CHEMICAL KINETICS WHY DO WE CARE Acetominophen Tylenol








![ZERO ORDER REACTIONS Rate is independent of concentration � Rate = k[A]0 = k ZERO ORDER REACTIONS Rate is independent of concentration � Rate = k[A]0 = k](https://slidetodoc.com/presentation_image/346d43dcc9717f1e814a71338a15ab41/image-14.jpg)


![INTERGRATED SECOND AND ZERO ORDER RATE LAWS �For Rate = k[A]2 � 1/[A]t = INTERGRATED SECOND AND ZERO ORDER RATE LAWS �For Rate = k[A]2 � 1/[A]t =](https://slidetodoc.com/presentation_image/346d43dcc9717f1e814a71338a15ab41/image-28.jpg)




- Slides: 42

CHEMICAL KINETICS

WHY DO WE CARE? ? ? Acetominophen (Tylenol, 500 mg) Biological Half-life: 3 hours Recommended dosage: 2 pills every 6 hours Toxic if over 4, 000 mg are ingested in 24 hours Construct a line graph relating time elapsed after ingestion to amount of Tylenol present in the bloodstream.

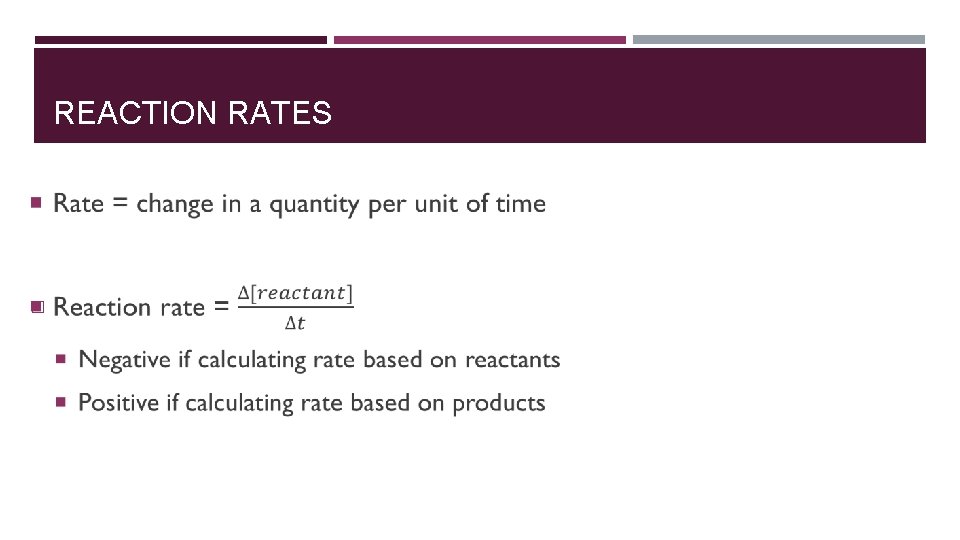
REACTION RATES �

Chemical Jargon Average rate of reaction = rate of reaction over total time elapsed (final – initial) � Instantaneous rate of reaction = rate of reaction at specific instant in time �



GENERAL EQUATION FOR A REACTION RATE �For a. A + b. B → c. C + d. D…

Practice Write the rate expressions for the following: � � I-(aq) + OCl-(aq) + OI-(aq) � 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g)

PRACTICE �For the reaction A + 2 B → C under a set of given conditions, the initial rate is 0. 100 M/s. What is the change in concentration of B under the same conditions?

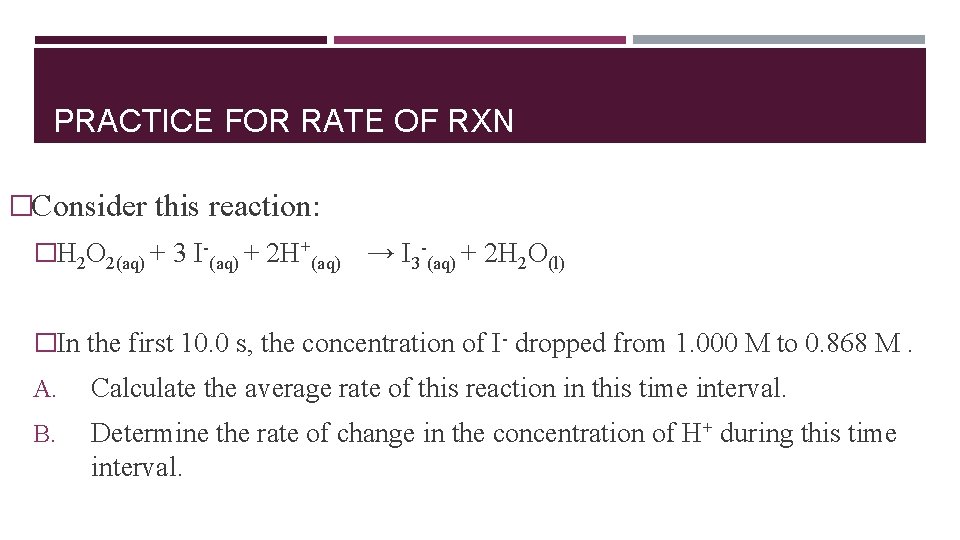
PRACTICE FOR RATE OF RXN �Consider this reaction: �H 2 O 2(aq) + 3 I-(aq) + 2 H+(aq) → I 3 -(aq) + 2 H 2 O(l) �In the first 10. 0 s, the concentration of I- dropped from 1. 000 M to 0. 868 M. A. Calculate the average rate of this reaction in this time interval. B. Determine the rate of change in the concentration of H+ during this time interval.

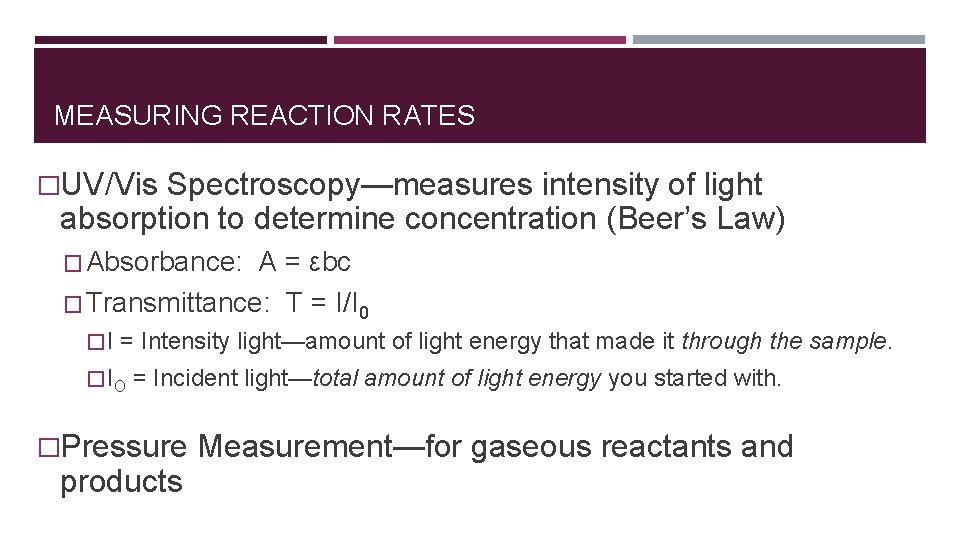
MEASURING REACTION RATES �UV/Vis Spectroscopy—measures intensity of light absorption to determine concentration (Beer’s Law) � Absorbance: A = εbc � Transmittance: T = I/I 0 � I = Intensity light—amount of light energy that made it through the sample. � IO = Incident light—total amount of light energy you started with. �Pressure Measurement—for gaseous reactants and products

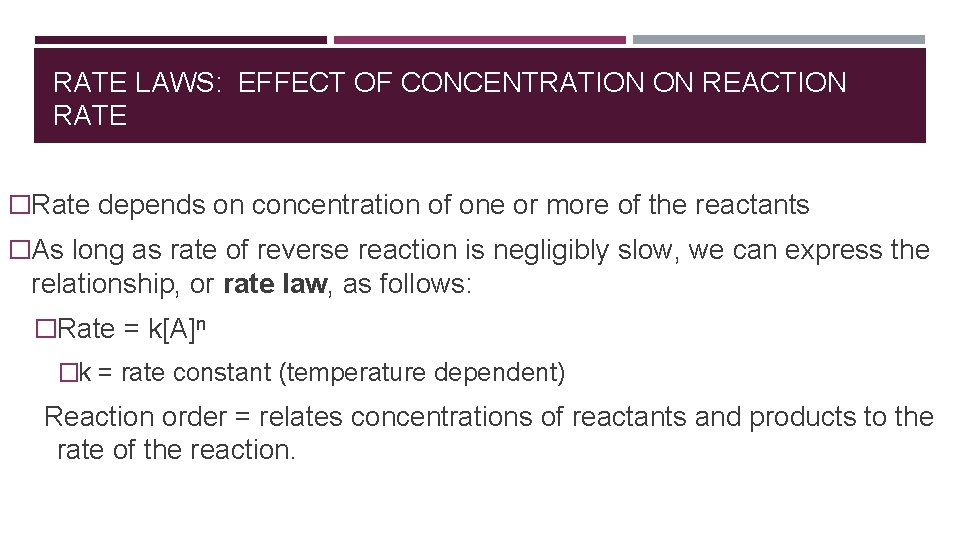
RATE LAWS: EFFECT OF CONCENTRATION ON REACTION RATE �Rate depends on concentration of one or more of the reactants �As long as rate of reverse reaction is negligibly slow, we can express the relationship, or rate law, as follows: �Rate = k[A]n �k = rate constant (temperature dependent) Reaction order = relates concentrations of reactants and products to the rate of the reaction.

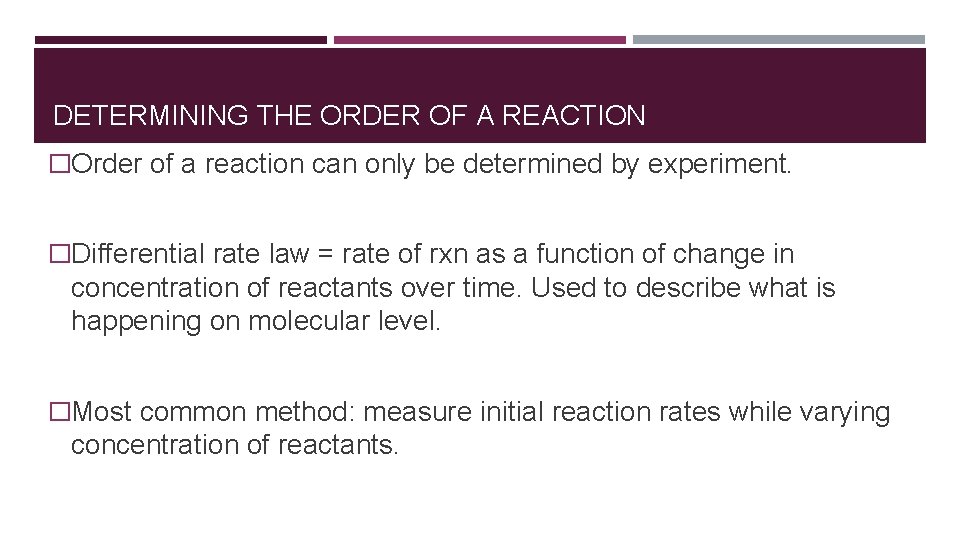
DETERMINING THE ORDER OF A REACTION �Order of a reaction can only be determined by experiment. �Differential rate law = rate of rxn as a function of change in concentration of reactants over time. Used to describe what is happening on molecular level. �Most common method: measure initial reaction rates while varying concentration of reactants.
![ZERO ORDER REACTIONS Rate is independent of concentration Rate kA0 k ZERO ORDER REACTIONS Rate is independent of concentration � Rate = k[A]0 = k](https://slidetodoc.com/presentation_image/346d43dcc9717f1e814a71338a15ab41/image-14.jpg)
ZERO ORDER REACTIONS Rate is independent of concentration � Rate = k[A]0 = k � Example: Sublimation—number of particles available to sublime remains constant (theoretically) [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20 0. 015 0. 40 0. 015

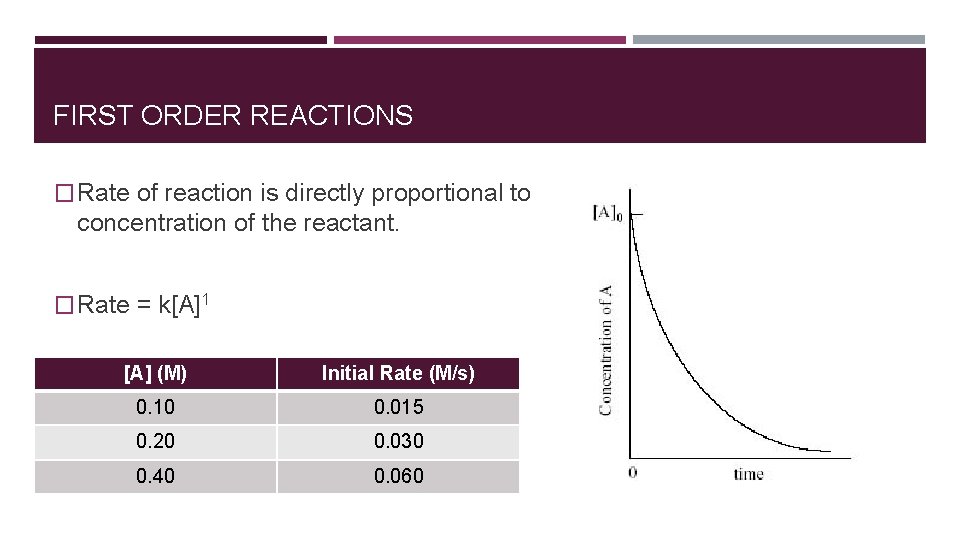
FIRST ORDER REACTIONS � Rate of reaction is directly proportional to concentration of the reactant. � Rate = k[A]1 [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20 0. 030 0. 40 0. 060

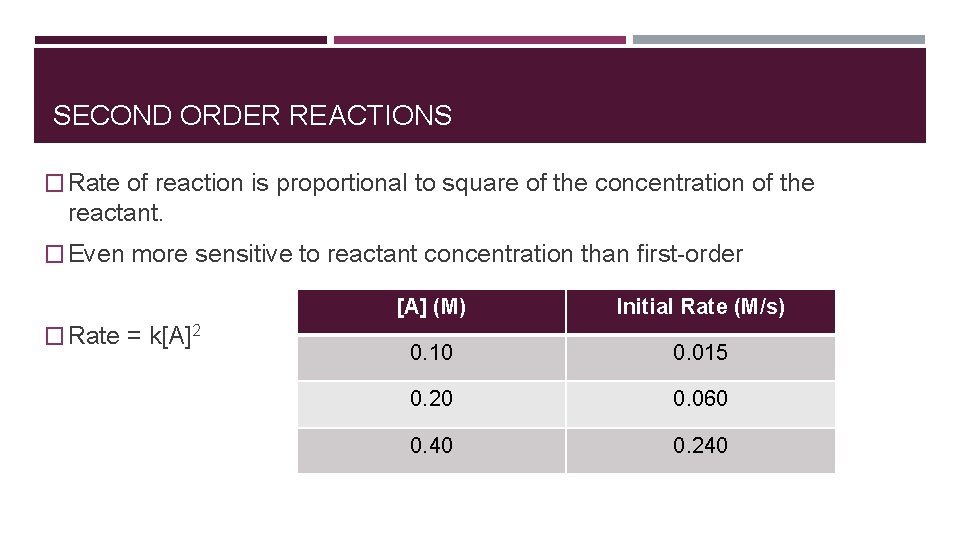
SECOND ORDER REACTIONS � Rate of reaction is proportional to square of the concentration of the reactant. � Even more sensitive to reactant concentration than first-order � Rate = k[A]2 [A] (M) Initial Rate (M/s) 0. 10 0. 015 0. 20 0. 060 0. 40 0. 240

PRACTICE �The reaction A → B has been experimentally determined to be second order. The initial rate is 0. 0100 M/s at an initial concentration of A of 0. 100 M. What is the initial rate at [A] = 0. 500 M?

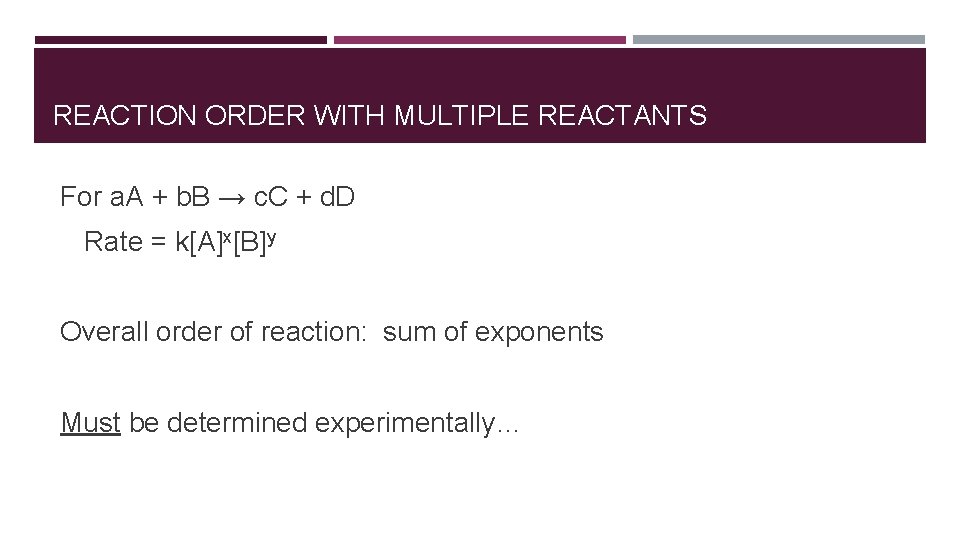
REACTION ORDER WITH MULTIPLE REACTANTS For a. A + b. B → c. C + d. D Rate = k[A]x[B]y Overall order of reaction: sum of exponents Must be determined experimentally…

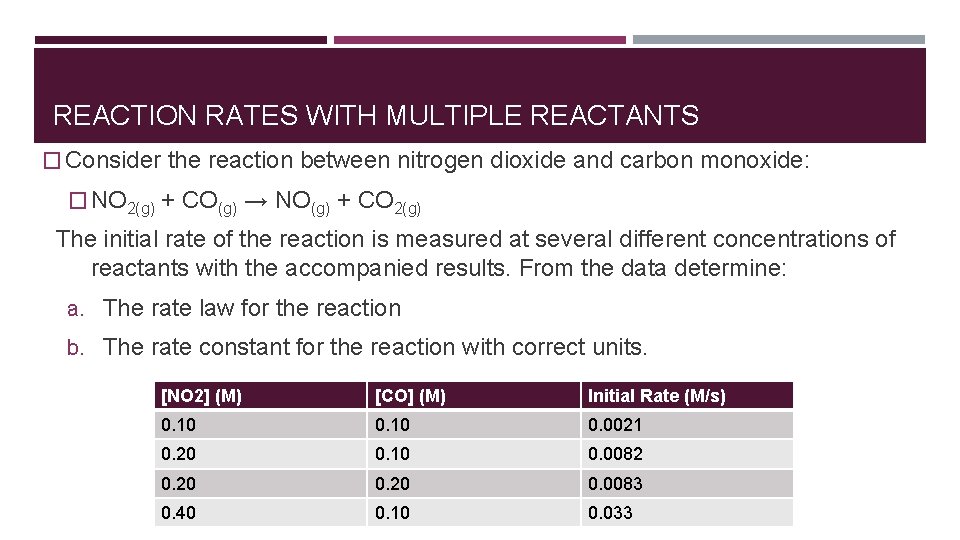
REACTION RATES WITH MULTIPLE REACTANTS � Consider the reaction between nitrogen dioxide and carbon monoxide: � NO 2(g) + CO(g) → NO(g) + CO 2(g) The initial rate of the reaction is measured at several different concentrations of reactants with the accompanied results. From the data determine: a. The rate law for the reaction b. The rate constant for the reaction with correct units. [NO 2] (M) [CO] (M) Initial Rate (M/s) 0. 10 0. 0021 0. 20 0. 10 0. 0082 0. 20 0. 0083 0. 40 0. 10 0. 033

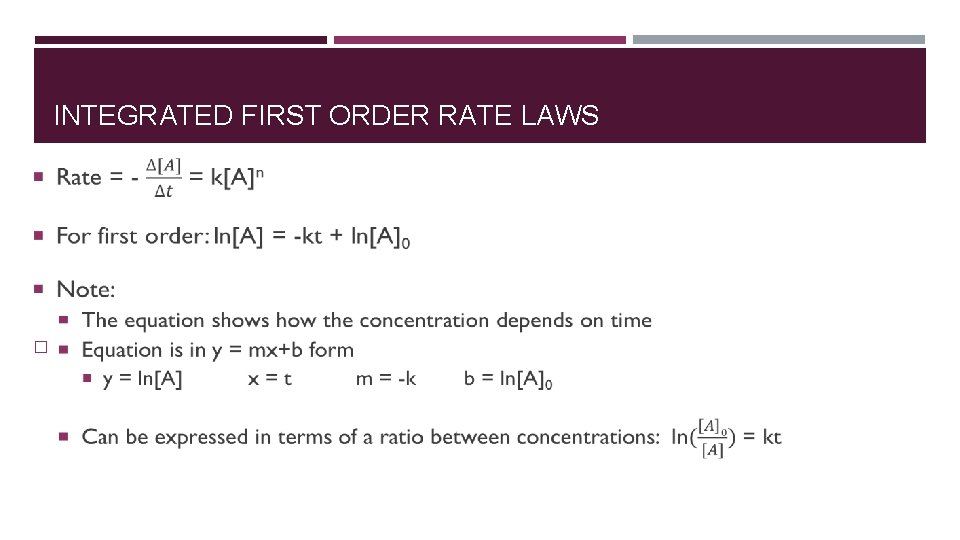
WHY INTEGRATED RATE LAWS? � What are they? � Relates concentrations of reactions and time. � Found by integrating the differential rate laws Allows us to answer the following: � How long does it take? � What was the initial concentration? � How much remains after a given amount of time?

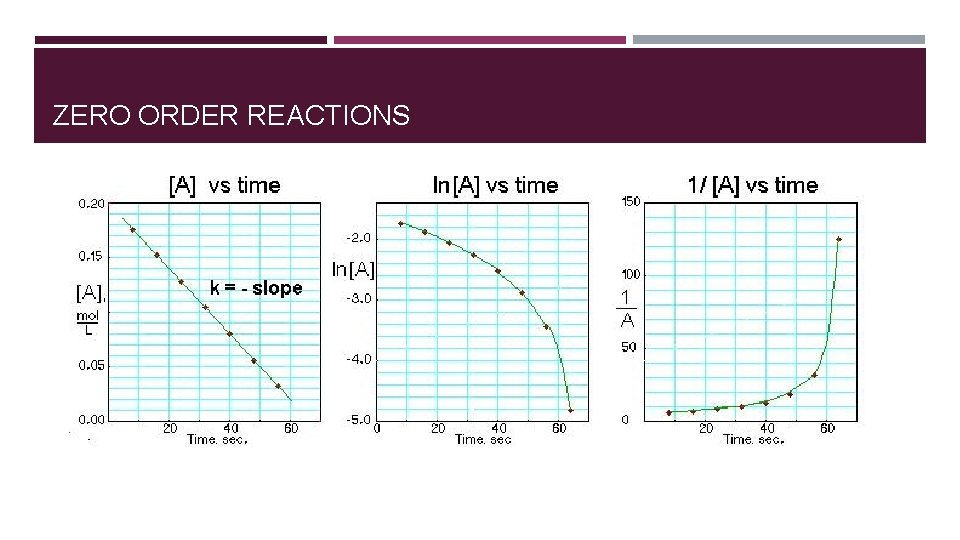
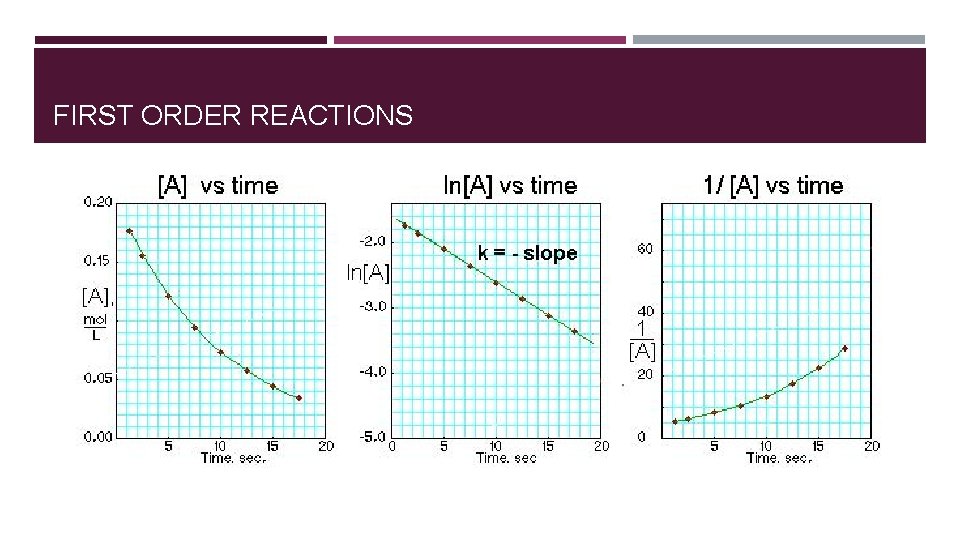
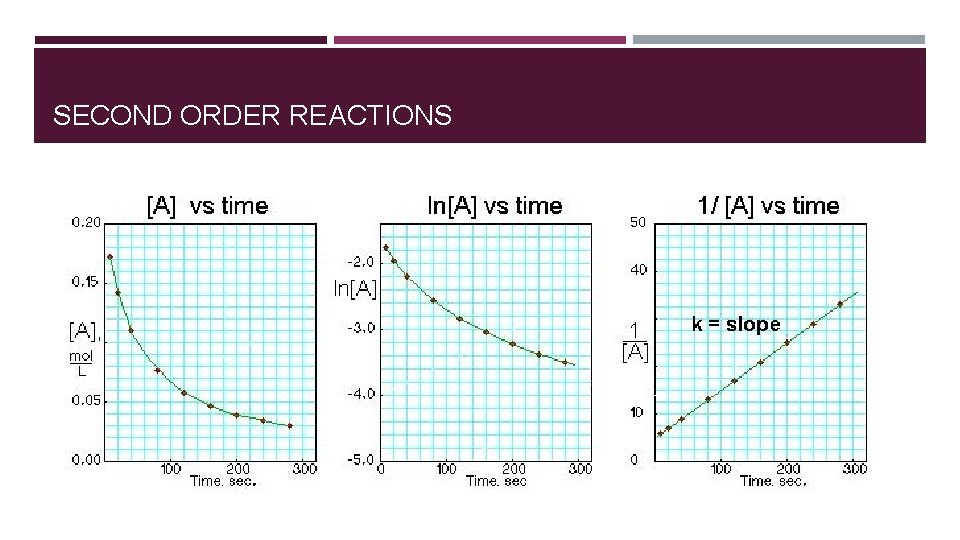
ORDER OF REACTION DETERMINED GRAPHICALLY Each reaction order rate equation can be integrated to relate time and concentration. � A plot of 1/[A] versus t yields a straight line with a slope of k for a second- order reaction. � A plot of ln[A] versus t yields a straight line with a slope of -k for a first-order reaction. � A plot of [A] versus t gives a straight line with a slope of -k for a zero-order reaction.

ZERO ORDER REACTIONS

FIRST ORDER REACTIONS

SECOND ORDER REACTIONS

INTEGRATED FIRST ORDER RATE LAWS �

PRACTICE �The decomposition of a certain insecticide in water follows first order kinetics and has a rate constant of 1. 45 yr-1 at 12 o. C. A quantity of this insecticide is washed into a lake on June 1, leading to a concentration of 5. 0× 10 -7 g/cm 3. What is the concentration of the insecticide on June 1 of the following year? Assume the average temperature of the lake is 12 o. C. �How long will it take for the concentration of insecticide to drop to 3. 0× 10 -7 g/cm 3?

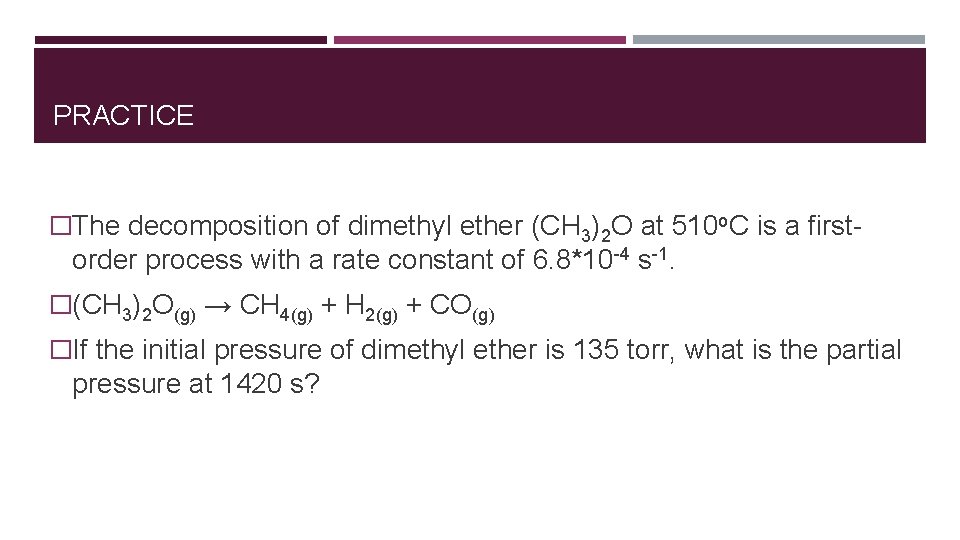
PRACTICE �The decomposition of dimethyl ether (CH 3)2 O at 510 o. C is a first- order process with a rate constant of 6. 8*10 -4 s-1. �(CH 3)2 O(g) → CH 4(g) + H 2(g) + CO(g) �If the initial pressure of dimethyl ether is 135 torr, what is the partial pressure at 1420 s?
![INTERGRATED SECOND AND ZERO ORDER RATE LAWS For Rate kA2 1At INTERGRATED SECOND AND ZERO ORDER RATE LAWS �For Rate = k[A]2 � 1/[A]t =](https://slidetodoc.com/presentation_image/346d43dcc9717f1e814a71338a15ab41/image-28.jpg)
INTERGRATED SECOND AND ZERO ORDER RATE LAWS �For Rate = k[A]2 � 1/[A]t = kt + 1/[A]0 �For Rate = k[A]0 �[A]t = -kt + [A]0

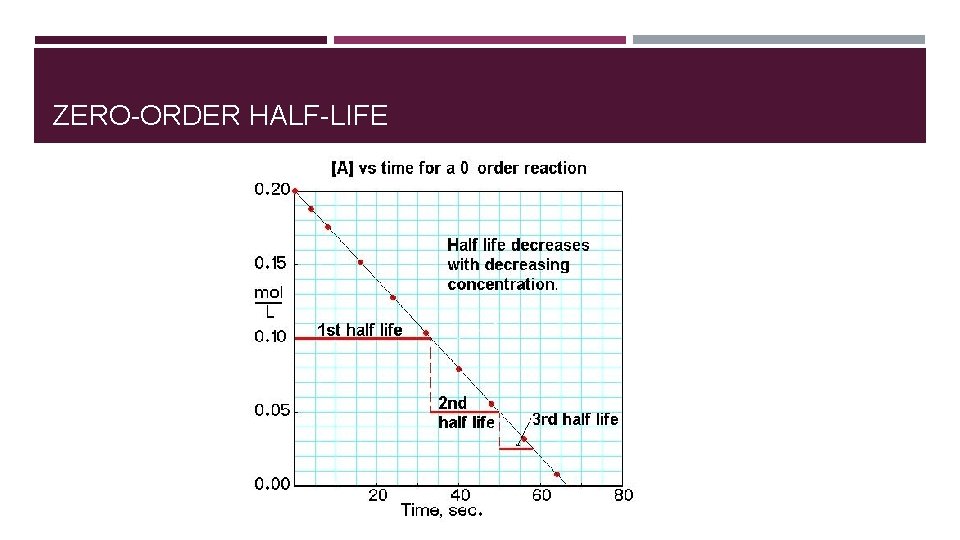
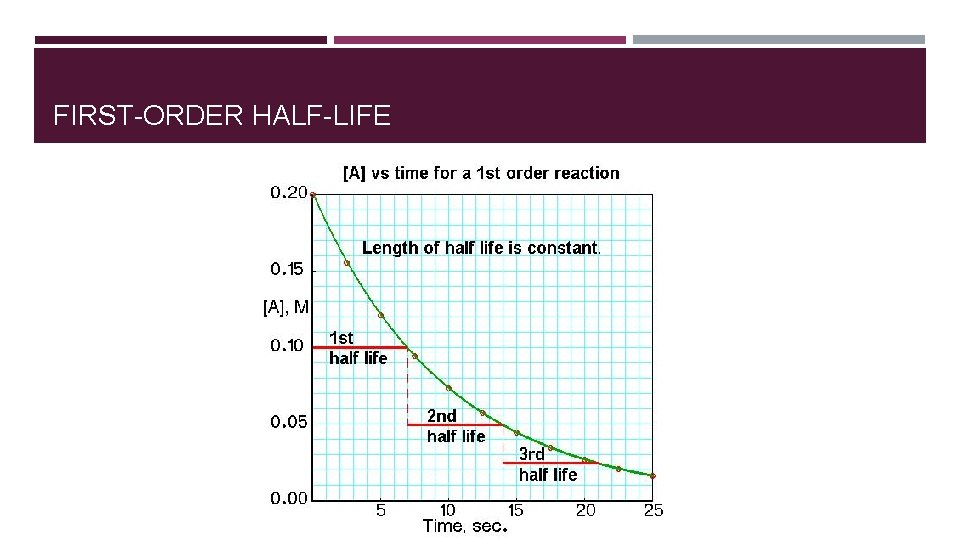
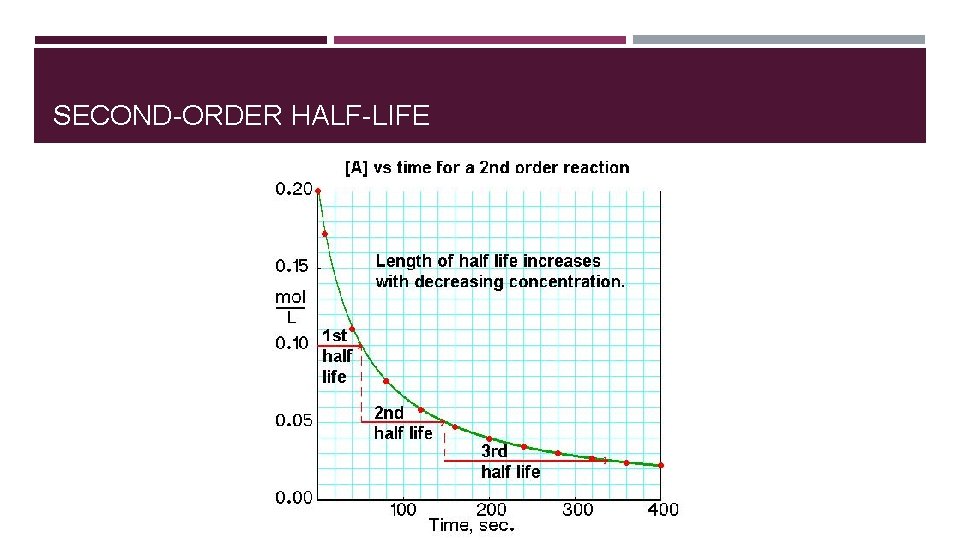
HALF LIFE FOR FIRST-ORDER REACTIONS � Half-life = time required for concentrations of a reactant to fall to one half of its initial value. � For zero-order reaction half-life: � t 1/2 = [A]0/2 k � For first-order reaction half-life: � ln([A]t/[A]0) = -kt � t 1/2 = 0. 693/k � For second-order reaction half-life: � t 1/2 = 1/k[A]0

ZERO-ORDER HALF-LIFE

FIRST-ORDER HALF-LIFE

SECOND-ORDER HALF-LIFE

PRACTICE �Molecular iodine dissociates at 625 K with a first-order rate constant of 0. 271 s-1. What is the half-life of this reaction? �A first-order reaction has a half-life of 26. 4 seconds How long does it take for the concentration of the reactant in the reaction to fall to oneeighth of its initial value?

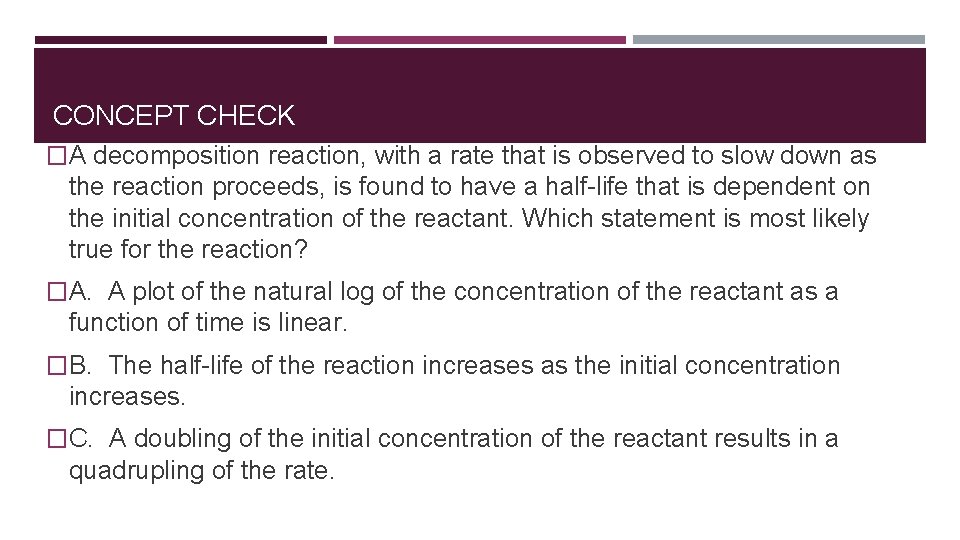
CONCEPT CHECK �A decomposition reaction, with a rate that is observed to slow down as the reaction proceeds, is found to have a half-life that is dependent on the initial concentration of the reactant. Which statement is most likely true for the reaction? �A. A plot of the natural log of the concentration of the reactant as a function of time is linear. �B. The half-life of the reaction increases as the initial concentration increases. �C. A doubling of the initial concentration of the reactant results in a quadrupling of the rate.

SUMMARY FOR REACTION ORDER AND RATES �Order and rate law have to be determined experimentally �Rate law relates the rate of the reaction to the concentration of the reactants �Integrated rate law (derived from differential rate law) relates concentration of reactants to time. �Half-life for a first-order reaction is independent of initial concentration. �Half-lives for zero- and second-order reaction depend on the initial concentration.

FACTORS THAT AFFECT THE RATE OF A REACTION surface area of a solid reactant � concentration or pressure of a reactant � temperature � nature of the reactants (state of matter, molecular size, bond type, bond strength. ) � presence/absence of a catalyst �

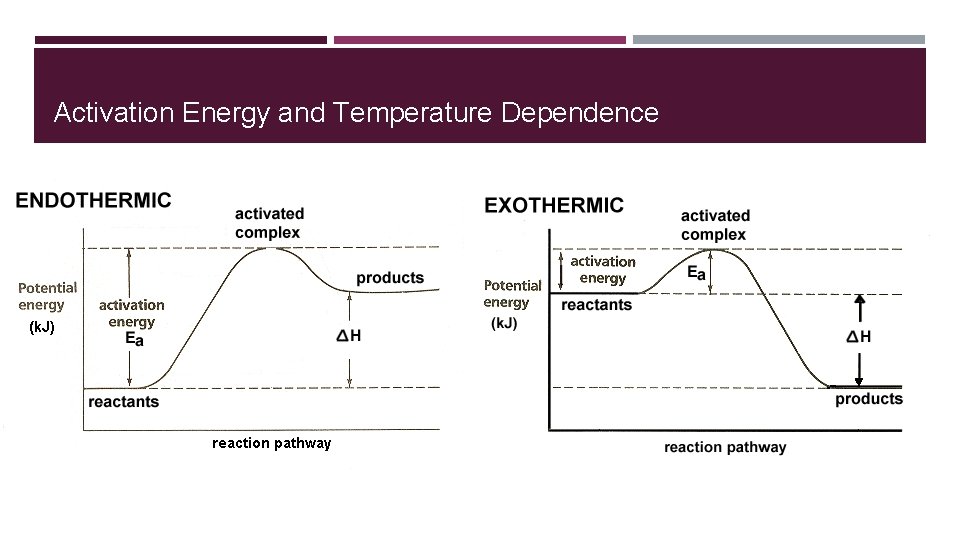
Activation Energy and Temperature Dependence

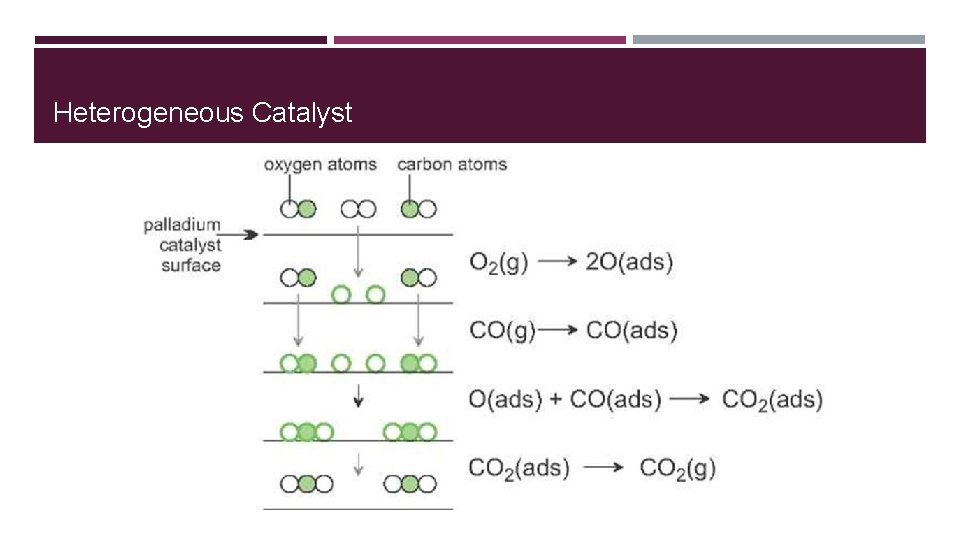
Catalysts Lower activation energy of reactions by putting the reactants into the proper orientation for more effective collisions � 2 types: � � Heterogeneous � Homogenous

Heterogeneous Catalyst

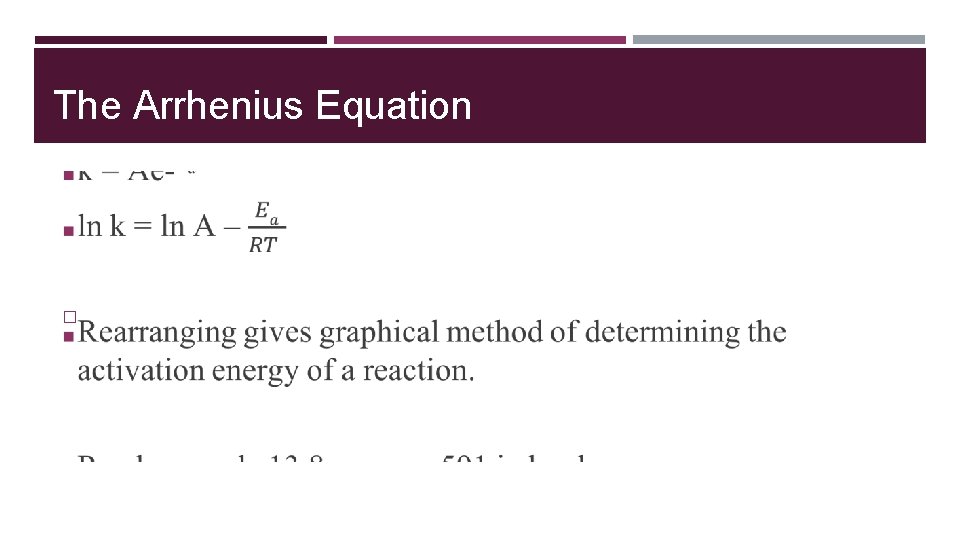
The Arrhenius Equation �

Practice 1. Consider the decomposition of NO 2. 2 NO 2(g) → 2 NO(g) + O 2(g) At 650 K, the rate constant is 1. 66 sec-1. At 700 K, the constant is 7. 39 sec-1. Calculate the activation energy.

Practice The activation energy for the isomerization of cyclopropane to propene is 274 k. J/mol. By what factor does the rate of reaction increase as the temperature rises from 500°C to 550°C? �