DisclosureDisclaimer All material is intended for your medical





- Slides: 14

Disclosure/Disclaimer • All material is intended for your medical education purposes only 1

DURATION Program: Exenatide Once Weekly Effects on the Cardiovascular System Amylin Pharmaceuticals, Inc. , San Diego, CA, USA Eli Lilly and Company, Indianapolis, IN, USA 2

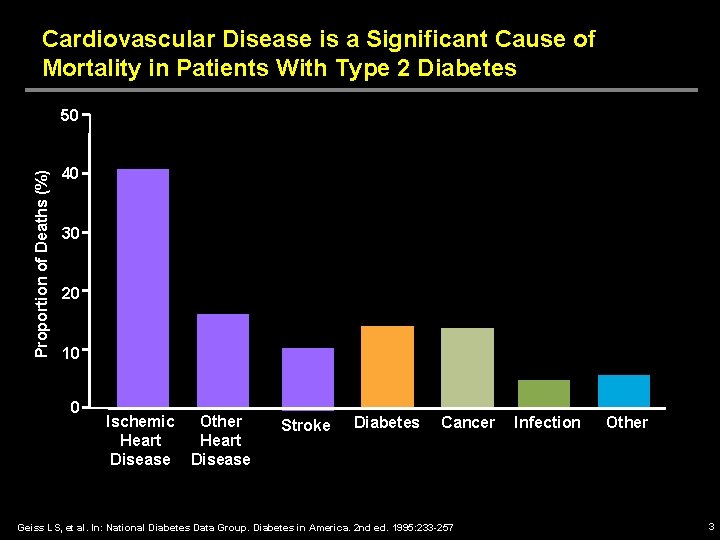
Cardiovascular Disease is a Significant Cause of Mortality in Patients With Type 2 Diabetes Proportion of Deaths (%) 50 40 30 20 10 0 Ischemic Other Heart Disease Stroke Diabetes Cancer Geiss LS, et al. In: National Diabetes Data Group. Diabetes in America. 2 nd ed. 1995: 233 -257 Infection Other 3

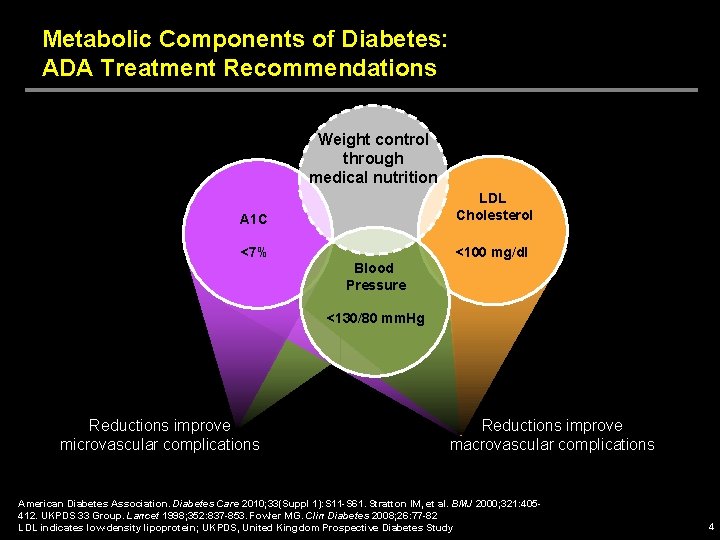
Metabolic Components of Diabetes: ADA Treatment Recommendations Weight control through medical nutrition A 1 C LDL Cholesterol <7% <100 mg/dl Blood Pressure <130/80 mm. Hg Reductions improve microvascular complications Reductions improve macrovascular complications American Diabetes Association. Diabetes Care 2010; 33(Suppl 1): S 11 -S 61. Stratton IM, et al. BMJ 2000; 321: 405412. UKPDS 33 Group. Lancet 1998; 352: 837 -853. Fowler MG. Clin Diabetes 2008; 26: 77 -82 LDL indicates low-density lipoprotein; UKPDS, United Kingdom Prospective Diabetes Study 4

GLP-1 in Tissue and Animal Models of Cardiac Function GLP-1 receptor expression in: GLP-1 R agonism: • myocytes • • • pericardium increases myocardial glucose uptake • vascular epithelium • attenuates ischemic injury • vascular smooth muscle • limits infarct size • Improves: leads to dose-dependent vasodilatation – – Ban K, et al. Circulation 2008; 117: 2340 -2350; Bose AK, et al. Diabetes 2005; 54: 146 -151; Nikolaidis LA, • et al. Circulation 2004; 110: 955 -961; Sonne DP, et al. Regulatory Peptides 2008; 146: 243 -249; Timmers L, et al. J Am Coll Cardiol 2009; 53: 501 -10; Zhao T, et al. JPET. 2006; 317: 1106 -1113. stroke volume cardiac output ejection fraction systemic resistance effects are diminished by receptor antagonism 5

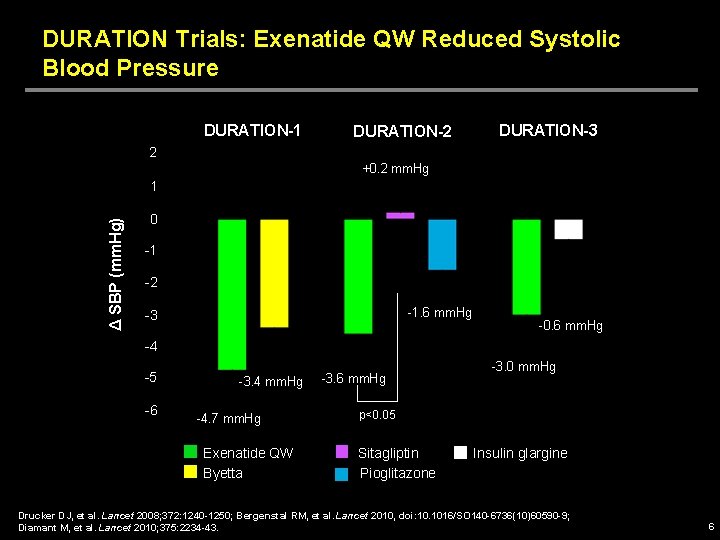
DURATION Trials: Exenatide QW Reduced Systolic Blood Pressure DURATION-1 DURATION-2 DURATION-3 2 +0. 2 mm. Hg Δ SBP (mm. Hg) 1 0 -1 -2 -1. 6 mm. Hg -3 -0. 6 mm. Hg -4 -5 -6 -3. 4 mm. Hg -4. 7 mm. Hg Exenatide QW Byetta -3. 6 mm. Hg -3. 0 mm. Hg p<0. 05 Sitagliptin Pioglitazone Insulin glargine Drucker DJ, et al. Lancet 2008; 372: 1240 -1250; Bergenstal RM, et al. Lancet 2010, doi: 10. 1016/SO 140 -6736(10)60590 -9; Diamant M, et al. Lancet 2010; 375: 2234 -43. 6

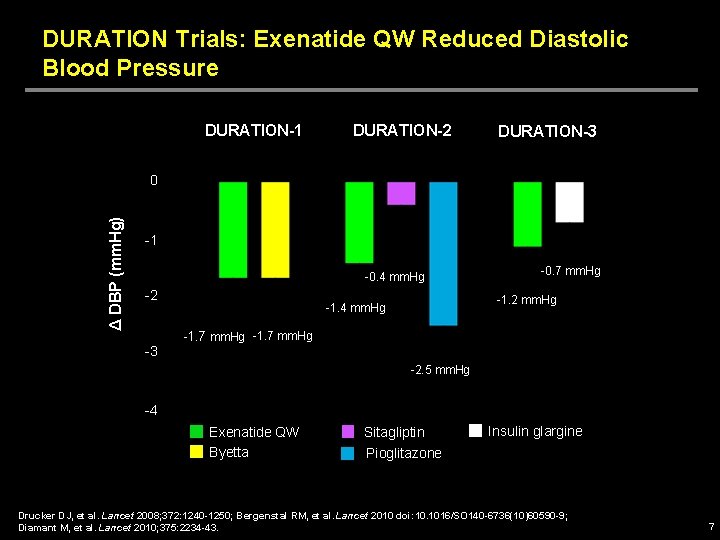
DURATION Trials: Exenatide QW Reduced Diastolic Blood Pressure DURATION-1 DURATION-2 DURATION-3 Δ DBP (mm. Hg) 0 -1 -0. 4 mm. Hg -2 -3 -0. 7 mm. Hg -1. 2 mm. Hg -1. 4 mm. Hg -1. 7 mm. Hg -2. 5 mm. Hg -4 Exenatide QW Byetta Sitagliptin Pioglitazone Insulin glargine Drucker DJ, et al. Lancet 2008; 372: 1240 -1250; Bergenstal RM, et al. Lancet 2010 doi: 10. 1016/SO 140 -6736(10)60590 -9; Diamant M, et al. Lancet 2010; 375: 2234 -43. 7

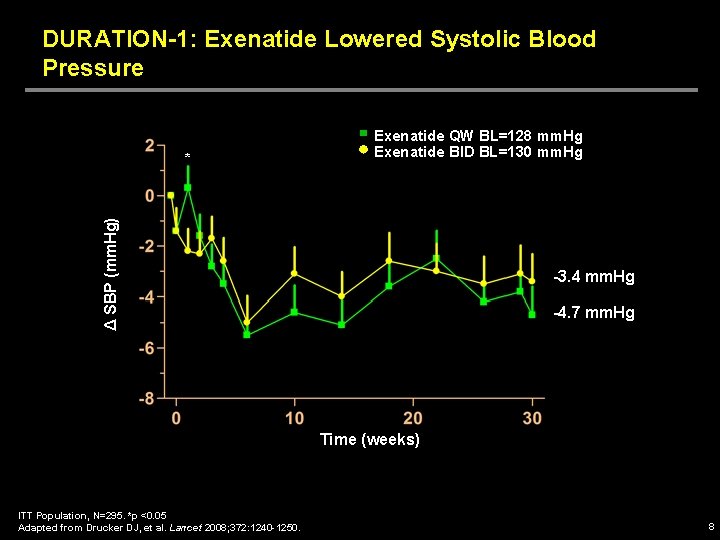
DURATION-1: Exenatide Lowered Systolic Blood Pressure Δ SBP (mm. Hg) * Exenatide QW BL=128 mm. Hg Exenatide BID BL=130 mm. Hg -3. 4 mm. Hg -4. 7 mm. Hg Time (weeks) ITT Population, N=295. *p <0. 05 Adapted from Drucker DJ, et al. Lancet 2008; 372: 1240 -1250. 8

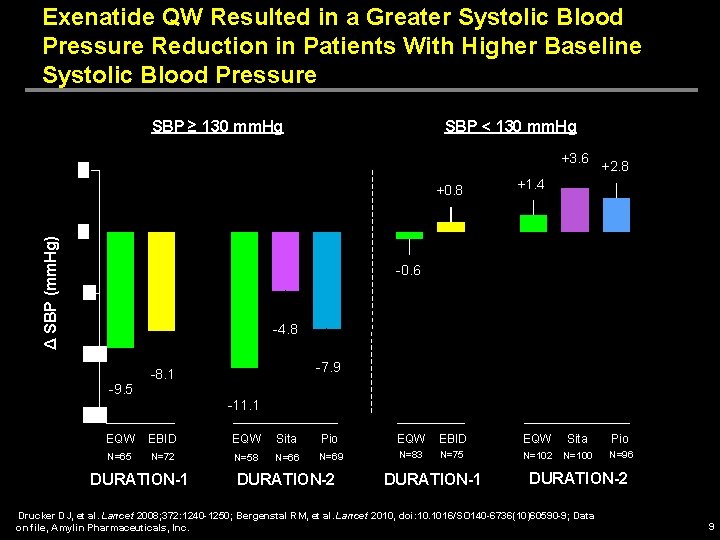
Exenatide QW Resulted in a Greater Systolic Blood Pressure Reduction in Patients With Higher Baseline Systolic Blood Pressure SBP ≥ 130 mm. Hg SBP < 130 mm. Hg +3. 6 Δ SBP (mm. Hg) +0. 8 +2. 8 +1. 4 -0. 6 -4. 8 -9. 5 -7. 9 -8. 1 -11. 1 EQW EBID EQW Sita Pio N=65 N=72 N=58 N=66 N=69 N=83 N=75 N=102 N=100 N=96 DURATION-1 DURATION-2 Drucker DJ, et al. Lancet 2008; 372: 1240 -1250; Bergenstal RM, et al. Lancet 2010, doi: 10. 1016/SO 140 -6736(10)60590 -9; Data on file, Amylin Pharmaceuticals, Inc. 9

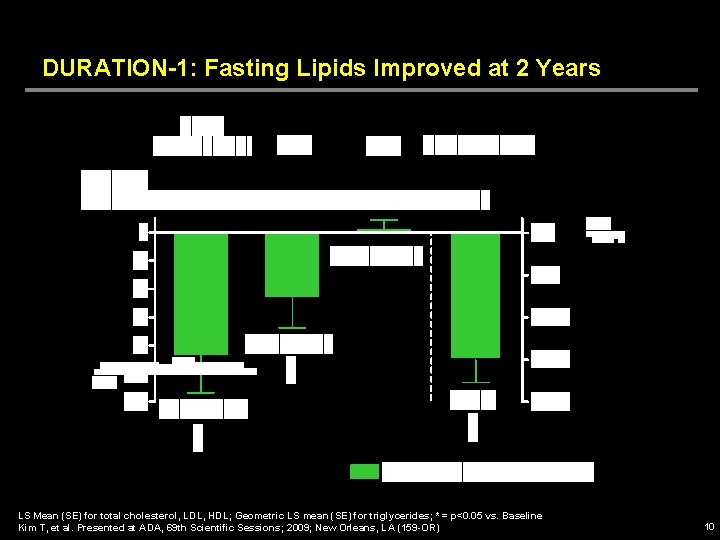
DURATION-1: Fasting Lipids Improved at 2 Years LS Mean (SE) for total cholesterol, LDL, HDL; Geometric LS mean (SE) for triglycerides; * = p<0. 05 vs. Baseline Kim T, et al. Presented at ADA, 69 th Scientific Sessions; 2009; New Orleans, LA (159 -OR) 10

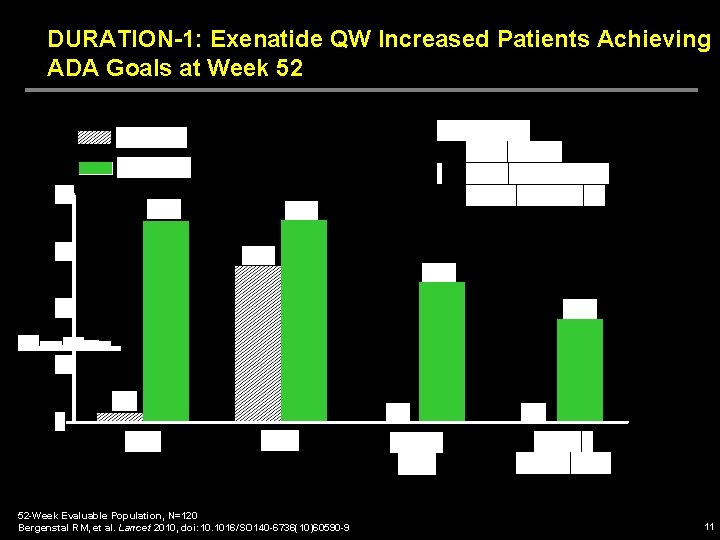
DURATION-1: Exenatide QW Increased Patients Achieving ADA Goals at Week 52 52 -Week Evaluable Population, N=120 Bergenstal RM, et al. Lancet 2010, doi: 10. 1016/SO 140 -6736(10)60590 -9 11

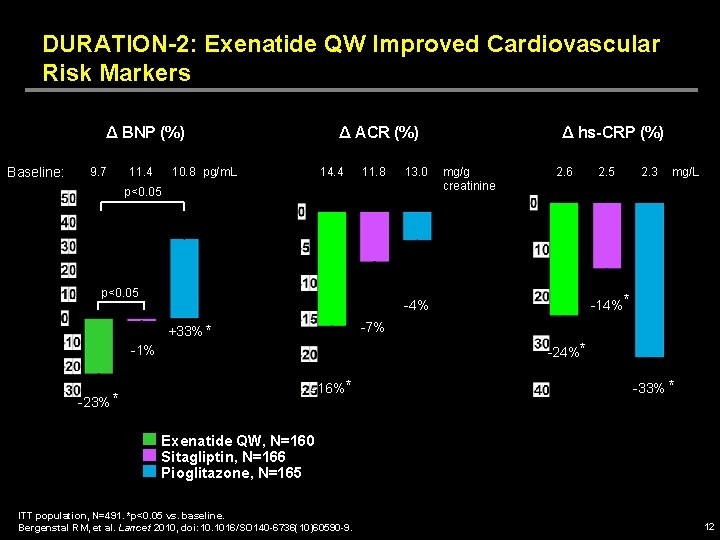
DURATION-2: Exenatide QW Improved Cardiovascular Risk Markers Δ BNP (%) Baseline: 9. 7 11. 4 Δ ACR (%) 10. 8 pg/m. L 14. 4 11. 8 13. 0 p<0. 05 mg/g creatinine 2. 6 2. 5 2. 3 mg/L -14%* -4% -7% +33% * -24%* -1% -23% * Δ hs-CRP (%) -16% * -33% * Exenatide QW, N=160 Sitagliptin, N=166 Pioglitazone, N=165 ITT population, N=491. *p<0. 05 vs. baseline. Bergenstal RM, et al. Lancet 2010, doi: 10. 1016/SO 140 -6736(10)60590 -9. 12

EXSCEL Trial EXenatide Study of Cardiovascular Event Lowering • Pragmatic, placebo-controlled, double-blinded trial evaluating cardiovascular outcomes in patients with type 2 diabetes • Hypothesis: EQW, when used as part of usual care, lowers the risk for major macrovascular events compared to usual care alone • Global trial, 9, 500 patients, ~4. 5 years median exposure • Run jointly by Duke Clinical Research Institute and University of Oxford Diabetes Trial Unit 13

Cardiovascular Risk and Exenatide QW Systolic Blood Pressure Reduced Lipid profile Improved Cardiovascular risk markers Reduced 14
 Name three lines
Name three lines Gdt symbol
Gdt symbol Standard costing and variance analysis formula
Standard costing and variance analysis formula Non material culture definition
Non material culture definition Whats cultural lag
Whats cultural lag Material and non material culture examples
Material and non material culture examples Useful and harmful materials
Useful and harmful materials Give us your hungry your tired your poor
Give us your hungry your tired your poor Iso 22301 utbildning
Iso 22301 utbildning Novell typiska drag
Novell typiska drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Särskild löneskatt för pensionskostnader
Särskild löneskatt för pensionskostnader