CHEMICAL QUANTITIES Chapter 9 CHEMICAL EQUATIONS Coefficients in









- Slides: 36

CHEMICAL QUANTITIES Chapter 9

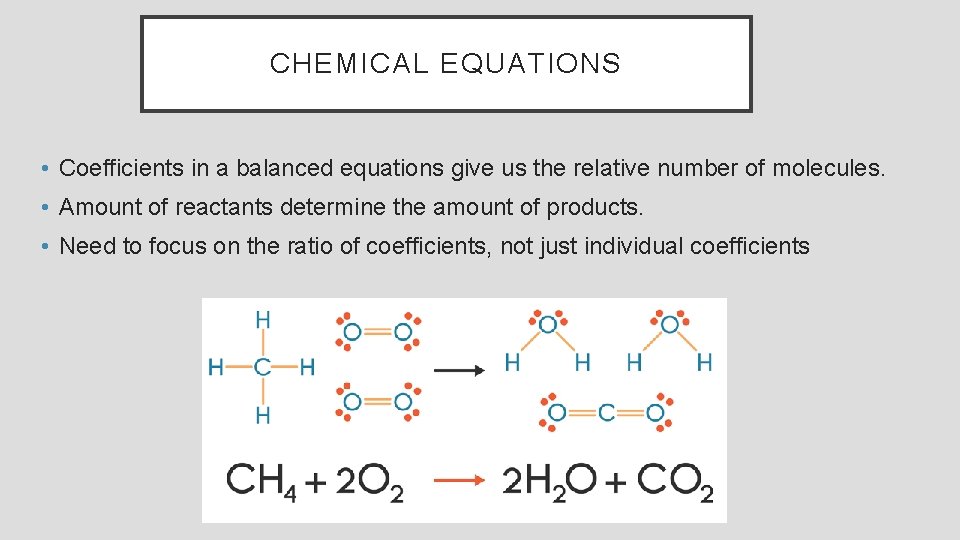
CHEMICAL EQUATIONS • Coefficients in a balanced equations give us the relative number of molecules. • Amount of reactants determine the amount of products. • Need to focus on the ratio of coefficients, not just individual coefficients

REAL WORLD EXAMPLE • To make a hamburger, what would your need: • 1 bun • 1 hamburger patty • 4 slices of bacon • 1 slice of cheese • 2 pieces of lettuce • 3 slices of tomatoes • 5 pickle slices • 8 pieces of grilled onions • How would you figure out how much of each ingredients would you need if you were making 237 hamburgers?

REAL WORLD EXAMPLE • How would you figure out how much of each ingredients would you need if you were making 237 hamburgers? • 1 bun x 237 burgers = 237 buns • 1 hamburger patty x 237 burgers = 237 patties • 4 slices of bacon x 237 burgers = 948 slices of bacon • 1 slice of cheese x 237 burgers = 237 slices of cheese • 2 pieces of lettuce x 237 burgers = 474 pieces of lettuce • 3 slices of tomatoes x 237 burgers = 711 slices of tomatoes • 5 pickle slices x 237 burgers = 1185 pickle slices • 8 pieces of grilled onions x 237 burgers = 1896 pieces of grilled onion

REAL WORLD EXAMPLE • 237 Bn + 237 P + 948 Ba + 237 C + 474 L + 711 T +1185 P +1896 O = 237 HB • 1 Bn + 1 P + 4 Ba + 1 C + 2 L + 3 T + 5 P + 8 O = 1 HB • Notice the ratios • 237: 948: 237: 474: 711: 1185: 1896: 237 is the same as • 1: 1: 4: 1: 2: 3: 5: 8: 1

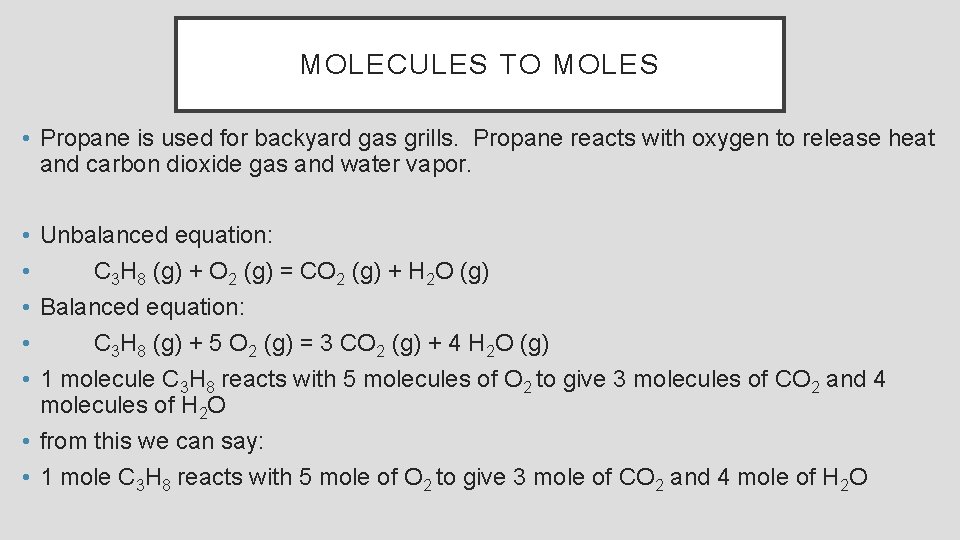
MOLECULES TO MOLES • Propane is used for backyard gas grills. Propane reacts with oxygen to release heat and carbon dioxide gas and water vapor. • Unbalanced equation: • C 3 H 8 (g) + O 2 (g) = CO 2 (g) + H 2 O (g) • Balanced equation: • C 3 H 8 (g) + 5 O 2 (g) = 3 CO 2 (g) + 4 H 2 O (g) • 1 molecule C 3 H 8 reacts with 5 molecules of O 2 to give 3 molecules of CO 2 and 4 molecules of H 2 O • from this we can say: • 1 mole C 3 H 8 reacts with 5 mole of O 2 to give 3 mole of CO 2 and 4 mole of H 2 O

PICK A CARD, ANY CARD… X ? P

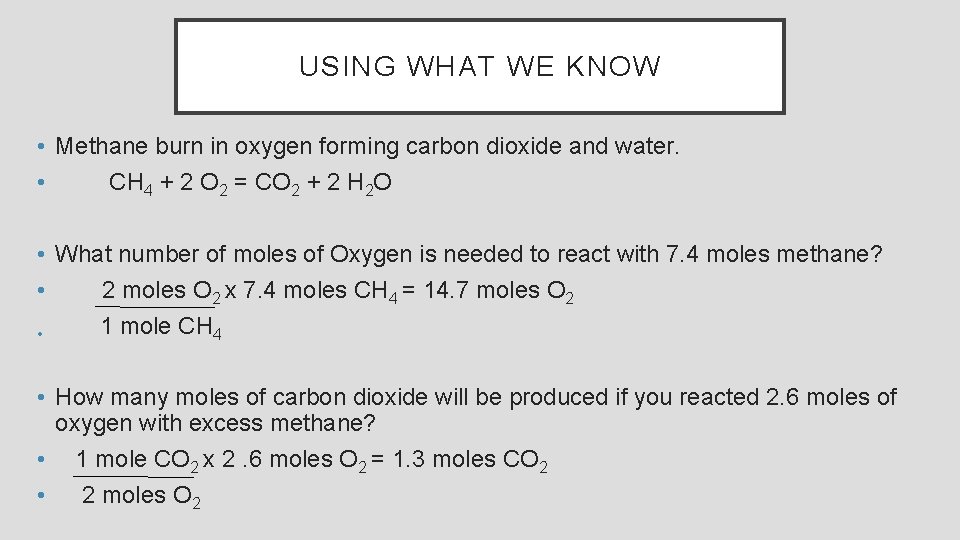
USING WHAT WE KNOW • Methane burn in oxygen forming carbon dioxide and water. • CH 4 + 2 O 2 = CO 2 + 2 H 2 O • What number of moles of Oxygen is needed to react with 7. 4 moles methane? • 2 moles O 2 x 7. 4 moles CH 4 = 14. 7 moles O 2 1 mole CH 4 • • How many moles of carbon dioxide will be produced if you reacted 2. 6 moles of oxygen with excess methane? • 1 mole CO 2 x 2. 6 moles O 2 = 1. 3 moles CO 2 • 2 moles O 2

TABLE PROBLEMS • Calculate the number of moles of oxygen required to react exactly with 4. 3 moles of propane, given this reaction: • C 3 H 8 (g) + 5 O 2 (g) = 3 CO 2 (g) + 4 H 2 O (g) • 5 moles O 2 x 4. 3 moles C 3 H 8 = 21. 5 moles O 2 1 mole C 3 H 8 • Calculate the moles of CO 2 formed when 4. 3 moles C 3 H 8 reacts with the 21. 5 moles O 2. • 3 moles CO 2 x 4. 3 moles C 3 H 8 = 12. 9 moles CO 2 1 mole C 3 H 8

MASS CALCULATIONS • Given the following equation, how much iodine is needed if we have 35 g of aluminum? • 2 Al + 3 I 2 = 2 Al. I 3 • We need to find number of moles of Al and grams of I 2. grams • 1 moles Al x 35 grams Al = 1. 297 moles Al 26. 982 g Al 3 moles I 2 x 1. 297 moles Al = 1. 9455 moles I 2 2 moles Al 253 grams I 2 x 1. 9455 moles I 2 = 493. 78 grams I 2 1 mole I 2 From PT 1 mole Al = 26. 982

PICK A CARD, ANY CARD… X ? P

PROBLEMS FOR YOU… • Calculate the mass of carbon dioxide required to react with 10. 0 grams of sodium hydroxide. • 2 Na. OH + CO 2 = Na 2 CO 3 + H 2 O • 44. 01 grams CO 2 x 1 mole CO 2 x 1 mole Na. OH x 10 grams Na. OH = 5. 50 grams CO 2 • 1 mole CO 2 2 moles Na. OH 39. 998 grams Na. OH • Determine the mass of O 2 required to react with 96. 1 grams C 3 H 8. • C 3 H 8 (g) + O 2 (g) = CO 2 (g) + H 2 O (g) • 32 grams O 2 x 1 mole C 3 H 8 x 96. 1 grams C 3 H 8 = 69. 742 grams O 2 • 1 mole O 2 1 mole C 3 H 8 44. 094 grams

USING STOICHIOMETRY TO COMPARE REACTIONS • Compare Baking soda (Na. HCO 3) and milk of magnesia (Mg(OH)2 in neutralizing stomach acid. You have 1 g of each Na. HCO 3 and Mg(OH)2. • Na. HCO 3 + HCl = Na. Cl + H 2 O + CO 2 • Mg(OH)2 + 2 HCl = 2 H 2 O + Mg. Cl 2 • 1 moles HCl x 1 mole Na. HCO 3 x 1 gram Na. HCO 3 = 0. 0119 moles HCl • 1 mole Na. HCO 3 84. 008 gram Na. HCO 3 • 2 mole HCl x 1 mole Mg(OH)2 x 1 g Mg(OH)2 = 0. 0343 moles HCl • 1 mole Mg(OH)2 58. 321 grams Mg(OH)2

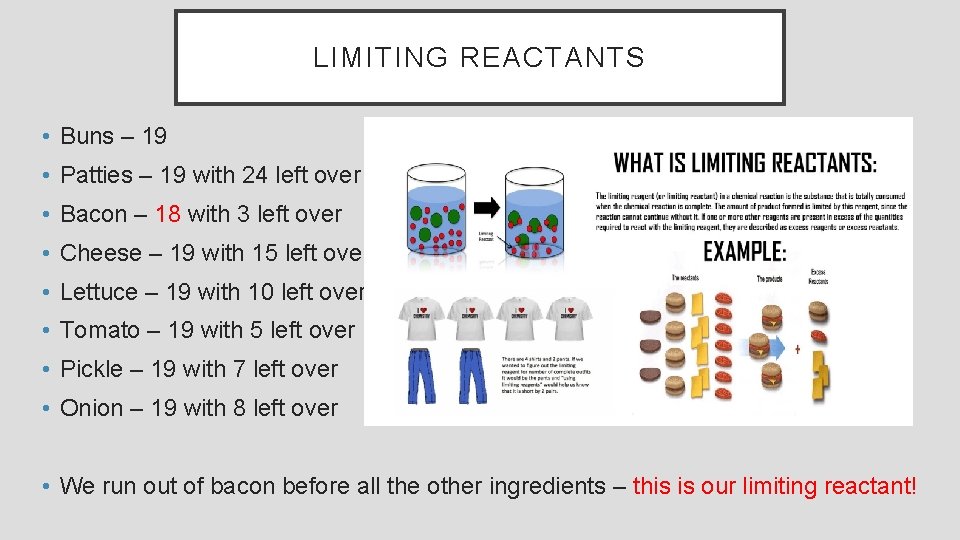
LIMITING REACTANTS • To make a hamburger, what would your need: • Suppose you have leftover from the BBQ, • 1 bun • 19 buns • 1 hamburger patty • 43 hamburger patties • 4 slices of bacon • 75 slices of bacon • 1 slice of cheese • 34 slices of cheese • 2 pieces of lettuce • 48 pieces of lettuce • 3 slices of tomatoes • 62 slices of tomatoes • 5 pickle slices • 102 pickle slices • 8 pieces of grilled onions • 160 pieces of grilled onions How many burgers can you make before you run out of

LIMITING REACTANTS • Buns – 19 • Patties – 19 with 24 left over • Bacon – 18 with 3 left over • Cheese – 19 with 15 left over • Lettuce – 19 with 10 left over • Tomato – 19 with 5 left over • Pickle – 19 with 7 left over • Onion – 19 with 8 left over • We run out of bacon before all the other ingredients – this is our limiting reactant!

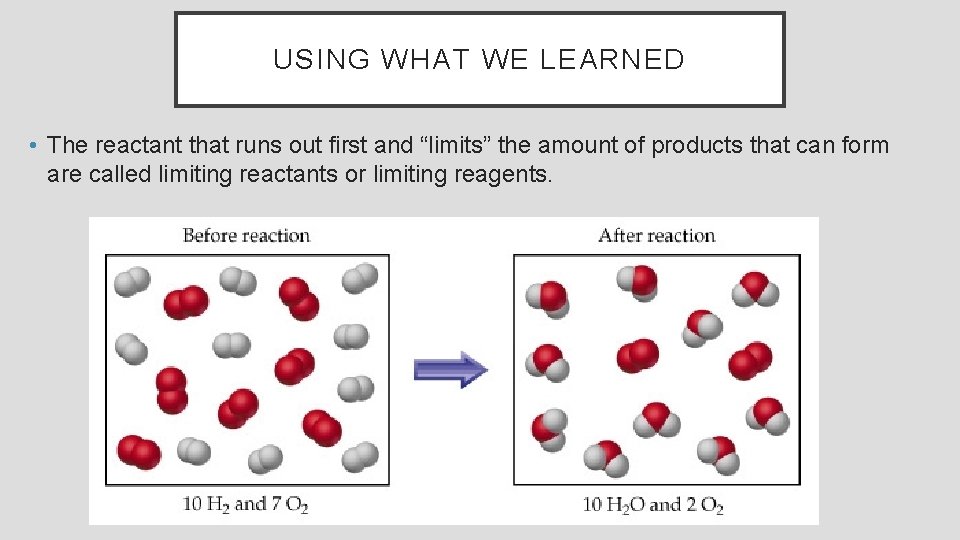
USING WHAT WE LEARNED • The reactant that runs out first and “limits” the amount of products that can form are called limiting reactants or limiting reagents.

STEPS FOR CALCULATING LIMITING REACTANTS • 1. Balance the equation. • 2. Convert masses of reactants or products to moles. • 3. Use balanced equation to get mole ratio. • 4. Use mole ratio to calculate number of moles of desired reactant or product. • 5. Convert mole back to mass.

IDENTIFYING LIMITING REACTANTS • Suppose 25 kg of nitrogen gas and 5 kg of hydrogen gas are mixed and react to form ammonia. Calculate the mass of ammonia produced. • 1. Write a balanced equation. • N 2 + 3 H 2 = 2 NH 3 • 2. Convert mass to moles • 1 mole N 2 x 1000 g x 25 kg = 892 moles N 2 • 28. 02 gr 1 kg • 1 mole H 2 x 1000 gr x 5 kg = 2480 moles H 2 • 2. 016 gr 1 kg

IDENTIFYING LIMITING REACTANTS • Suppose 25 kg of nitrogen gas and 5 kg of hydrogen gas are mixed and react to form ammonia. Calculate the mass of ammonia produced. • 3. Use the balanced equations to get molar ratios. N 2 + 3 H 2 = 2 NH 3 • 4. Use the molar ratios to calculate reactants or products. • 3 mole H 2 x 892 moles N 2 = 2680 moles H 2 • 1 mole N 2 • So we need 2680 moles H 2 but we only have 2480 moles H 2. So H 2 is the limiting reactant. • 2 moles NH 3 x 2480 moles H 2 = • 3 moles H 2 1650 moles NH 3

IDENTIFYING LIMITING REACTANTS • Suppose 25 kg of nitrogen gas and 5 kg of hydrogen gas are mixed and react to form ammonia. Calculate the mass of ammonia produced. • 5. Convert moles into mass. N 2 + 3 H 2 = 2 NH 3 • 17. 03 g NH 3 x 1650 moles NH 3 = 2810 grams NH 3 • 1 mole NH 3

PICK A CARD, ANY CARD… X ? P

YOUR TURN. . • Aluminum reacts with chlorine to form aluminum chloride. You have 10 grams of aluminum and 35 grams of chlorine. How many grams of Al. Cl 3 will be produced? • • • 1. Write a balanced equation. 2 Al + 3 Cl 2 = 2 Al. Cl 3 2. Convert mass to moles 1 mole Al x 10 g =. 3706 moles Al 26. 98 gr • 1 mole Cl 2 x 35 gr =. 4936 moles Cl 2 • 70. 9 gr

YOUR TURN. . • Aluminum reacts with chlorine to form aluminum chloride. You have 10 grams of aluminum and 35 grams of chlorine. How many grams of Al. Cl 3 will be produced? • 3. Use the balanced equations to get molar ratios. 2 Al + 3 Cl 2 = 2 Al. Cl 3 • 4. Use the molar ratios to calculate reactants or products. • 2 mole Al x. 4936 moles Cl 2 =. 3290 moles Al • 3 mole Cl 2 • 3 moles Cl 2 x. 3706 gr Al =. 5559 moles Cl 2 • 2 mole Al • So we need. 3290 moles Al &. 5559 Cl 2 moles Cl 2, but we only have. 3706 Al and. 4937 moles Cl 2. So Cl 2 is the limiting reactant.

YOUR TURN. . • Aluminum reacts with chlorine to form aluminum chloride. You have 10 grams of aluminum and 35 grams of chlorine. How many grams of Al. Cl 3 will be produced? • 5. Convert moles into mass. 2 Al + 3 Cl 2 = 2 Al. Cl 3 • 133. 332 gr Al. Cl 3 x 2 moles Al. Cl 3 x. 4937 moles Cl 2 = 43. 88 grams Al. Cl 3 1 mole Al. Cl 3 3 moles Cl 2

YOUR TURN. . • Aluminum reacts with chlorine to form aluminum chloride. You have 10 grams of aluminum and 35 grams of chlorine. How many grams of Al left over? • 2 Al + 3 Cl 2 = 2 Al. Cl 3 • 26. 982 gr Al x 2 moles Al x. 4937 moles Cl 2 = 8. 88 grams Al 1 mole Al 3 moles Cl 2 • But since we started with 10 grams, • 10 – 8. 88 = 1. 12 grams Al left over

PICK A CARD, ANY CARD… X ? P

PERCENT YIELD • Theoretical yield – amount of product calculated to result from a given equation (what we did last section!) • maximum amount available to be produced from the given reactants. • Unfortunately, this yield is rarely attained! • Percent yield – actual yield of products, actual amount of products produced. • Tells the efficiency of the reaction. • Actual yield x 100% = percent yield • Theoretical Yield

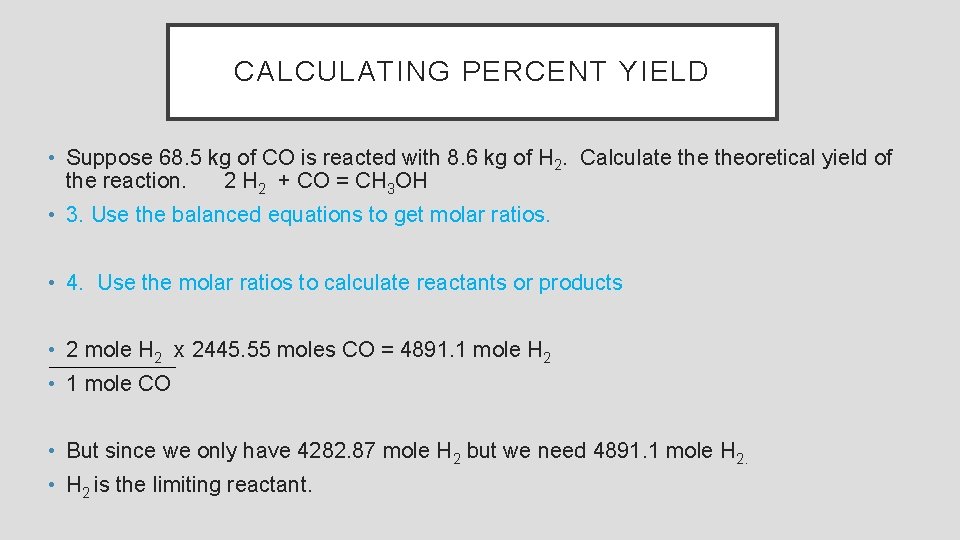
CALCULATING PERCENT YIELD • Suppose 68. 5 kg of CO is reacted with 8. 6 kg of H 2. Calculate theoretical yield of the reaction. 2 H 2 + CO = CH 3 OH • 1. Write a balanced equation. 2 H 2 + CO = CH 3 OH • 2. Convert mass to moles • 1 mole CO x 1000 g x 68. 5 kg = 2445. 55 mole CO • 28. 01 g CO 1 kg • 1 mole H 2 x 1000 g x 8. 6 kg = 4282. 87 mole H 2 • 2. 008 g 1 kg

CALCULATING PERCENT YIELD • Suppose 68. 5 kg of CO is reacted with 8. 6 kg of H 2. Calculate theoretical yield of the reaction. 2 H 2 + CO = CH 3 OH • 3. Use the balanced equations to get molar ratios. • 4. Use the molar ratios to calculate reactants or products • 2 mole H 2 x 2445. 55 moles CO = 4891. 1 mole H 2 • 1 mole CO • But since we only have 4282. 87 mole H 2 but we need 4891. 1 mole H 2. • H 2 is the limiting reactant.

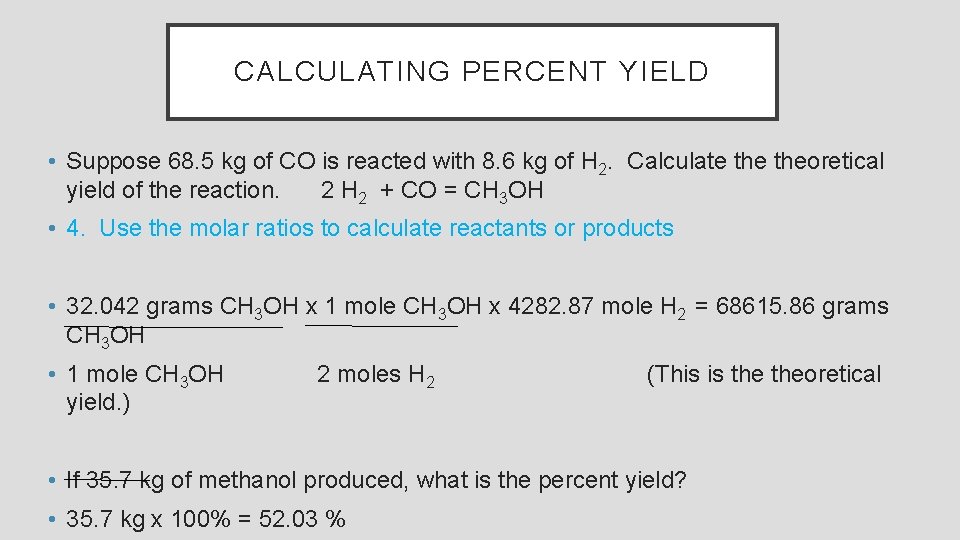
CALCULATING PERCENT YIELD • Suppose 68. 5 kg of CO is reacted with 8. 6 kg of H 2. Calculate theoretical yield of the reaction. 2 H 2 + CO = CH 3 OH • 4. Use the molar ratios to calculate reactants or products • 32. 042 grams CH 3 OH x 1 mole CH 3 OH x 4282. 87 mole H 2 = 68615. 86 grams CH 3 OH • 1 mole CH 3 OH yield. ) 2 moles H 2 (This is theoretical • If 35. 7 kg of methanol produced, what is the percent yield? • 35. 7 kg x 100% = 52. 03 %

PICK A CARD, ANY CARD… X ? P

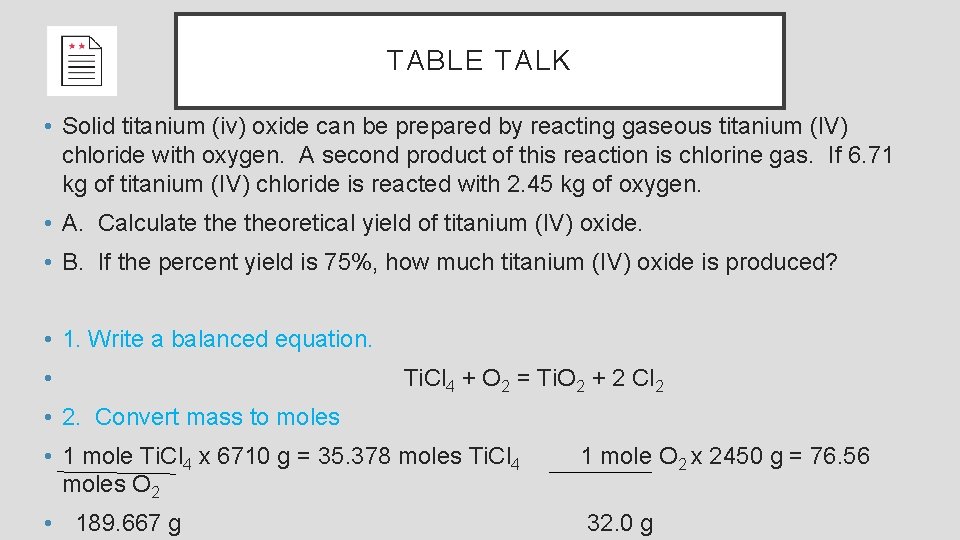
TABLE TALK • Solid titanium (iv) oxide can be prepared by reacting gaseous titanium (IV) chloride with oxygen. A second product of this reaction is chlorine gas. If 6. 71 kg of titanium (IV) chloride is reacted with 2. 45 kg of oxygen. • A. Calculate theoretical yield of titanium (IV) oxide. • B. If the percent yield is 75%, how much titanium (IV) oxide is produced? • 1. Write a balanced equation. • Ti. Cl 4 + O 2 = Ti. O 2 + 2 Cl 2 • 2. Convert mass to moles • 1 mole Ti. Cl 4 x 6710 g = 35. 378 moles Ti. Cl 4 moles O 2 • 189. 667 g 1 mole O 2 x 2450 g = 76. 56 32. 0 g

TABLE TALK • Solid titanium (iv) oxide can be prepared by reacting gaseous titanium (IV) chloride with oxygen. A second product of this reaction is chlorine gas. If 6. 71 kg of titanium (IV) chloride is reacted with 2. 45 kg of oxygen. • A. Calculate theoretical yield of titanium (IV) oxide. • B. If the percent yield is 75%, how much titanium (IV) oxide is produced? • 3. Use the balanced equations to get molar ratios. Ti. Cl 4 + O 2 = Ti. O 2 + 2 Cl 2 • 4. Use the molar ratios to calculate reactants or products • 1 mole Ti. Cl 4 x 76. 56 = 76. 56 • 1 mole O 2 but we only have 35. 378 moles Ti. Cl 4

TABLE TALK • Solid titanium (iv) oxide can be prepared by reacting gaseous titanium (IV) chloride with oxygen. A second product of this reaction is chlorine gas. If 6. 71 kg of titanium (IV) chloride is reacted with 2. 45 kg of oxygen. • A. Calculate theoretical yield of titanium (IV) oxide. • B. If the percent yield is 75%, how much titanium (IV) oxide is produced? • 3. Use the balanced equations to get molar ratios. Ti. Cl 4 + O 2 = Ti. O 2 + 2 Cl 2 • 4. Use the molar ratios to calculate reactants or products • 1 mole Ti. Cl 4 x 76. 56 = 76. 56 • 1 mole O 2 but we only have 35. 378 moles Ti. Cl 4

TABLE TALK • If 6. 71 kg of titanium (IV) chloride is reacted with 2. 45 kg of oxygen. Ti. Cl 4 + O 2 = Ti. O 2 + 2 Cl 2 • A. Calculate theoretical yield of titanium (IV) oxide. • B. If the percent yield is 75%, how much titanium (IV) oxide is produced? • • • 4. Use the molar ratios to calculate reactants or products 79. 867 g Ti. O 2 x 1 mole Ti. O 2 x 35. 378 moles Ti. Cl 4 = 2825. 53 grams Ti. O 2 1 mole Ti. Cl 4 Actual yield x 100% = percent yield 2119. 148 gr • Theoretical yield AY x 100% = 75% 2825. 53 AY =

WORKS CITED • https: //encrypted-tbn 0. gstatic. com/images? q=tbn: ANd 9 Gc. Sy 3 m. S 4 hr. Sb 4 N-w 8 -OF 9 YBOGw 3 s. VPga_N-n 4 ntbp-vk. DQ 38 ec. E • https: //www. learner. org/courses/chemistry/images/lrg_img/methane_rxn. jpg • https: //i. ytimg. com/vi/d. PBSQMc 6 dn. Q/maxresdefault. jpg • http: //wps. prenhall. com/wps/media/objects/165/169519/GIFS/AAAUAUT 0. JPG • https: //d 2 gne 97 vdumgn 3. cloudfront. net/api/file/BWc. Rc 9 id. QQCclt. Py. NO 7 l • https: //ssc. oureducation. in/wp-content/uploads/2017/06/quiz-940 x 530 -704 x 400. jpg • https: //whitespark. ca/wp-content/uploads/2016/01/review-handout-generator-1. jpg