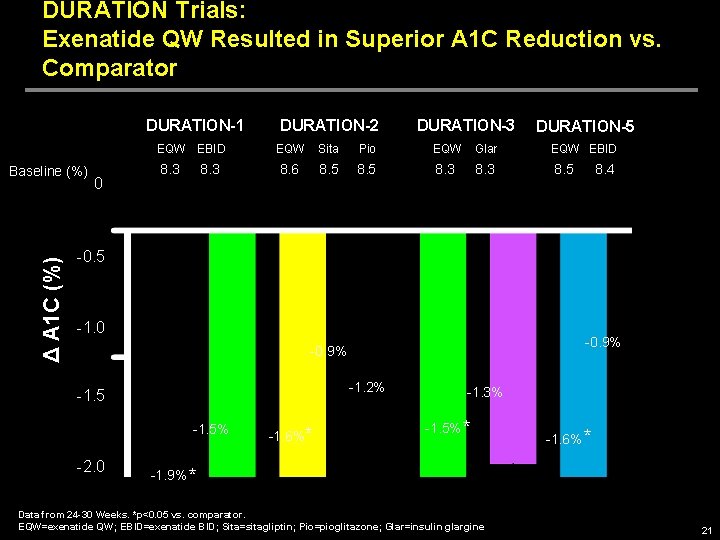
DURATION Trials: Exenatide QW Resulted in Superior A 1 C Reduction vs. Comparator DURATION-1 Δ A 1 C (%) Baseline (%) 0 DURATION-2 DURATION-3 DURATION-5 EQW EBID EQW Sita Pio EQW Glar EQW EBID 8. 3 8. 6 8. 5 8. 3 8. 4 -0. 5 -1. 0 -0. 9% -1. 2% -1. 5% -2. 0 -1. 6%* -1. 3% -1. 5% * -1. 6% * -1. 9% * Data from 24 -30 Weeks. *p<0. 05 vs. comparator. EQW=exenatide QW; EBID=exenatide BID; Sita=sitagliptin; Pio=pioglitazone; Glar=insulin glargine 21

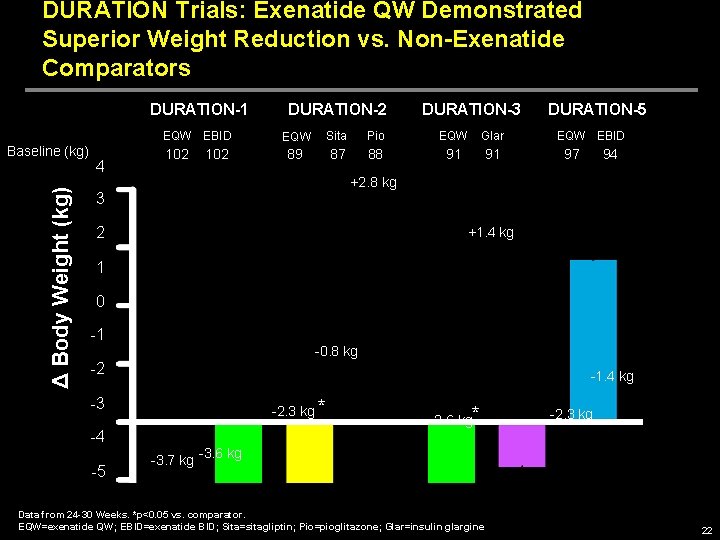
DURATION Trials: Exenatide QW Demonstrated Superior Weight Reduction vs. Non-Exenatide Comparators DURATION-1 EQW EBID Δ Body Weight (kg) Baseline (kg) 4 102 DURATION-2 DURATION-3 EQW Sita Pio EQW Glar 89 87 88 91 91 DURATION-5 EQW EBID 97 94 +2. 8 kg 3 2 +1. 4 kg 1 0 -1 -0. 8 kg -2 -1. 4 kg -3 -2. 3 kg * -4 -5 -2. 6 kg* -2. 3 kg -3. 7 kg -3. 6 kg Data from 24 -30 Weeks. *p<0. 05 vs. comparator. EQW=exenatide QW; EBID=exenatide BID; Sita=sitagliptin; Pio=pioglitazone; Glar=insulin glargine 22

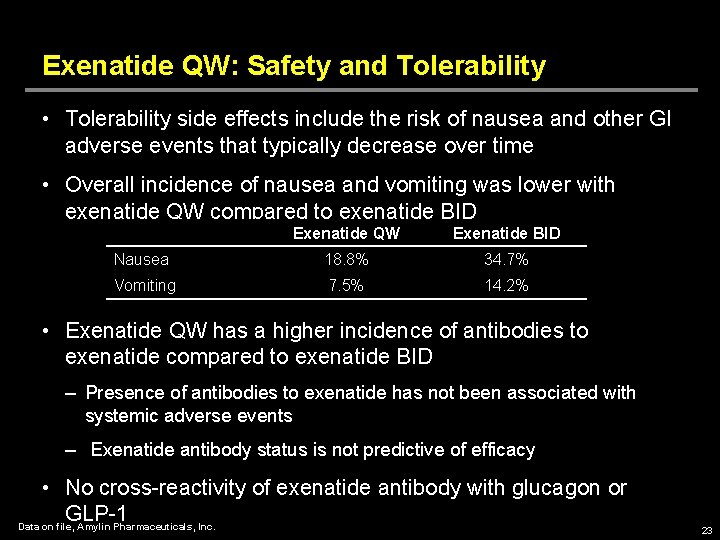
Exenatide QW: Safety and Tolerability • Tolerability side effects include the risk of nausea and other GI adverse events that typically decrease over time • Overall incidence of nausea and vomiting was lower with exenatide QW compared to exenatide BID Exenatide QW Exenatide BID Nausea 18. 8% 34. 7% Vomiting 7. 5% 14. 2% • Exenatide QW has a higher incidence of antibodies to exenatide compared to exenatide BID – Presence of antibodies to exenatide has not been associated with systemic adverse events – Exenatide antibody status is not predictive of efficacy • No cross-reactivity of exenatide antibody with glucagon or GLP-1 Data on file, Amylin Pharmaceuticals, Inc. 23

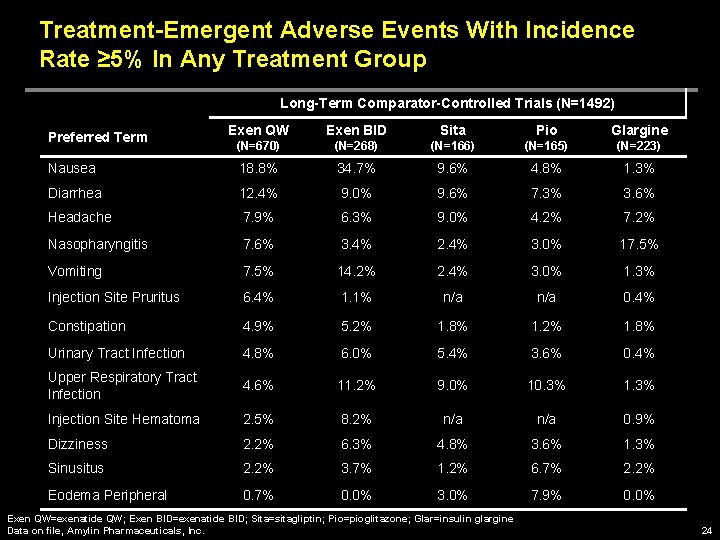
Treatment-Emergent Adverse Events With Incidence Rate ≥ 5% In Any Treatment Group Long-Term Comparator-Controlled Trials (N=1492) Exen QW Exen BID Sita Pio Glargine (N=670) (N=268) (N=166) (N=165) (N=223) Nausea 18. 8% 34. 7% 9. 6% 4. 8% 1. 3% Diarrhea 12. 4% 9. 0% 9. 6% 7. 3% 3. 6% Headache 7. 9% 6. 3% 9. 0% 4. 2% 7. 2% Nasopharyngitis 7. 6% 3. 4% 2. 4% 3. 0% 17. 5% Vomiting 7. 5% 14. 2% 2. 4% 3. 0% 1. 3% Injection Site Pruritus 6. 4% 1. 1% n/a 0. 4% Constipation 4. 9% 5. 2% 1. 8% 1. 2% 1. 8% Urinary Tract Infection 4. 8% 6. 0% 5. 4% 3. 6% 0. 4% Upper Respiratory Tract Infection 4. 6% 11. 2% 9. 0% 10. 3% 1. 3% Injection Site Hematoma 2. 5% 8. 2% n/a 0. 9% Dizziness 2. 2% 6. 3% 4. 8% 3. 6% 1. 3% Sinusitus 2. 2% 3. 7% 1. 2% 6. 7% 2. 2% Eodema Peripheral 0. 7% 0. 0% 3. 0% 7. 9% 0. 0% Preferred Term Exen QW=exenatide QW; Exen BID=exenatide BID; Sita=sitagliptin; Pio=pioglitazone; Glar=insulin glargine Data on file, Amylin Pharmaceuticals, Inc. 24

Exenatide QW: Safety and Tolerability • Injection site related adverse events occurred more frequently with exenatide QW than comparators also administered by injection (exenatide BID and insulin glargine) – Events were mild, transient and resolved without interruption of exenatide once weekly treatment • The risk of hypoglycemia with exenatide QW was similar to non-insulin comparators (exenatide BID, sitagliptin, pioglitazone) and less compared to insulin glargine • Incidence of hypoglycemia with exenatide QW was low and was related to the concomitant use of an SU agent – No increased risk of hypoglycemia among patients using exenatide QW with metformin, a TZD or diet and exercise • A small (2. 7 beats/min), persistent increase in heart rate was associated with exenatide QW therapy, without an identifiable effect on clinical outcomes or cardiovascular events. Data on file, Amylin Pharmaceuticals, Inc. 25

Exenatide QW: Safety and Tolerability • Data from the exenatide BID and QW clinical programs and exenatide BID epidemiologic studies demonstrated no increased risk of pancreatitis in patients receiving exenatide relative to comparator treatment or placebo • Nonclinical carcinogenicity studies have shown an increased incidence of thyroid c-cell adenoma and carcinomas in rats with exenatide QW compared to controls – The human relevance of this finding is unknown • Clinical data with exenatide QW or exenatide BID, and spontaneously reported and epidemiological data from exenatide BID use have not suggested a risk of MTC with exenatide use in humans Data on file, Amylin Pharmaceuticals, Inc. 26

Exenatide QW Summary • Superior glycemic control with EQW compared to maximum daily doses of BYETTA, sitagliptin, pioglitazone and treat-to-target insulin glargine • EQW weight reduction was superior compared to all nonexenatide comparators • EQW demonstrated durable improvements in A 1 C and weight through 52 weeks of treatment • EQW safety profile was consistent with that of BYETTA and was generally well tolerated • EQW was associated with low incidence of hypoglycemia 27
 Name
Name Gd and t symbols
Gd and t symbols Time variance
Time variance Relativism
Relativism Material and non material culture examples
Material and non material culture examples Example of material culture
Example of material culture Useful and harmful materials found at home
Useful and harmful materials found at home Give us your hungry your tired your poor
Give us your hungry your tired your poor Fspos vägledning för kontinuitetshantering
Fspos vägledning för kontinuitetshantering Novell typiska drag
Novell typiska drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Ekologiskt fotavtryck
Ekologiskt fotavtryck Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidbok yrkesförare
Tidbok yrkesförare Anatomi organ reproduksi
Anatomi organ reproduksi Densitet vatten
Densitet vatten Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Debattartikel mall
Debattartikel mall Magnetsjukhus
Magnetsjukhus Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Lufttryck formel
Lufttryck formel Publik sektor
Publik sektor Kyssande vind analys
Kyssande vind analys Presentera för publik crossboss
Presentera för publik crossboss