Clicker Questions Chapter 20 Organic Chemistry Allison Soult



- Slides: 16

Clicker Questions Chapter 20 Organic Chemistry Allison Soult University of Kentucky © 2014 Pearson Education, Inc.

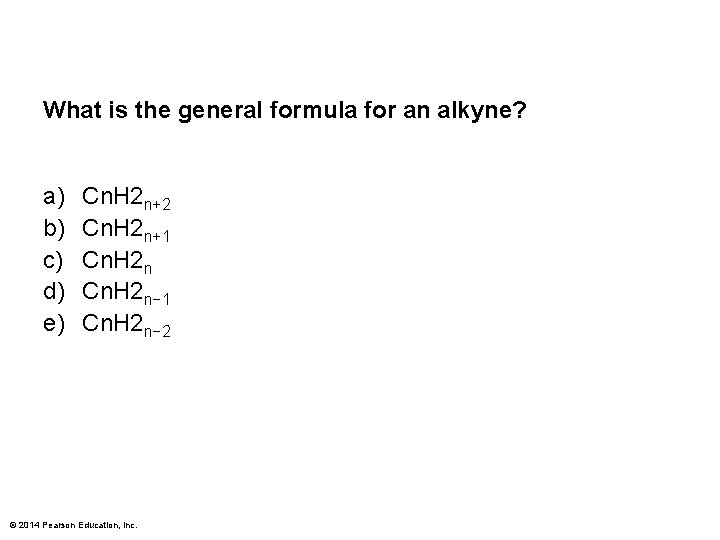
What is the general formula for an alkyne? a) b) c) d) e) Cn. H 2 n+2 Cn. H 2 n+1 Cn. H 2 n− 2 © 2014 Pearson Education, Inc.

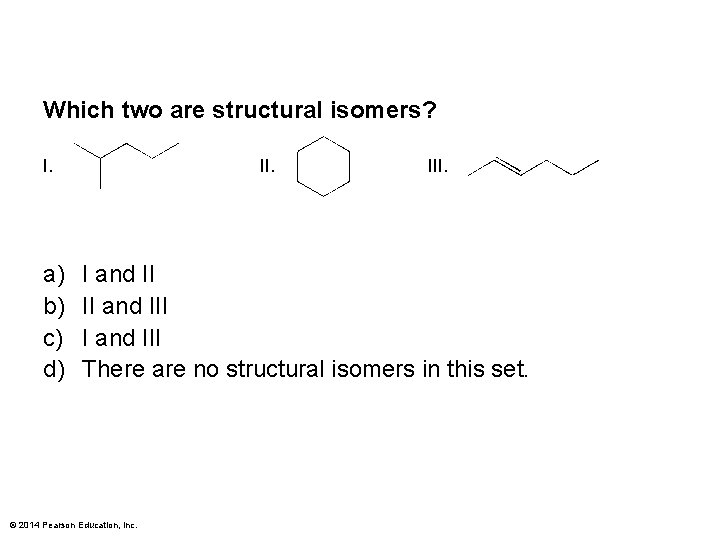
Which two are structural isomers? I. a) b) c) d) II. I and II II and III There are no structural isomers in this set. © 2014 Pearson Education, Inc.

Which of the following can exhibit optical isomerism? a) b) c) d) © 2014 Pearson Education, Inc.

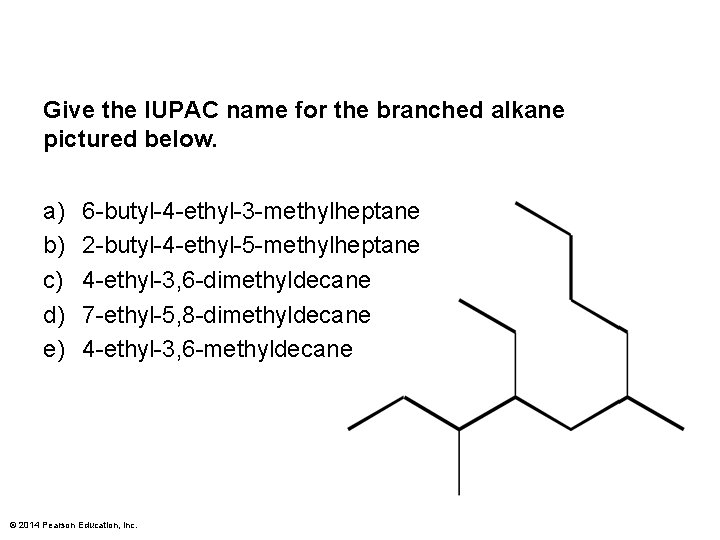
Give the IUPAC name for the branched alkane pictured below. a) b) c) d) e) 6 -butyl-4 -ethyl-3 -methylheptane 2 -butyl-4 -ethyl-5 -methylheptane 4 -ethyl-3, 6 -dimethyldecane 7 -ethyl-5, 8 -dimethyldecane 4 -ethyl-3, 6 -methyldecane © 2014 Pearson Education, Inc.

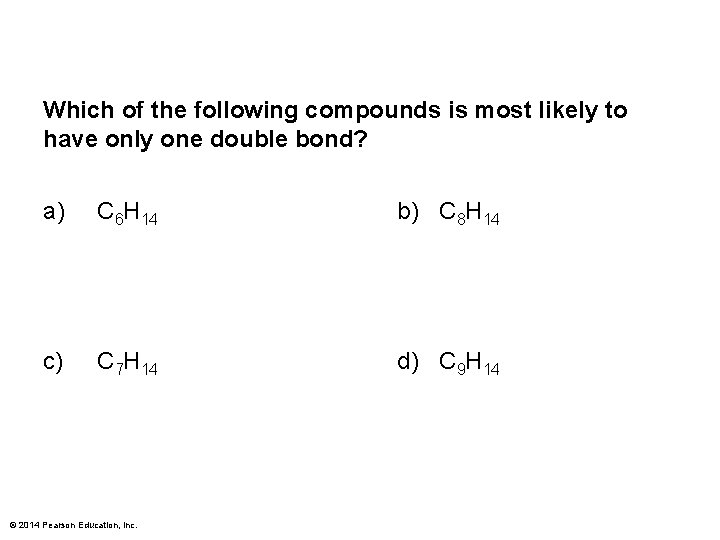
Which of the following compounds is most likely to have only one double bond? a) C 6 H 14 b) C 8 H 14 c) C 7 H 14 d) C 9 H 14 © 2014 Pearson Education, Inc.

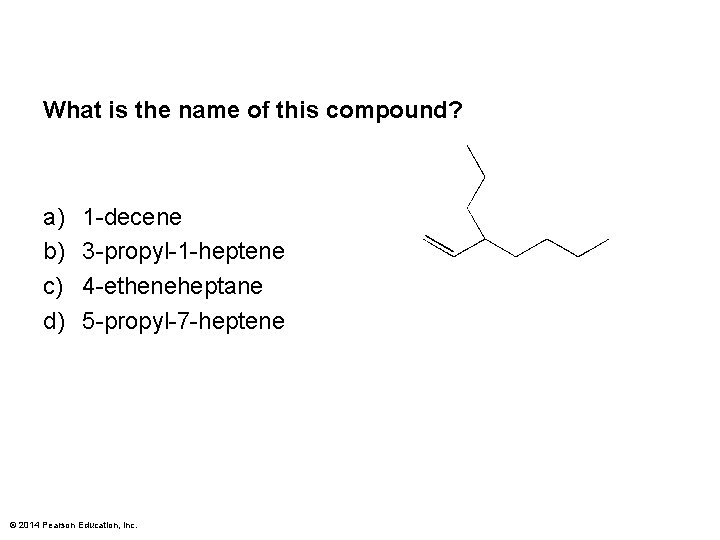
What is the name of this compound? a) b) c) d) 1 -decene 3 -propyl-1 -heptene 4 -etheneheptane 5 -propyl-7 -heptene © 2014 Pearson Education, Inc.

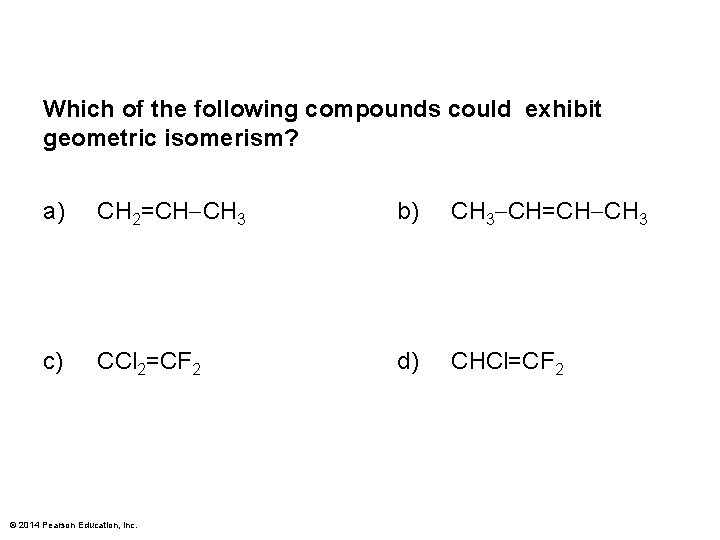
Which of the following compounds could exhibit geometric isomerism? a) CH 2=CH CH 3 b) CH 3 CH=CH CH 3 c) CCl 2=CF 2 d) CHCl=CF 2 © 2014 Pearson Education, Inc.

Which class of compounds is the least reactive? a) b) c) d) e) alkanes alkenes alkynes They are all the same. There is no trend in the reactivity. © 2014 Pearson Education, Inc.

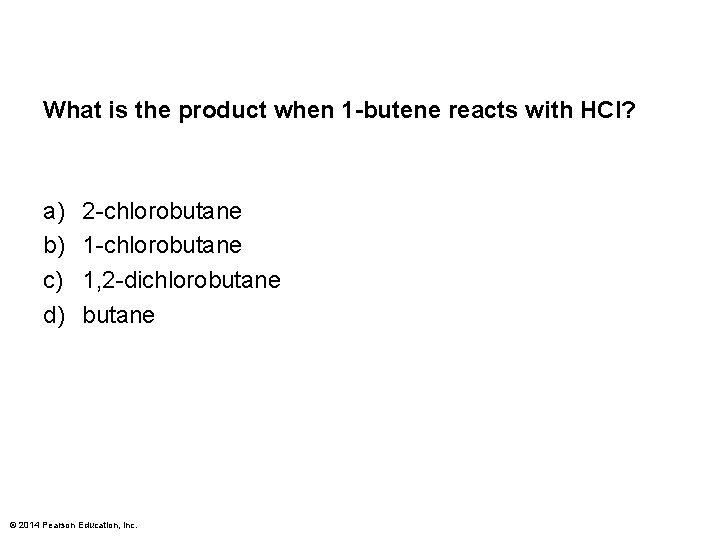
What is the product when 1 -butene reacts with HCl? a) b) c) d) 2 -chlorobutane 1, 2 -dichlorobutane © 2014 Pearson Education, Inc.

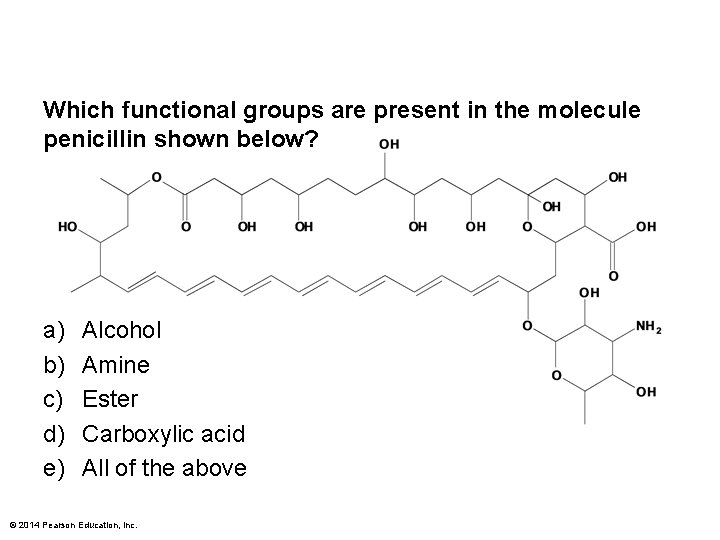
Which functional groups are present in the molecule penicillin shown below? a) b) c) d) e) Alcohol Amine Ester Carboxylic acid All of the above © 2014 Pearson Education, Inc.

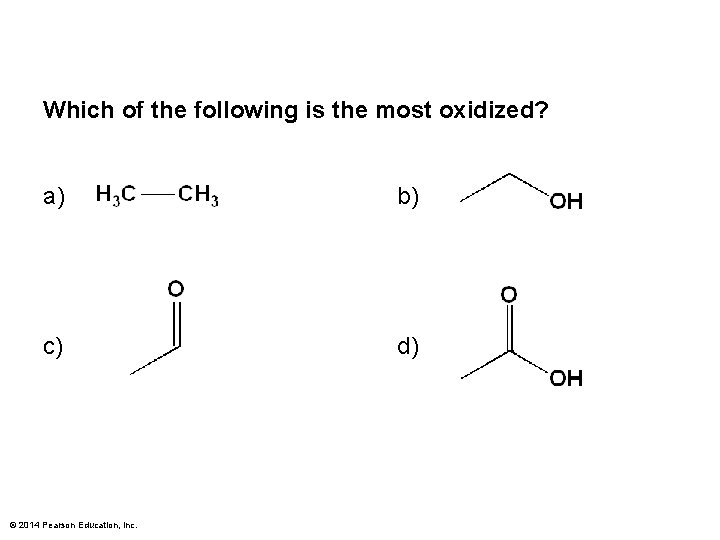
Which of the following is the most oxidized? a) b) c) d) © 2014 Pearson Education, Inc.

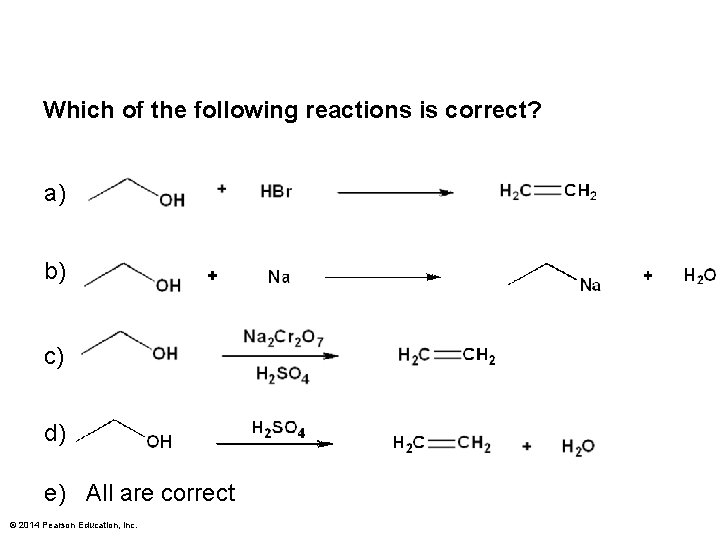
Which of the following reactions is correct? a) b) c) d) e) All are correct © 2014 Pearson Education, Inc.

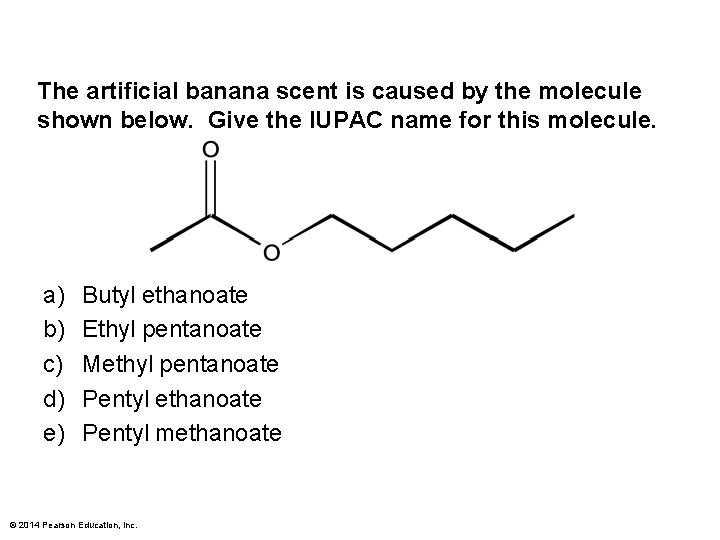
The artificial banana scent is caused by the molecule shown below. Give the IUPAC name for this molecule. a) b) c) d) e) Butyl ethanoate Ethyl pentanoate Methyl pentanoate Pentyl ethanoate Pentyl methanoate © 2014 Pearson Education, Inc.

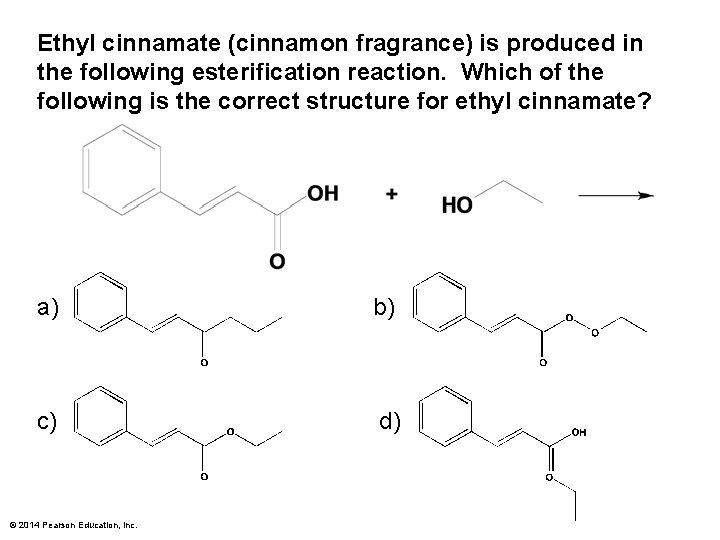
Ethyl cinnamate (cinnamon fragrance) is produced in the following esterification reaction. Which of the following is the correct structure for ethyl cinnamate? a) b) c) d) © 2014 Pearson Education, Inc.

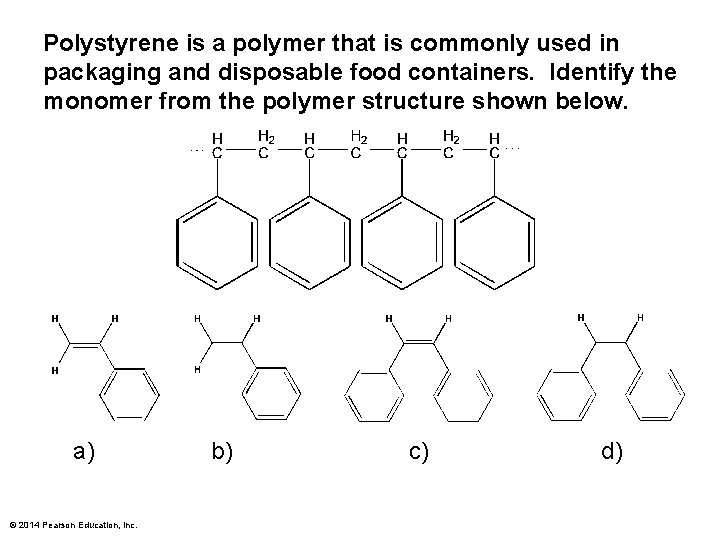
Polystyrene is a polymer that is commonly used in packaging and disposable food containers. Identify the monomer from the polymer structure shown below. a) © 2014 Pearson Education, Inc. b) c) d)
 Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Clicker questions physics
Clicker questions physics Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Importance of organic chemistry
Importance of organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Carboxylic acid h3o+ reaction
Carboxylic acid h3o+ reaction Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 chemistry review
Chapter 7 chemistry review Nonene
Nonene Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Organic chemistry (3rd) edition chapter 2 problem 17s
Organic chemistry (3rd) edition chapter 2 problem 17s Founder of organic chemistry
Founder of organic chemistry Chemistry of soap making
Chemistry of soap making Ester organic chemistry
Ester organic chemistry Define organic chemistry
Define organic chemistry Rearranged most stable carbocation is
Rearranged most stable carbocation is






























