Clicker Questions Chapter 18 Electrochemistry Allison Soult University





- Slides: 31

Clicker Questions Chapter 18 Electrochemistry Allison Soult University of Kentucky © 2014 Pearson Education, Inc.

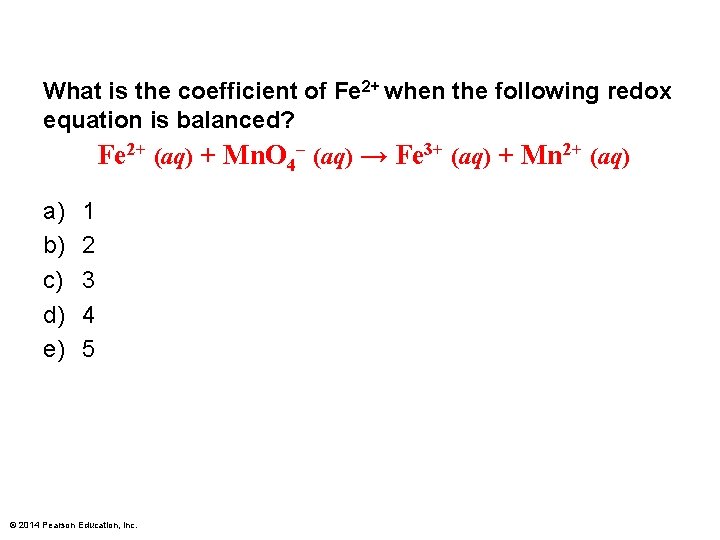
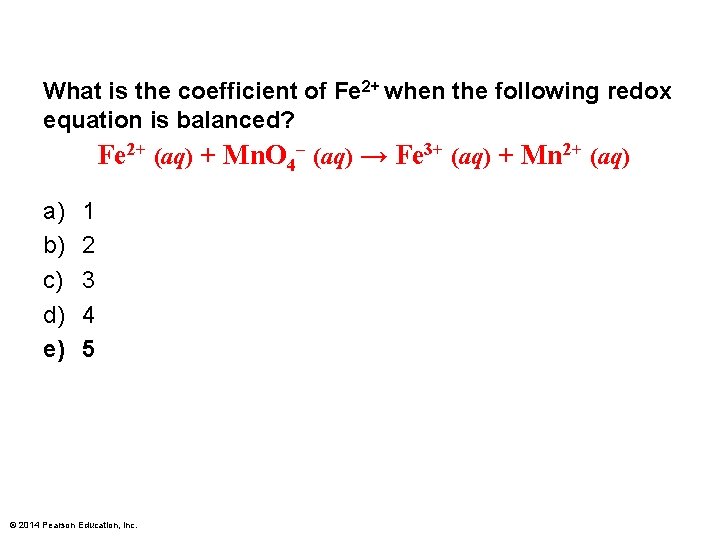
What is the coefficient of Fe 2+ when the following redox equation is balanced? Fe 2+ (aq) + Mn. O 4– (aq) → Fe 3+ (aq) + Mn 2+ (aq) a) b) c) d) e) 1 2 3 4 5 © 2014 Pearson Education, Inc.

What is the coefficient of Fe 2+ when the following redox equation is balanced? Fe 2+ (aq) + Mn. O 4– (aq) → Fe 3+ (aq) + Mn 2+ (aq) a) b) c) d) e) 1 2 3 4 5 © 2014 Pearson Education, Inc.

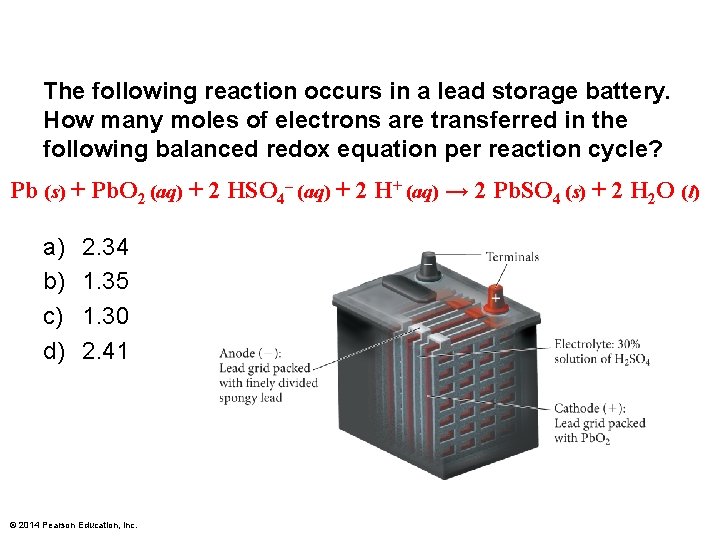
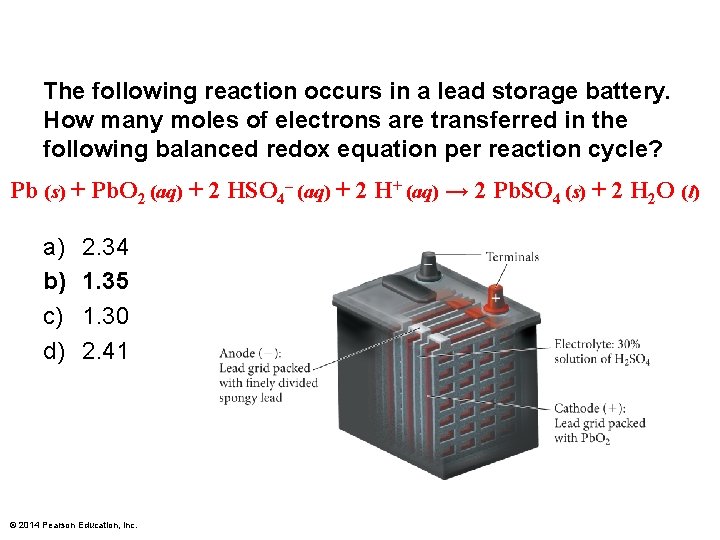
The following reaction occurs in a lead storage battery. How many moles of electrons are transferred in the following balanced redox equation per reaction cycle? Pb (s) + Pb. O 2 (aq) + 2 HSO 4– (aq) + 2 H+ (aq) → 2 Pb. SO 4 (s) + 2 H 2 O (l) a) b) c) d) 2. 34 1. 35 1. 30 2. 41 © 2014 Pearson Education, Inc.

The following reaction occurs in a lead storage battery. How many moles of electrons are transferred in the following balanced redox equation per reaction cycle? Pb (s) + Pb. O 2 (aq) + 2 HSO 4– (aq) + 2 H+ (aq) → 2 Pb. SO 4 (s) + 2 H 2 O (l) a) b) c) d) 2. 34 1. 35 1. 30 2. 41 © 2014 Pearson Education, Inc.

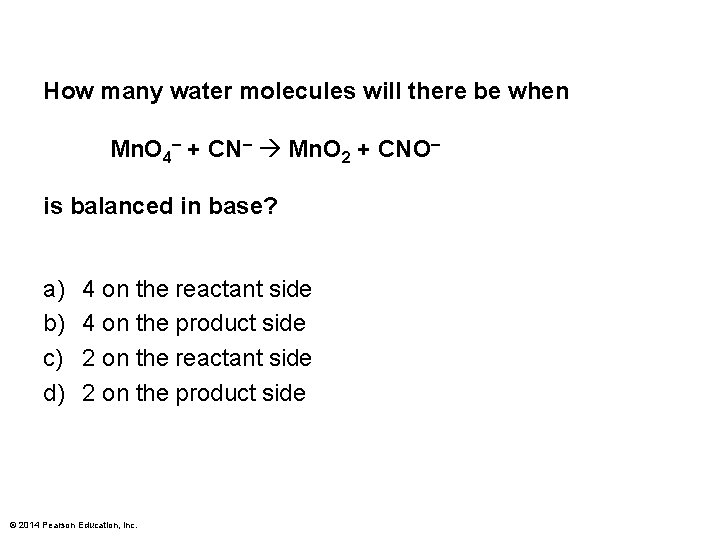
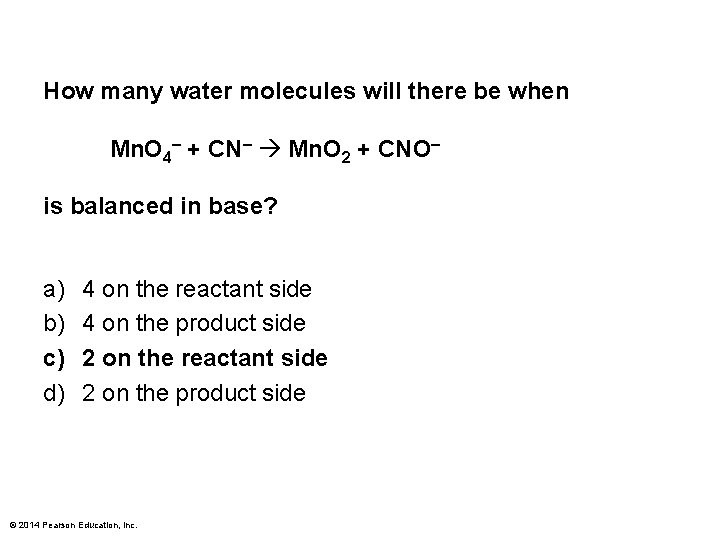
How many water molecules will there be when Mn. O 4– + CN– Mn. O 2 + CNO– is balanced in base? a) b) c) d) 4 on the reactant side 4 on the product side 2 on the reactant side 2 on the product side © 2014 Pearson Education, Inc.

How many water molecules will there be when Mn. O 4– + CN– Mn. O 2 + CNO– is balanced in base? a) b) c) d) 4 on the reactant side 4 on the product side 2 on the reactant side 2 on the product side © 2014 Pearson Education, Inc.

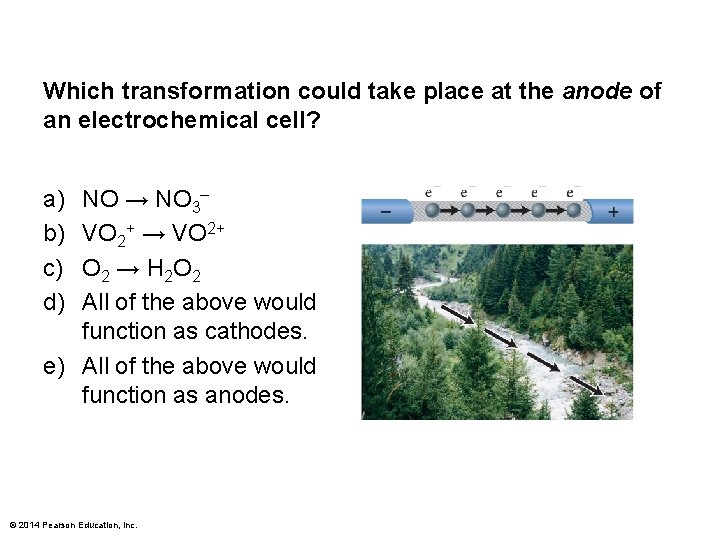
Which transformation could take place at the anode of an electrochemical cell? a) b) c) d) NO → NO 3– VO 2+ → VO 2+ O 2 → H 2 O 2 All of the above would function as cathodes. e) All of the above would function as anodes. © 2014 Pearson Education, Inc.

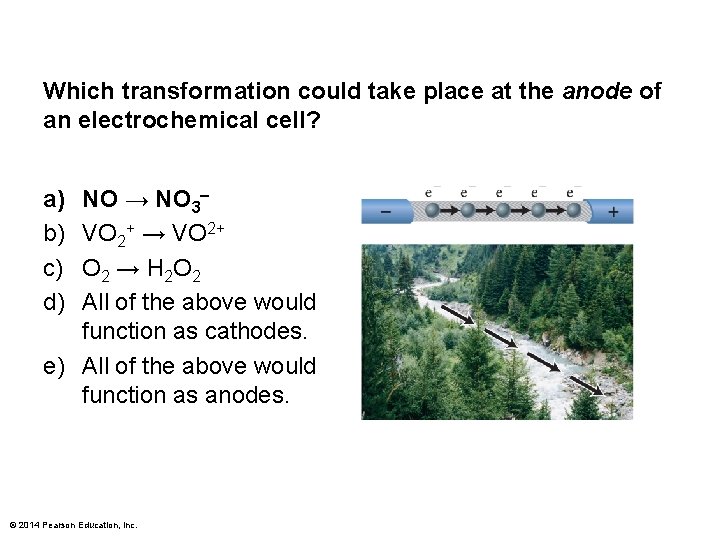
Which transformation could take place at the anode of an electrochemical cell? a) b) c) d) NO → NO 3– VO 2+ → VO 2+ O 2 → H 2 O 2 All of the above would function as cathodes. e) All of the above would function as anodes. © 2014 Pearson Education, Inc.

The purpose of the salt bridge in an electrochemical cell is a) to maintain electrical neutrality in the half-cells via migration of ions. b) to provide a source of ions to react at the anode and cathode. c) to provide oxygen to facilitate oxidation at the anode. d) to provide a means for electrons to travel from the anode to the cathode. e) to provide a means for electrons to travel from the cathode to the anode. © 2014 Pearson Education, Inc.

The purpose of the salt bridge in an electrochemical cell is a) to maintain electrical neutrality in the half-cells via migration of ions. b) to provide a source of ions to react at the anode and cathode. c) to provide oxygen to facilitate oxidation at the anode. d) to provide a means for electrons to travel from the anode to the cathode. e) to provide a means for electrons to travel from the cathode to the anode. © 2014 Pearson Education, Inc.

What is the reducing agent in the following electrochemical cell? Ni (s) | Ni 2+ (aq) || Ag+ (aq) | Ag (s) a) b) c) d) e) Ni Ni 2+ Ag Ag+ There is no reducing agent in an electrochemical cell. © 2014 Pearson Education, Inc.

What is the reducing agent in the following electrochemical cell? Ni (s) | Ni 2+ (aq) || Ag+ (aq) | Ag (s) a) b) c) d) e) Ni Ni 2+ Ag Ag+ There is no reducing agent in an electrochemical cell. © 2014 Pearson Education, Inc.

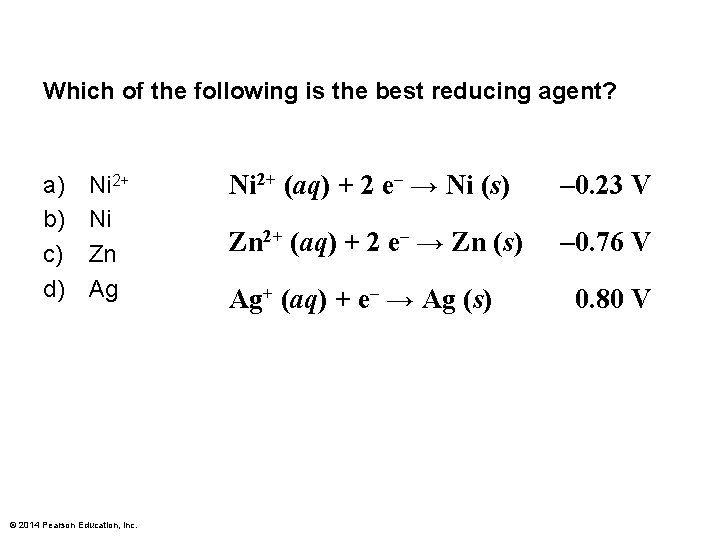
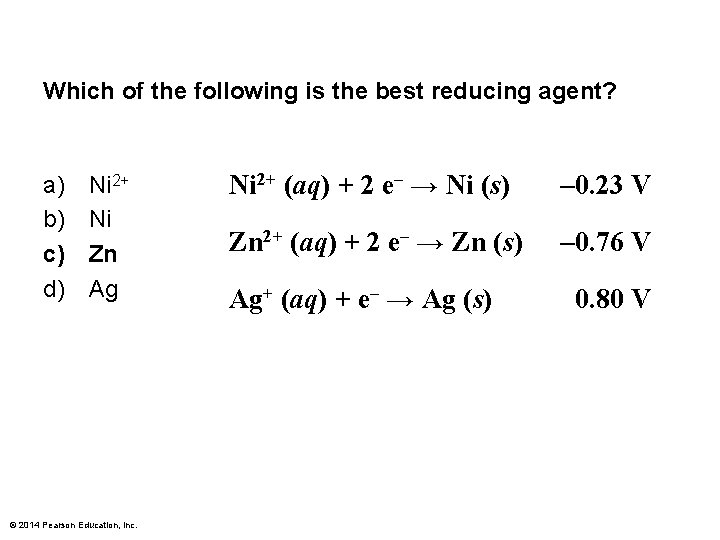
Which of the following is the best reducing agent? a) b) c) d) Ni 2+ Ni Zn Ag © 2014 Pearson Education, Inc. Ni 2+ (aq) + 2 e– → Ni (s) – 0. 23 V Zn 2+ (aq) + 2 e– → Zn (s) – 0. 76 V Ag+ (aq) + e– → Ag (s) 0. 80 V

Which of the following is the best reducing agent? a) b) c) d) Ni 2+ Ni Zn Ag © 2014 Pearson Education, Inc. Ni 2+ (aq) + 2 e– → Ni (s) – 0. 23 V Zn 2+ (aq) + 2 e– → Zn (s) – 0. 76 V Ag+ (aq) + e– → Ag (s) 0. 80 V

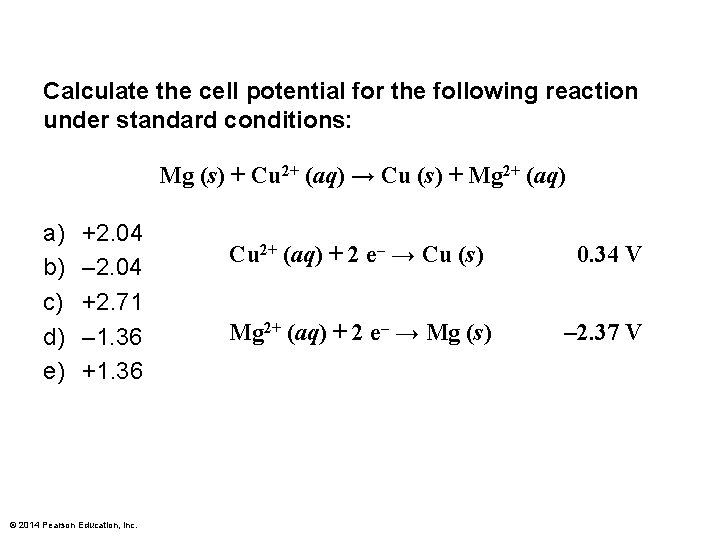
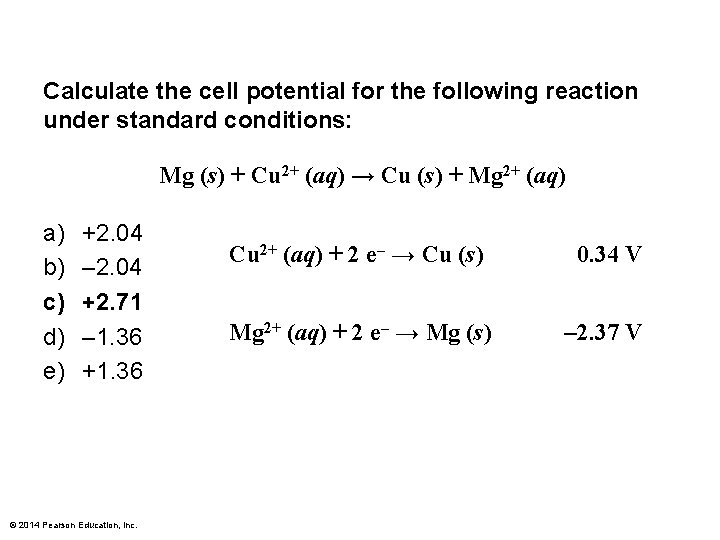
Calculate the cell potential for the following reaction under standard conditions: Mg (s) + Cu 2+ (aq) → Cu (s) + Mg 2+ (aq) a) b) c) d) e) +2. 04 – 2. 04 +2. 71 – 1. 36 +1. 36 © 2014 Pearson Education, Inc. Cu 2+ (aq) + 2 e– → Cu (s) 0. 34 V Mg 2+ (aq) + 2 e– → Mg (s) – 2. 37 V

Calculate the cell potential for the following reaction under standard conditions: Mg (s) + Cu 2+ (aq) → Cu (s) + Mg 2+ (aq) a) b) c) d) e) +2. 04 – 2. 04 +2. 71 – 1. 36 +1. 36 © 2014 Pearson Education, Inc. Cu 2+ (aq) + 2 e– → Cu (s) 0. 34 V Mg 2+ (aq) + 2 e– → Mg (s) – 2. 37 V

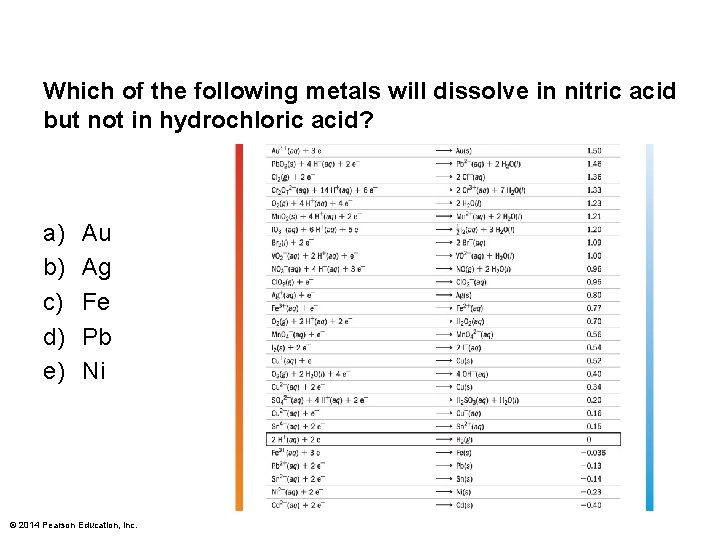
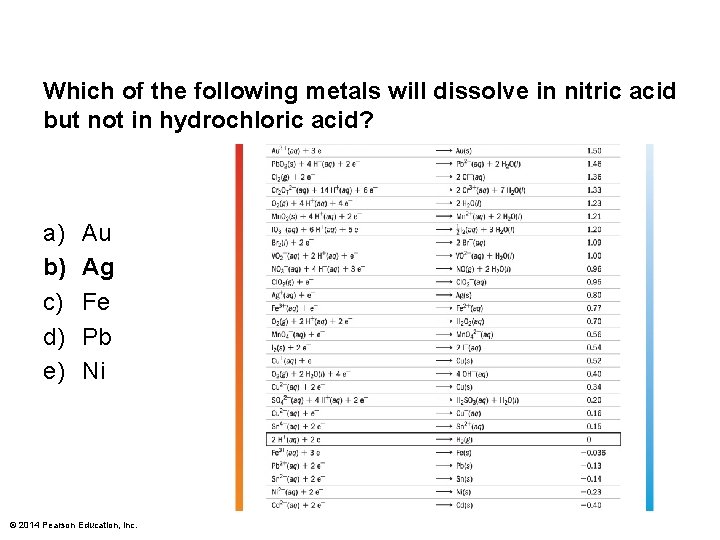
Which of the following metals will dissolve in nitric acid but not in hydrochloric acid? a) b) c) d) e) Au Ag Fe Pb Ni © 2014 Pearson Education, Inc.

Which of the following metals will dissolve in nitric acid but not in hydrochloric acid? a) b) c) d) e) Au Ag Fe Pb Ni © 2014 Pearson Education, Inc.

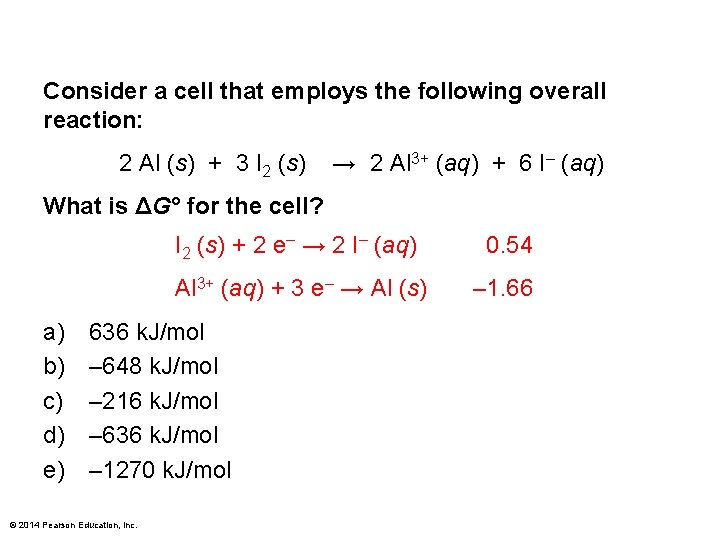
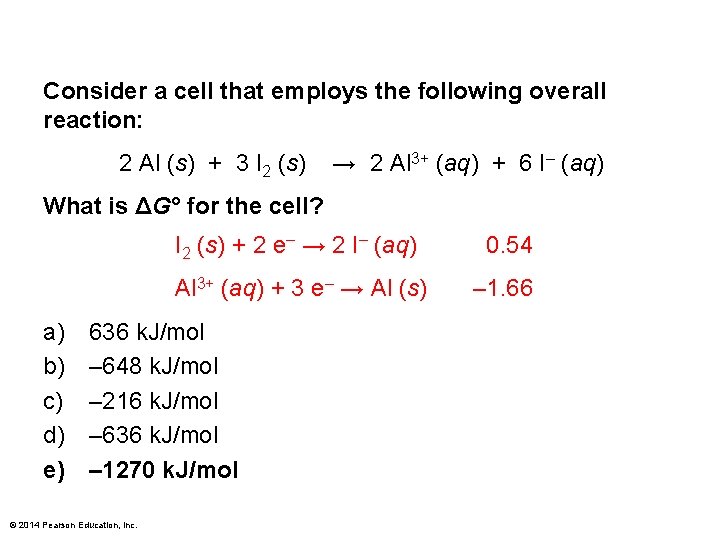
Consider a cell that employs the following overall reaction: 2 Al (s) + 3 I 2 (s) → 2 Al 3+ (aq) + 6 I– (aq) What is ΔG° for the cell? a) b) c) d) e) I 2 (s) + 2 e– → 2 I– (aq) 0. 54 Al 3+ (aq) + 3 e– → Al (s) – 1. 66 636 k. J/mol – 648 k. J/mol – 216 k. J/mol – 636 k. J/mol – 1270 k. J/mol © 2014 Pearson Education, Inc.

Consider a cell that employs the following overall reaction: 2 Al (s) + 3 I 2 (s) → 2 Al 3+ (aq) + 6 I– (aq) What is ΔG° for the cell? a) b) c) d) e) I 2 (s) + 2 e– → 2 I– (aq) 0. 54 Al 3+ (aq) + 3 e– → Al (s) – 1. 66 636 k. J/mol – 648 k. J/mol – 216 k. J/mol – 636 k. J/mol – 1270 k. J/mol © 2014 Pearson Education, Inc.

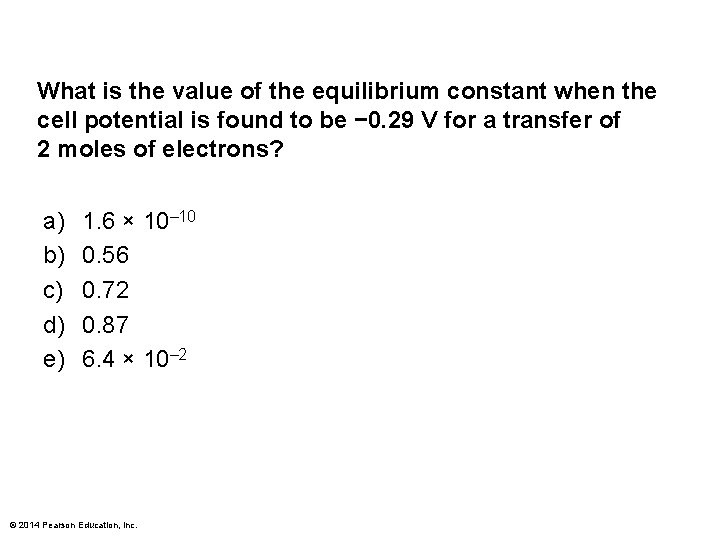
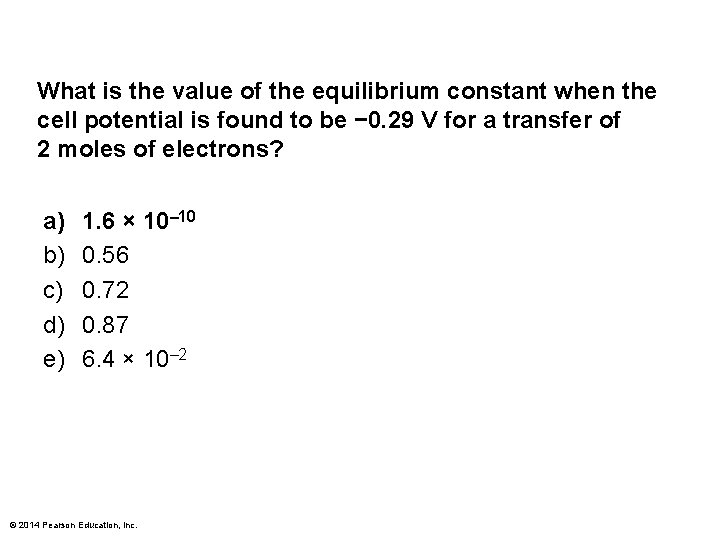
What is the value of the equilibrium constant when the cell potential is found to be − 0. 29 V for a transfer of 2 moles of electrons? a) b) c) d) e) 1. 6 × 10– 10 0. 56 0. 72 0. 87 6. 4 × 10– 2 © 2014 Pearson Education, Inc.

What is the value of the equilibrium constant when the cell potential is found to be − 0. 29 V for a transfer of 2 moles of electrons? a) b) c) d) e) 1. 6 × 10– 10 0. 56 0. 72 0. 87 6. 4 × 10– 2 © 2014 Pearson Education, Inc.

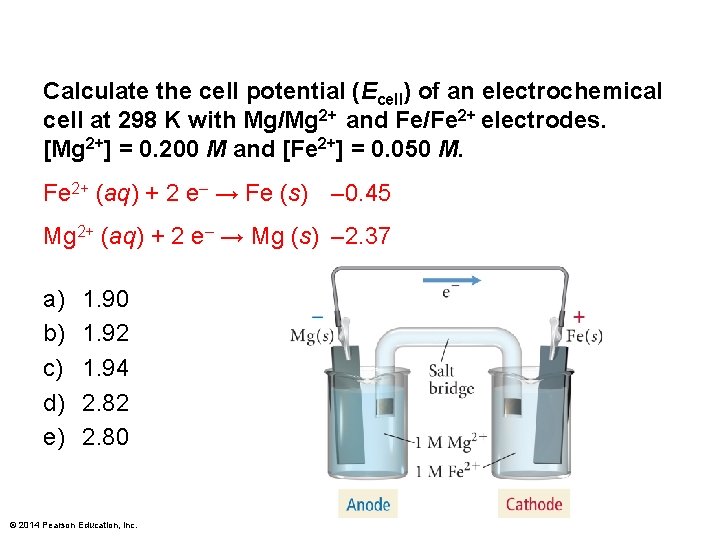
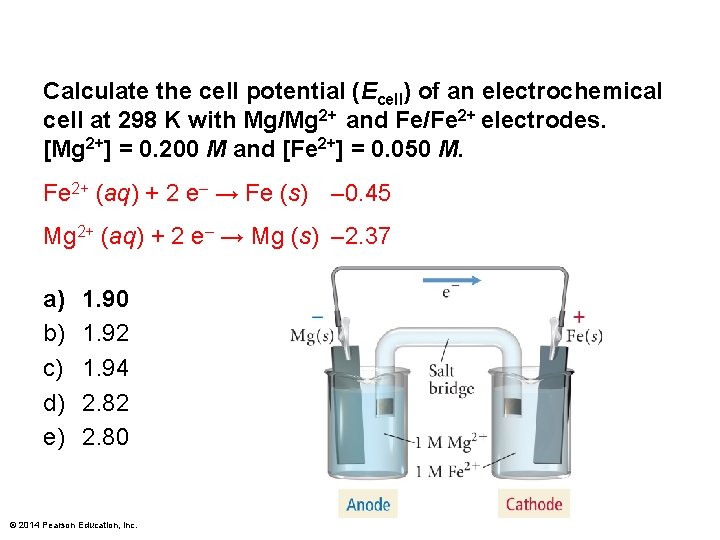
Calculate the cell potential (Ecell) of an electrochemical cell at 298 K with Mg/Mg 2+ and Fe/Fe 2+ electrodes. [Mg 2+] = 0. 200 M and [Fe 2+] = 0. 050 M. Fe 2+ (aq) + 2 e– → Fe (s) – 0. 45 Mg 2+ (aq) + 2 e– → Mg (s) – 2. 37 a) b) c) d) e) 1. 90 1. 92 1. 94 2. 82 2. 80 © 2014 Pearson Education, Inc.

Calculate the cell potential (Ecell) of an electrochemical cell at 298 K with Mg/Mg 2+ and Fe/Fe 2+ electrodes. [Mg 2+] = 0. 200 M and [Fe 2+] = 0. 050 M. Fe 2+ (aq) + 2 e– → Fe (s) – 0. 45 Mg 2+ (aq) + 2 e– → Mg (s) – 2. 37 a) b) c) d) e) 1. 90 1. 92 1. 94 2. 82 2. 80 © 2014 Pearson Education, Inc.

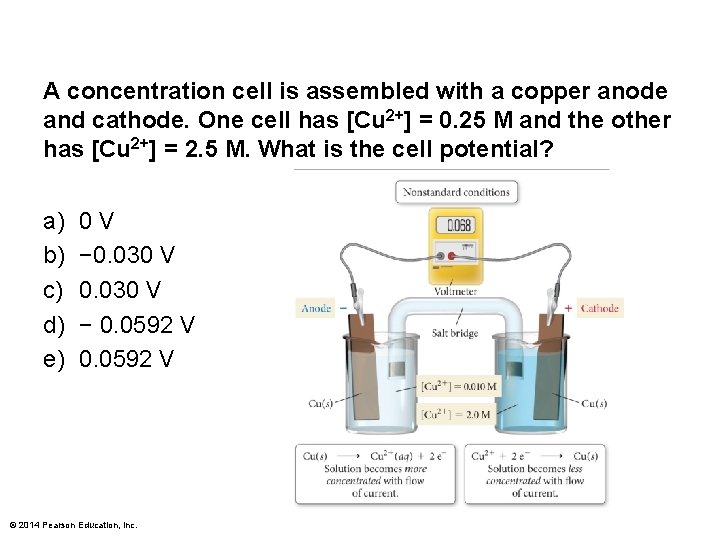
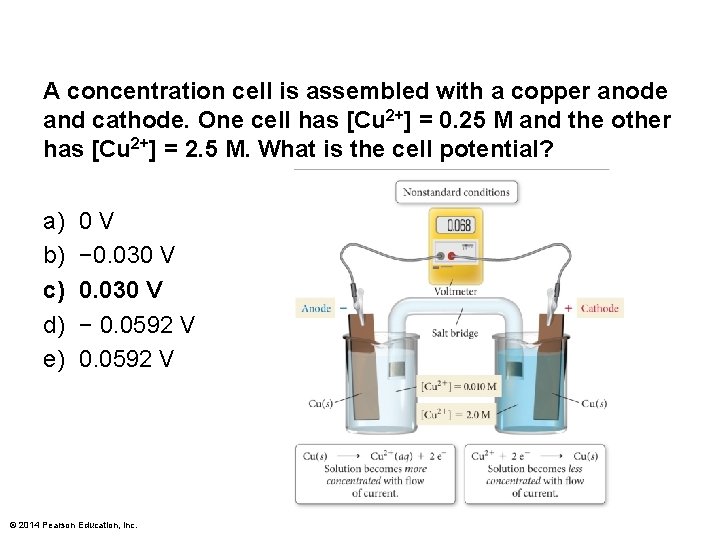
A concentration cell is assembled with a copper anode and cathode. One cell has [Cu 2+] = 0. 25 M and the other has [Cu 2+] = 2. 5 M. What is the cell potential? a) b) c) d) e) 0 V − 0. 030 V − 0. 0592 V © 2014 Pearson Education, Inc.

A concentration cell is assembled with a copper anode and cathode. One cell has [Cu 2+] = 0. 25 M and the other has [Cu 2+] = 2. 5 M. What is the cell potential? a) b) c) d) e) 0 V − 0. 030 V − 0. 0592 V © 2014 Pearson Education, Inc.

What mass of nickel can be plated from a solution containing Ni 2+ with a current of 3. 6 A for 14 minutes? a) b) c) d) e) 0. 92 g 1. 8 g 3. 7 g 1. 0 g 0. 071 g © 2014 Pearson Education, Inc.

What mass of nickel can be plated from a solution containing Ni 2+ with a current of 3. 6 A for 14 minutes? a) b) c) d) e) 0. 92 g 1. 8 g 3. 7 g 1. 0 g 0. 071 g © 2014 Pearson Education, Inc.

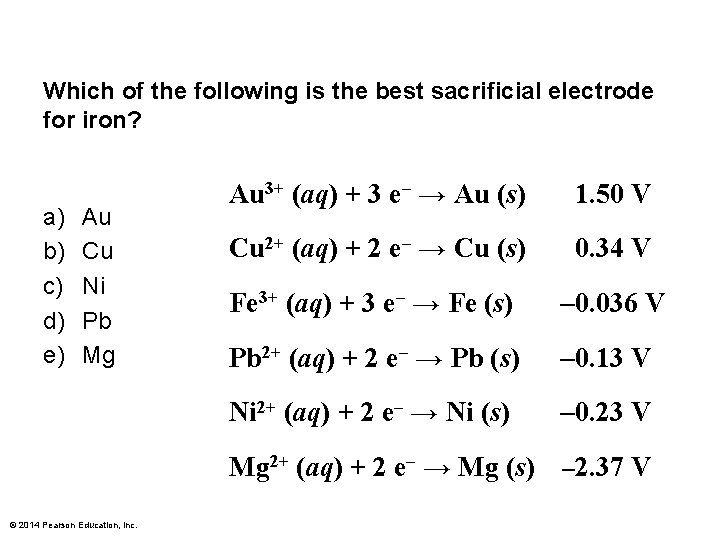
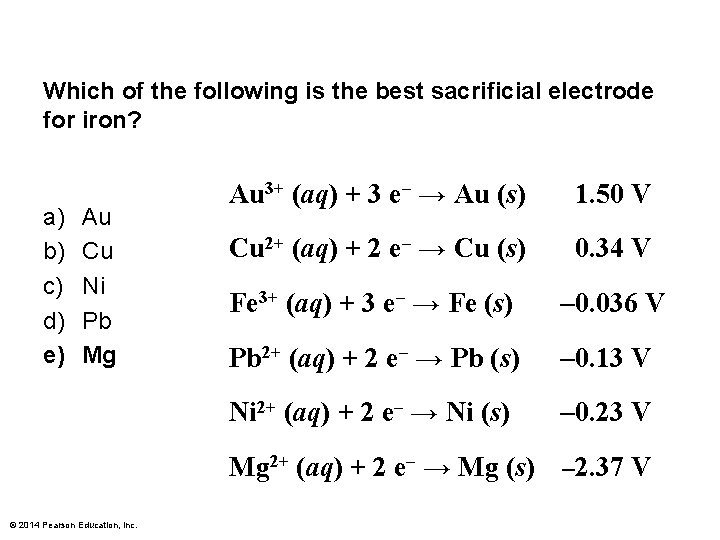
Which of the following is the best sacrificial electrode for iron? a) b) c) d) e) Au Cu Ni Pb Mg Au 3+ (aq) + 3 e– → Au (s) 1. 50 V Cu 2+ (aq) + 2 e– → Cu (s) 0. 34 V Fe 3+ (aq) + 3 e– → Fe (s) – 0. 036 V Pb 2+ (aq) + 2 e– → Pb (s) – 0. 13 V Ni 2+ (aq) + 2 e– → Ni (s) – 0. 23 V Mg 2+ (aq) + 2 e– → Mg (s) – 2. 37 V © 2014 Pearson Education, Inc.

Which of the following is the best sacrificial electrode for iron? a) b) c) d) e) Au Cu Ni Pb Mg Au 3+ (aq) + 3 e– → Au (s) 1. 50 V Cu 2+ (aq) + 2 e– → Cu (s) 0. 34 V Fe 3+ (aq) + 3 e– → Fe (s) – 0. 036 V Pb 2+ (aq) + 2 e– → Pb (s) – 0. 13 V Ni 2+ (aq) + 2 e– → Ni (s) – 0. 23 V Mg 2+ (aq) + 2 e– → Mg (s) – 2. 37 V © 2014 Pearson Education, Inc.
 Clicker questions physics
Clicker questions physics Mount allison university bookstore
Mount allison university bookstore Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Chapter 20 electrochemistry
Chapter 20 electrochemistry Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Chapter 21 electrochemistry
Chapter 21 electrochemistry Chapter 21 electrochemistry
Chapter 21 electrochemistry Electrons flow from anode to cathode
Electrons flow from anode to cathode Math clicker
Math clicker E clicker
E clicker Turning point audience response system
Turning point audience response system Lesson 5 building an app clicker game
Lesson 5 building an app clicker game Earth clicker
Earth clicker Heart rate clicker
Heart rate clicker Hitler clicker
Hitler clicker Clicker stop motion
Clicker stop motion Cooi clicker
Cooi clicker Umbc clicker
Umbc clicker Clicker box
Clicker box Clicker
Clicker Clicker stop motion
Clicker stop motion Clicker
Clicker Phys 172
Phys 172 Hlyniany
Hlyniany Salt clicker
Salt clicker Clicker gravel transport
Clicker gravel transport Atomic clicker
Atomic clicker Spacebar click
Spacebar click Fibonacci clicker
Fibonacci clicker Clicker
Clicker Presentation clicker challenger
Presentation clicker challenger Soh cah toa rules
Soh cah toa rules