DisclosureDisclaimer All material is intended for your medical







- Slides: 15

Disclosure/Disclaimer • All material is intended for your medical education purposes only 1

Effects Of Exenatide Versus Sitagliptin On Postprandial Glucose, Insulin and Glucagon Secretion, Gastric Emptying, And Caloric Intake: A Randomized, Cross-Over Study Ralph A. De. Fronzo 1; Ted Okerson 2; Prabhakar Viswanathan 2; Xuesong Guan 2; John H. Holcombe 3; Leigh Mac. Conell 2 1 Division of Diabetes, University of Texas Health Science Center, San Antonio, TX, USA; 2 Amylin Pharmaceuticals, Inc. , San Diego, CA, USA; 3 Eli Lilly and Company, Indianapolis, IN, USA

Introduction • This study provides the first head-to-head comparison of incretin-based therapies developed to treat T 2 DM 1: – The GLP-1 receptor agonist, exenatide – The dipeptidyl peptidase-4 (DPP-4) inhibitor, sitagliptin • Exenatide binds directly to the GLP-1 receptor and is at least equipotent, if not more potent, than GLP‑ 12, 3 in: – Stimulation of glucose-dependent secretion of insulin – Restoration of first-phase and second-phase insulin secretion – Suppression of inappropriately elevated postprandial glucagon secretion – Slowing of gastric emptying – Reduction in food intake – Promotion of β-cell proliferation and islet neogenesis from precursor cells in both in vitro and in vivo models of diabetes 1 3

Introduction • The DPP-4 inhibitor sitagliptin inhibits the proteolytic cleavage of GLP-1 in the circulation by binding to and reducing the activity of the DPP-4 enzyme by approximately 80%, increasing the concentration of endogenous GLP-1 by approximately 2 -fold, thereby: – Reducing plasma glucagon and increasing insulin and C-peptide concentrations, and increasing β -cell mass and function 1 4

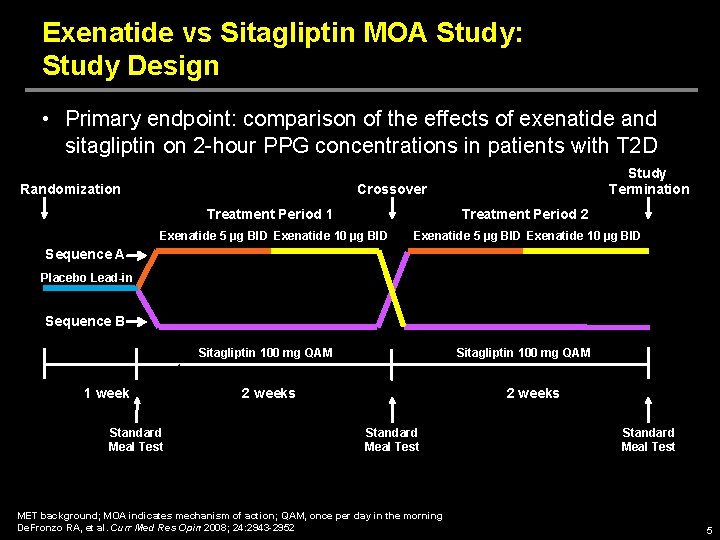
Exenatide vs Sitagliptin MOA Study: Study Design • Primary endpoint: comparison of the effects of exenatide and sitagliptin on 2 -hour PPG concentrations in patients with T 2 D Randomization Study Termination Crossover Treatment Period 1 Treatment Period 2 Exenatide 5 µg BID Exenatide 10 µg BID Sequence A Placebo Lead-in Sequence B Sitagliptin 100 mg QAM 1 week Standard Meal Test Sitagliptin 100 mg QAM 2 weeks Standard Meal Test MET background; MOA indicates mechanism of action; QAM, once per day in the morning De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 Standard Meal Test 5

Demographic and Baseline Characteristics Evaluable Patients (n = 61) Sex, Female/Male (%) 54/46 Age (y) 54 ± 9 Race, Caucasian/Black/Hispanic (%) 30/8/62 Height (cm) 167. 0 ± 9. 9 Body Weight (kg) 91. 5 ± 18. 8 BMI (kg/m 2) 32. 6 ± 5. 1 A 1 C (%) 8. 5 ± 1. 2 Duration of Diabetes (y) 7± 5 Fasting Triglycerides (mg/d. L) 166 ± 93 FPG (mg/d. L) 178 ± 48 2 -hr PPG (mg/d. L) 245 ± 65 Patients with T 2 D; MET background; Mean ± SD, unless otherwise indicated; BMI indicates body mass index De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 6

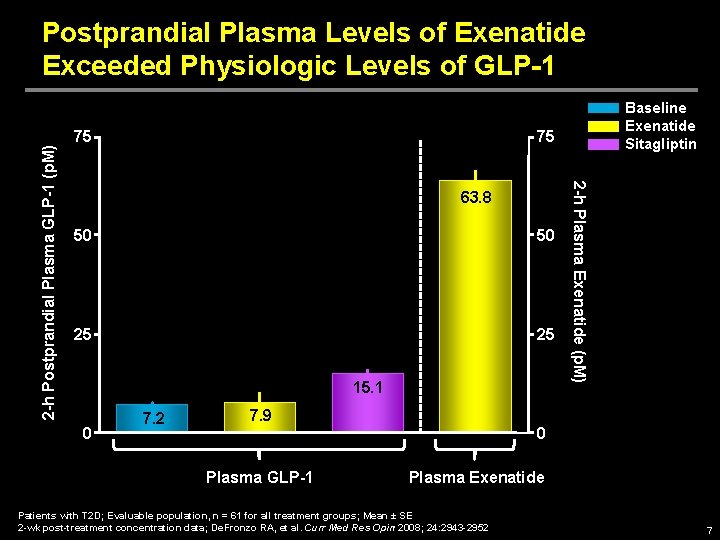
75 75 63. 8 50 50 25 25 15. 1 0 Baseline Exenatide Sitagliptin 7. 2 2 -h Plasma Exenatide (p. M) 2 -h Postprandial Plasma GLP-1 (p. M) Postprandial Plasma Levels of Exenatide Exceeded Physiologic Levels of GLP-1 7. 9 0 Plasma GLP-1 Plasma Exenatide Patients with T 2 D; Evaluable population, n = 61 for all treatment groups; Mean ± SE 2 -wk post-treatment concentration data; De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 7

Exenatide Reduced PPG Concentrations To a Greater Extent Than Sitagliptin Baseline Exenatide Sitagliptin PPG (mg/d. L) Primary Endpoint Standard Meal Time (min) Patients with T 2 D; Evaluable population, n = 61 for all treatment groups; Mean ± SE; * LS mean ± SE, P<0. 0001 De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 8

Reductions in 2 -Hour PPG Were Greater With Exenatide Than With Sitagliptin 2 -hr PPG (mg/d. L) Exenatide Sitagliptin Baseline End of Period 1 End of Period 2 • After Period 1, patients were switched to the otherapy Patients with T 2 D; Evaluable population: exenatide-sitagliptin, n = 29; sitagliptin-exenatide, n = 32 Mean ± SE; De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 9

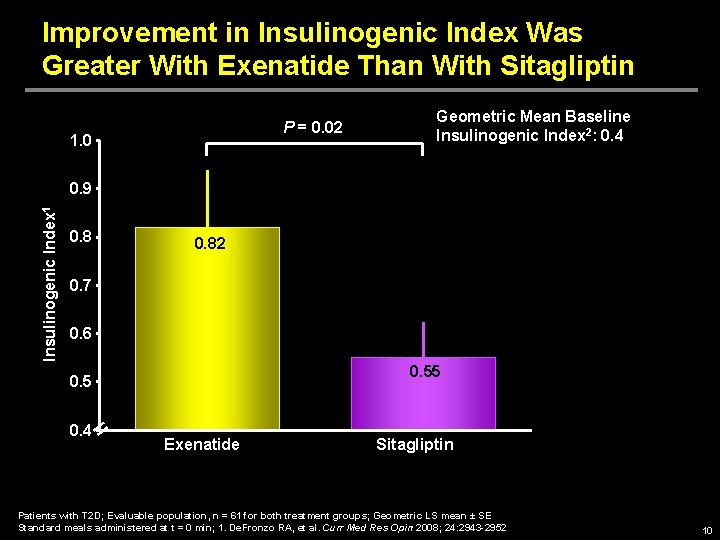
Improvement in Insulinogenic Index Was Greater With Exenatide Than With Sitagliptin P = 0. 02 1. 0 Geometric Mean Baseline Insulinogenic Index 2: 0. 4 Insulinogenic Index 1 0. 9 0. 82 0. 7 0. 6 0. 55 0. 4 Exenatide Sitagliptin Patients with T 2 D; Evaluable population, n = 61 for both treatment groups; Geometric LS mean ± SE Standard meals administered at t = 0 min; 1. De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 10

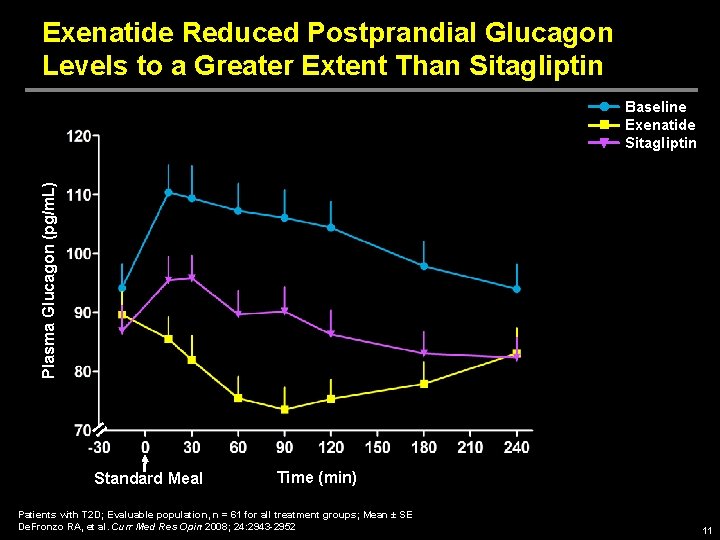
Exenatide Reduced Postprandial Glucagon Levels to a Greater Extent Than Sitagliptin Plasma Glucagon (pg/m. L) Baseline Exenatide Sitagliptin Standard Meal Time (min) Patients with T 2 D; Evaluable population, n = 61 for all treatment groups; Mean ± SE De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 11

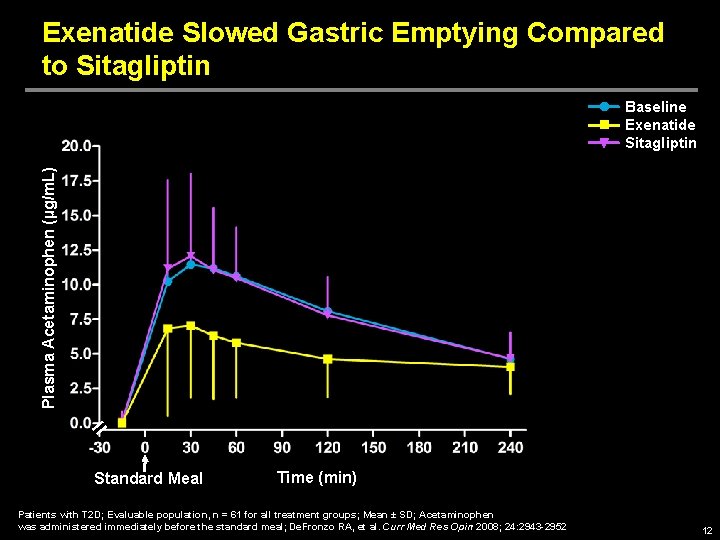
Exenatide Slowed Gastric Emptying Compared to Sitagliptin Plasma Acetaminophen (µg/m. L) Baseline Exenatide Sitagliptin Standard Meal Time (min) Patients with T 2 D; Evaluable population, n = 61 for all treatment groups; Mean ± SD; Acetaminophen was administered immediately before the standard meal; De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 12

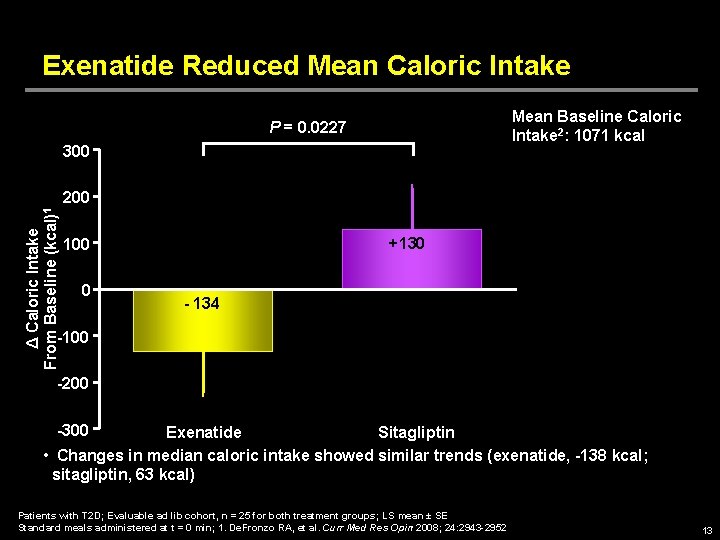
Exenatide Reduced Mean Caloric Intake Mean Baseline Caloric Intake 2: 1071 kcal P = 0. 0227 300 Δ Caloric Intake From Baseline (kcal)1 200 +130 100 0 -100 - 134 -200 -300 Exenatide Sitagliptin • Changes in median caloric intake showed similar trends (exenatide, -138 kcal; sitagliptin, 63 kcal) Patients with T 2 D; Evaluable ad lib cohort, n = 25 for both treatment groups; LS mean ± SE Standard meals administered at t = 0 min; 1. De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 13

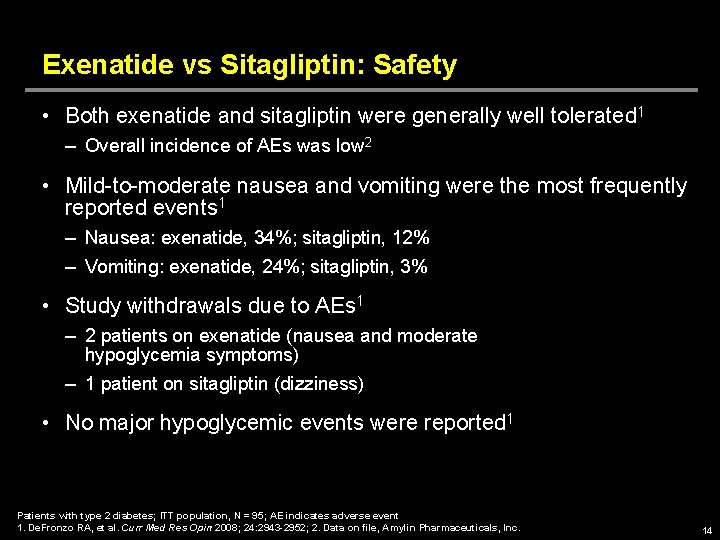
Exenatide vs Sitagliptin: Safety • Both exenatide and sitagliptin were generally well tolerated 1 – Overall incidence of AEs was low 2 • Mild-to-moderate nausea and vomiting were the most frequently reported events 1 – Nausea: exenatide, 34%; sitagliptin, 12% – Vomiting: exenatide, 24%; sitagliptin, 3% • Study withdrawals due to AEs 1 – 2 patients on exenatide (nausea and moderate hypoglycemia symptoms) – 1 patient on sitagliptin (dizziness) • No major hypoglycemic events were reported 1 Patients with type 2 diabetes; ITT population, N = 95; AE indicates adverse event 1. De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952; 2. Data on file, Amylin Pharmaceuticals, Inc. 14

Exenatide vs Sitagliptin MOA Study: Conclusions • 2 -hr PPG concentration was significantly reduced with exenatide compared with sitagliptin • Compared with sitagliptin treatment, exenatide treatment led to – Greater reductions in § PPG concentrations over time § Postprandial glucose excursions § Postprandial glucagon levels – Improved insulinogenic index – Delayed gastric emptying – Decreased caloric intake • Changes in FPG concentrations were comparable with exenatide and sitagliptin • Both exenatide and sitagliptin were generally well tolerated De. Fronzo RA, et al. Curr Med Res Opin 2008; 24: 2943 -2952 15
 Draw three noncollinear points j k and l
Draw three noncollinear points j k and l Profile tolerance symbol
Profile tolerance symbol Rsq formula in standard costing
Rsq formula in standard costing Popular culture examples
Popular culture examples Examples of non material culture
Examples of non material culture All groups create norms to enforce their cultural values.
All groups create norms to enforce their cultural values. Materials found at home that are useful and harmful
Materials found at home that are useful and harmful Give us your hungry your tired your poor
Give us your hungry your tired your poor Formuö
Formuö Typiska drag för en novell
Typiska drag för en novell Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Returpilarna
Returpilarna Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Underlag för särskild löneskatt på pensionskostnader
Underlag för särskild löneskatt på pensionskostnader Personlig tidbok för yrkesförare
Personlig tidbok för yrkesförare