Chapter 9 OTHER SEPARATION PROCESSES u PhysicalMechanical Separations














































- Slides: 80

Chapter 9 OTHER SEPARATION PROCESSES u Physical/Mechanical Separations u Diffusional/equilibration separations

Physical/Mechanical Separations u Filtration u Expression u Centrifugation u Cyclone

Diffusional/equilibration separations u Crystallization u Distillation u Absorption/Stripping u Extraction u Adsorption u Ion Exchange u Dialysis/Electrodialysis


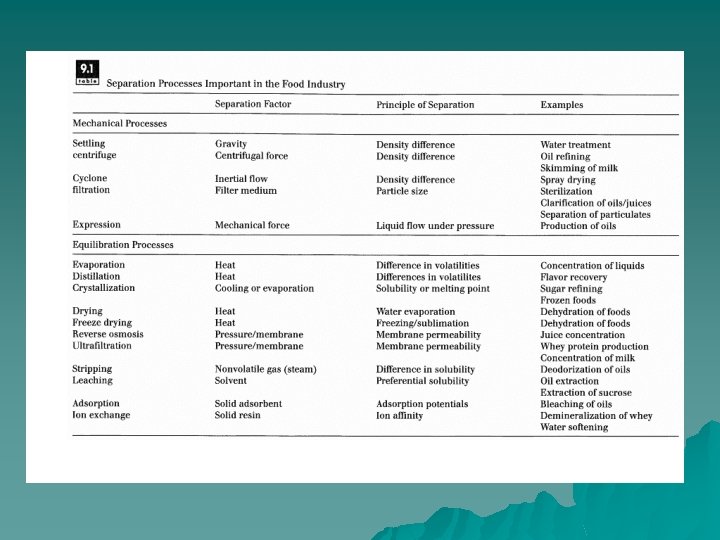
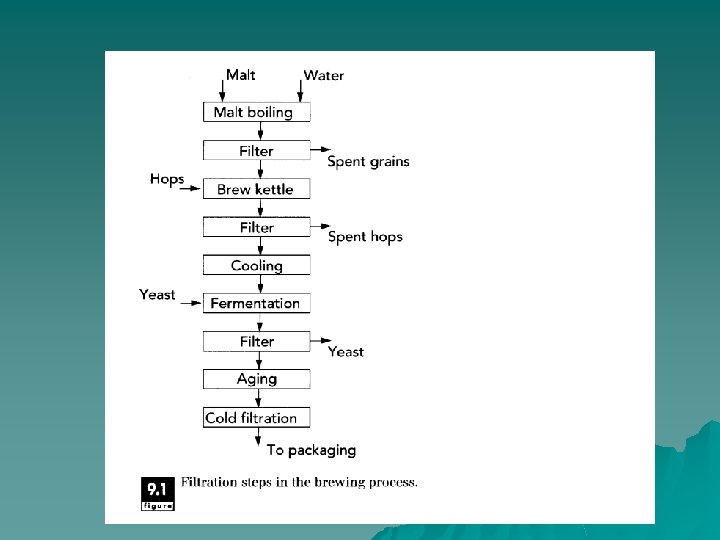
Filtration u Used to clarify fluid foods( fruit juices or vegetable oils) and to remove microorganisms from either air or fluid foods and to separate solid from liquid phases. In some cases, the material that remains on the filter is the valuable component (crystal slurry from liquid in sugar refining, separation of proteins or yeast). The material that passes through the filter is the desired product (fruit juice or vegetable oil Clarification, sterilized water or air).

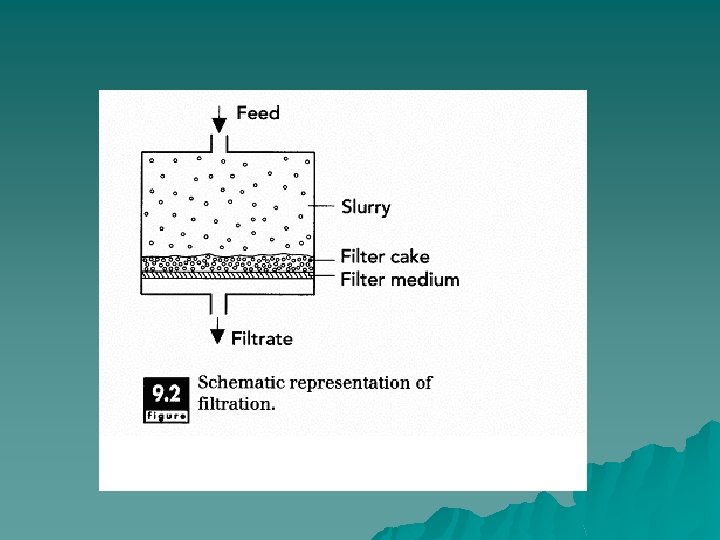
Principles of Operation. u Filtration involves passing a material through a filter medium that retains particles of a certain size, Filtration is distinguished from other membrane separations (ultrafiltration and reverse osmosis) in terms of the size of particles that are separated. A fluid containing solid matter is forced through a filter medium, with the solid particles being retained by the filter and the liquid passing through.


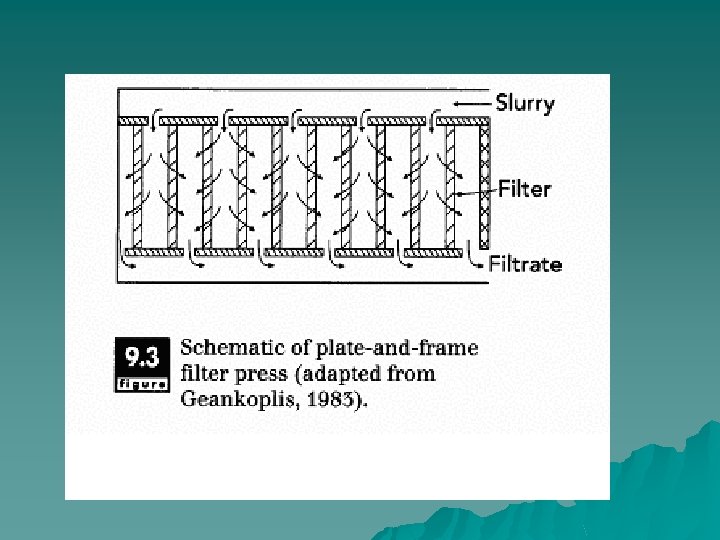
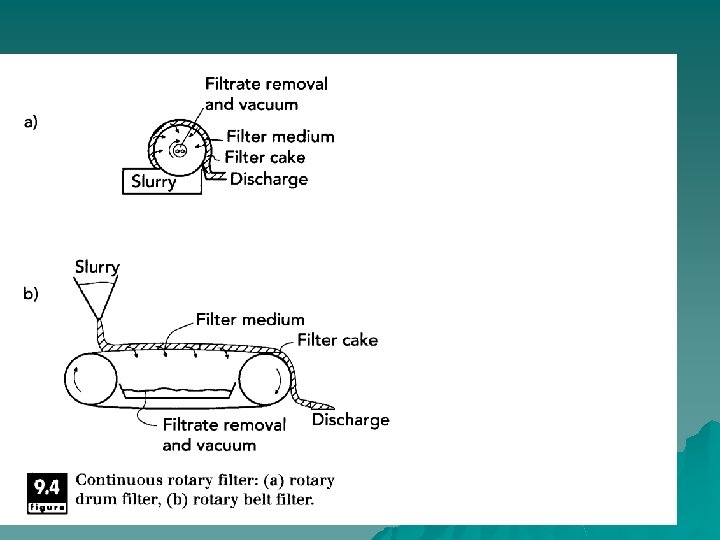
Configurations u plate-and-frame u continuous rotary filter

Factors influencing filtration u Size of the pores in the filter medium u The viscosity of the carrier fluid u The amount and characteristics of suspended solids to be filtered u The external pressure applied u Filter aids, such as diatomaceous earth or perlite





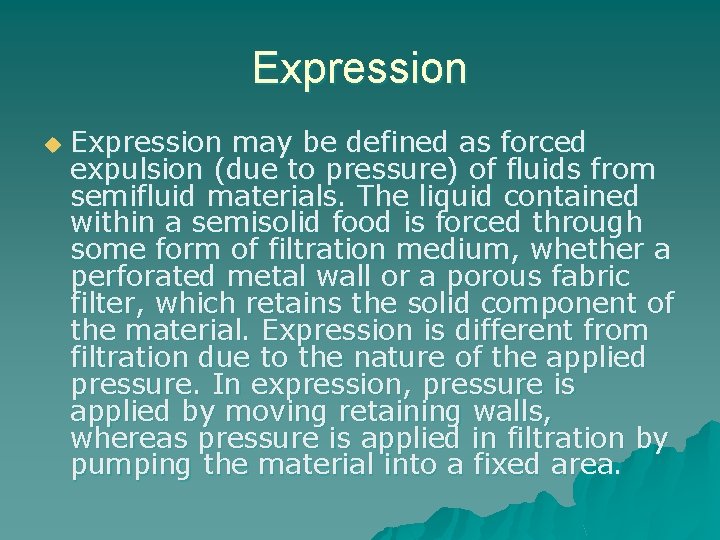
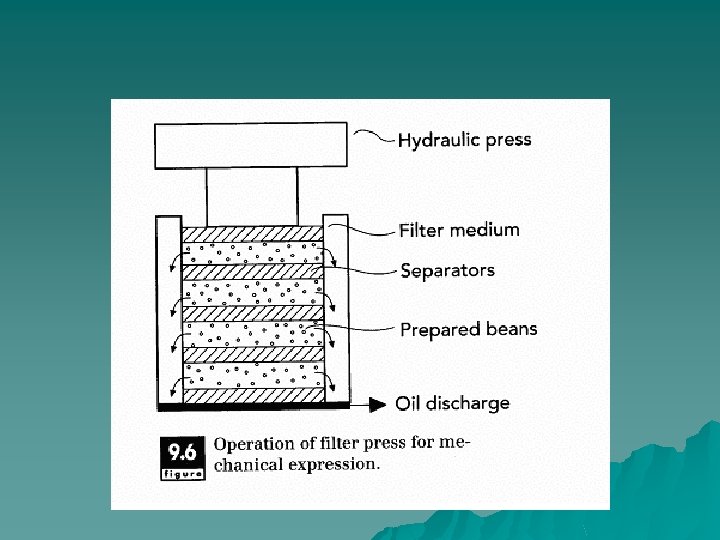
Expression u Expression may be defined as forced expulsion (due to pressure) of fluids from semifluid materials. The liquid contained within a semisolid food is forced through some form of filtration medium, whether a perforated metal wall or a porous fabric filter, which retains the solid component of the material. Expression is different from filtration due to the nature of the applied pressure. In expression, pressure is applied by moving retaining walls, whereas pressure is applied in filtration by pumping the material into a fixed area.

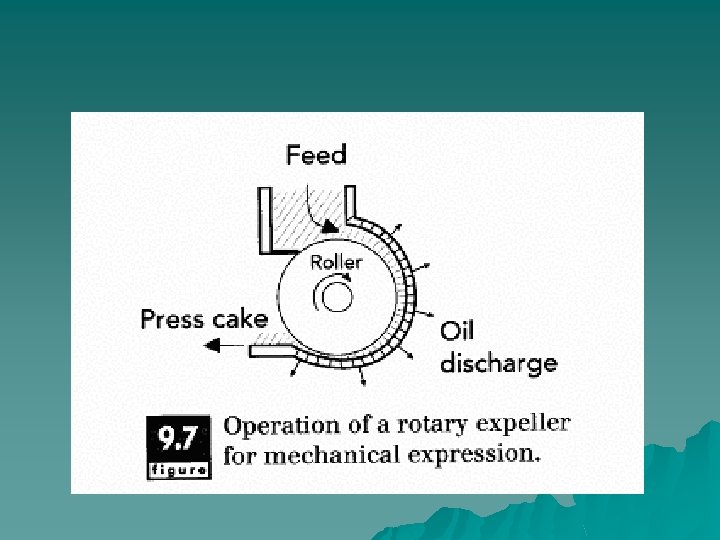
Principles of Operation u When placed under considerable pressure, the cells of the seeds are ruptured, and the oil contained within can flow out. By means of some pressure application device, the seeds are pressed against a plate or filter in order to rupture the internal structure. Pressure also may be applied by such devices as a piston, or a disk rotating within a chamber with narrowing width. A screw press, which simultaneously pumps and compresses, is often used to mechanically separate oil from seed cake.





Centrifugation u separation of cream from milk and refining of vegetable oils. Centrifugal separation is also employed in the brewing industry, processing of fish protein concentrate, and removal of cellular materials from fruit juices. Centrifugal filtration is also employed in the food industry for separation of crystalline products from a crystal slurry. In this case, centrifugal force causes the solid particles to form a filter cake on a screen in a rotating basket Rotation of the basket allows washing and drying of the separated slurry following centrifugation.

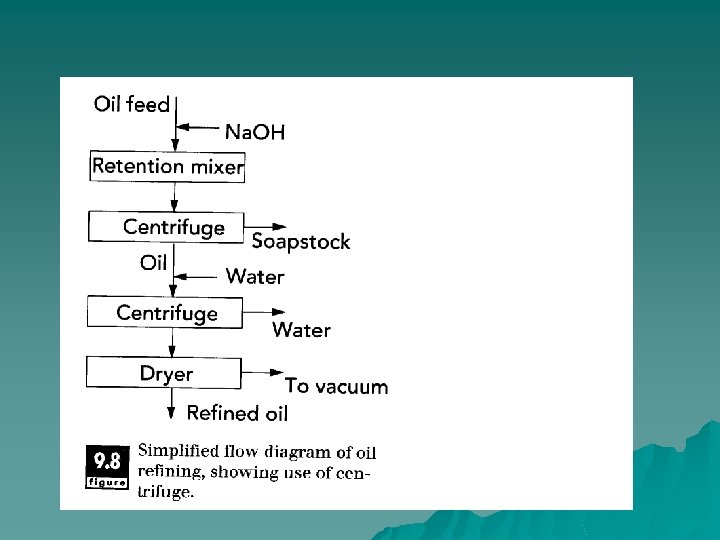
u In oil refining, alkali (base) is added to the liquid oil to aid in removing free fatty acids, which give rise to undesired odors. The alkali (sodium hydroxide) solution reacts with the free fatty acids to produce soaps, which are soluble in the aqueous phase. A centrifuge is then used to separate the aqueous phase containing soaps from the liquid oil phase. Separation of oil from water is accomplished based on their different densities.


u Separation of the fat in milk is also accomplished using a centrifugal separator, In this case, whole milk containing about 3. 2 to 3. 5% milk fat enters the centrifuge, where the fat phase is separated from the aqueous phase by centrifugal force based on their different densities. The fat phase is only concentrated, and not separated entirely, to produce cream with about 30% fat The remainder of the cream is basically the same as the skim milk, which is the other product from the centrifuge. In a centrifuge, the fat content of milk may be reduced to less 0. 1%.


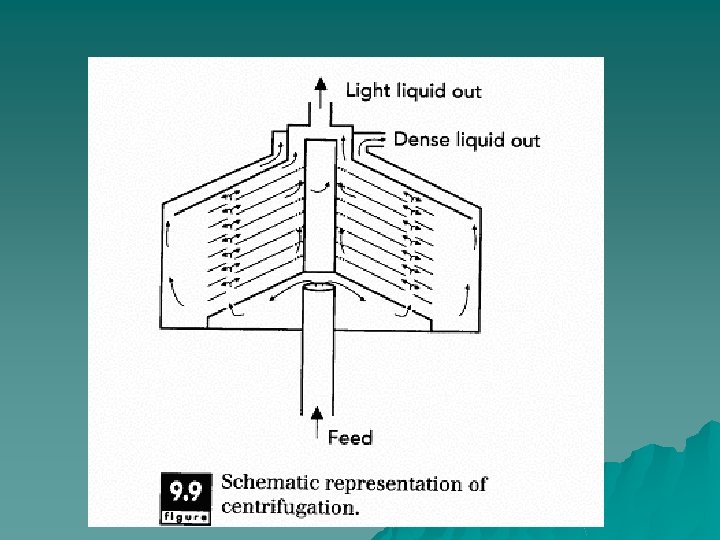
Principles of Operation u In a salad dressing made with oil and water, shaking is needed to mix the two phases (oil and water). After the bottle has sat for awhile, the oil separates from the aqueous phase. The oil rises to form the top layer, since it is less dense than the aqueous phase. The force of gravity causes this separation. In a centrifuge, rotational forces are applied to separate materials with different densities. The centrifuge is used to separate phases based on differences in density.

u In a typical centrifugal separator, there a number of disks set one on top of another with only a small spacing. The disks are shaped like funnels with a 45 -degree angle. This disk stack rotates at 5, 000 to 6, 000 rpm to generate considerable centrifugal force. The stream enters at the bottom of the stacks and makes its way into the stack through a series of holes in the disks. The phase which has greater density is forced to the outer regions of the centrifuge. The lighter phase migrates to the inner regions of the centrifuge and collects at the center of the disk stuck.

centrifugal clarification u Centrifugation of a slurry to separate a solid phase from a liquid carrier involves basically the same principle of operation. If there are only small amounts of insoluble solids to be removed (less than 5% or so), it is called centrifugal clarification.

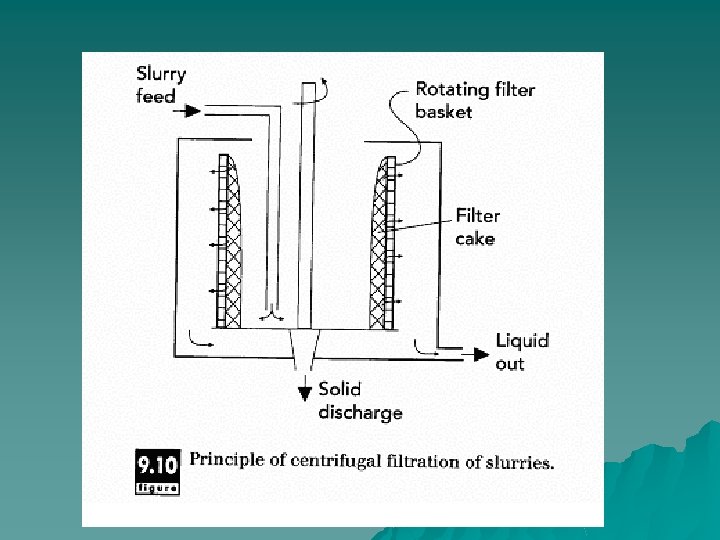
Decanting u Rotating a liquid containing solid particles at sufficiently high velocity causes the solid particles to separate from the fluid by centrifugal force. If the solids have higher density than the fluid, they are preferentially forced to the outside of the rotating chamber used to implement the centrifugal force. Typically, a basket with a screen for walls is used so that the solid particles form a filter cake on the walls of the basket. Centrifugal filters can be either batch or continuous. At the end of a batch filtration cycle, the filter cake on the basket is washed with pure liquid and then dried by rapid rotation. The product is then scraped off the basket for further processing.


Cyclone u It is often necessary to separate particulate material, whether solid particles or fine liquid droplets, suspended in a gas. This occurs, for example, when spray-dried powders must be separated from the air that was used to dry them. It also occurs during steam generation, when fine droplets of entrained water must be removed from the vapor to produce saturated steam.

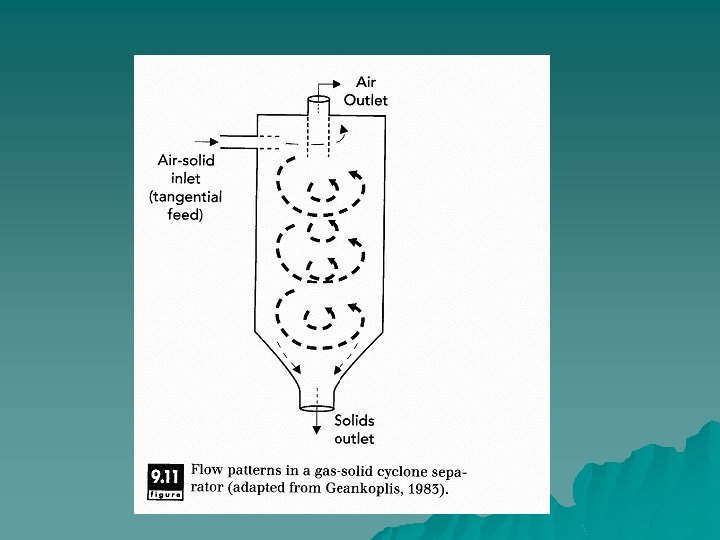
Principles of Operation u As with centrifugation, the driving force behind cyclone separation is centrifugal force and the difference in specific gravity between the particle and the carrier gas. In a Cyclone, the air or vapor containing particulate material is forced into along the tangential axis. A helical flow pattern is set up within the chamber. The centrifugal force causes the particles to migrate to the outside of the chamber. Here they fall down to the bottom of the cyclone by gravity. The air moves up the center of the cyclone and reaches the top.


DIFFUSIONAL EQUILIBRIUM SEPARATIONS u solid--liquid extraction u Distillation u Crystallization u liquid--liquid extraction

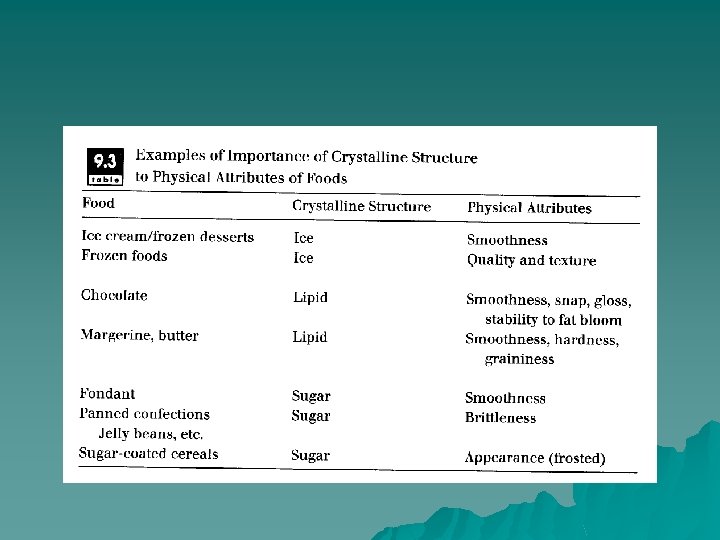
Crystallization u The crystalline phase has considerable importance in food processing. Controlling crystallization is critical to: (l) efficient processing for production foods and (2) to development of proper final product quality. Many components of food products can form the crystalline state in foods, including water (ice), sugars (sucrose, glucose, fructose, lactose), lipids, salts, starch, and protein.

u some products are crystalline in nature through separation of the crystalline material from the surrounding solution. For example, refined sugars (sucrose, lactose, etc. ) and organic acids (citric acid, etc. ) are processed to provide essentially pure crystalline material as the product. In a similar way, fats are fractionated by crystallization to produce components with different physical and chemical characteristics.

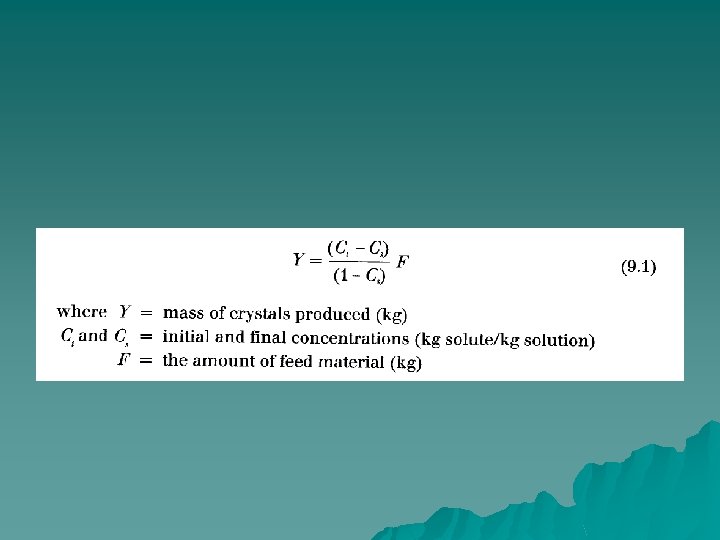
u In production of refined sugar, sugar crystals are produced by crystallization from concentrated solution. The slurry formed in the crystallizer is centrifuged to produce a saturated liquid and the crystalline product. After drying, the crystalline product is generally screened or sieved to classify the sugar crystals according to product size.




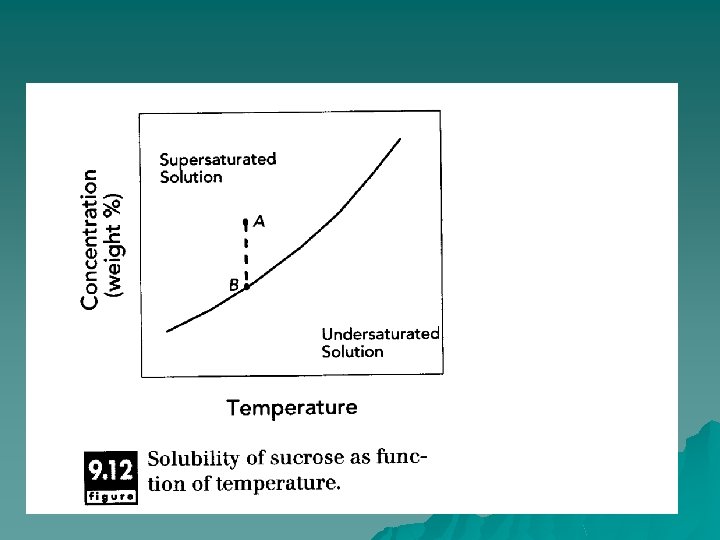
Principles of Operation u In order to form crystals, the liquid phase needs to be cooled below the freezing point or concentrated above the solubility point. To freeze ice in foods, the product must be frozen below the freezing point, which is determined by the concentration of small molecular-weight solutes (salts, sugars). Smaller molecules, like salts and simple sugars, have significant effect on the freezing point of water.

u In sugars and salts crystallization , the concentration of these components must exceed some equilibrium value. The solubility (or saturation) concentration represents the equilibrium between solute molecules in the liquid state and those in the crystalline state. This equilibrium often depends on temperature, and for many systems, the solubility concentration increases with increasing temperature.


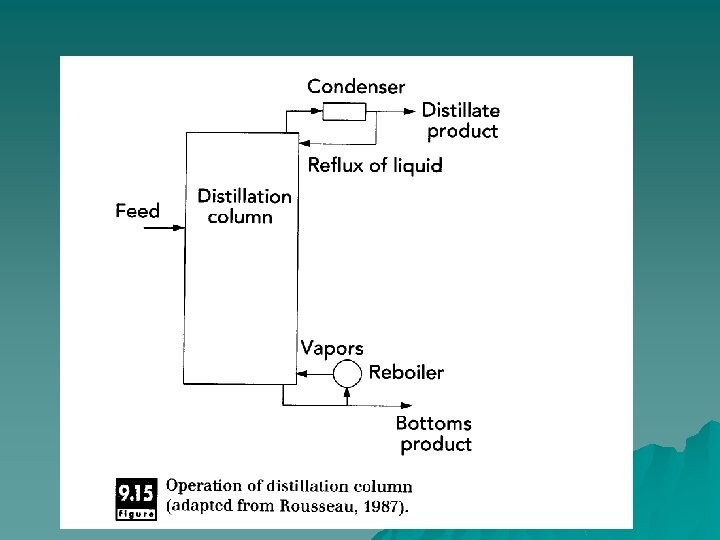
Distillation Separating components with different volatilities. Distillation is used for separation of volatile components like flavors. In processing of orange juice, the vapors coming off an evaporator contain a significant amount of volatile flavors and aromas. These are separated from the condensed vapors in a distillation column. u The products from distillation are a stream rich in the volatile components (distillate) and another stream that has low volatile concentration (residue or bottoms). u




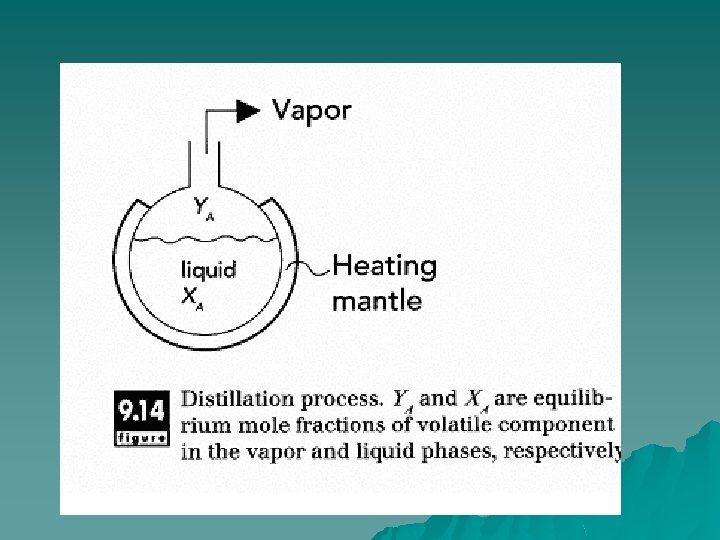
Principles of Operation u When a material containing components with different vaporization pressures is heated, the more-volatile components vaporize more readily and can be separated from the less volatile components. In simple batch distillation, a liquid mixture is heated to boiling, and the vapor formed is separated and condensed to produce a product high in the volatile components (distillate). The liquid remaining (residue) is low in these volatile components.

Industrial distillation operations u The feed enters a distillation column, where it is heated as it flows continuously through the system. The vapors rise to the top of the column and are removed, while the residual liquid falls to the bottom. As the liquid and vapor streams pass each other in the distillation column, equilibrium concentrations in both vapor and liquid phases are approached. These equilibrium concentrations are based on the relative volatilities of the components. Flow of vapors goes up and flow of liquid goes down in the column.

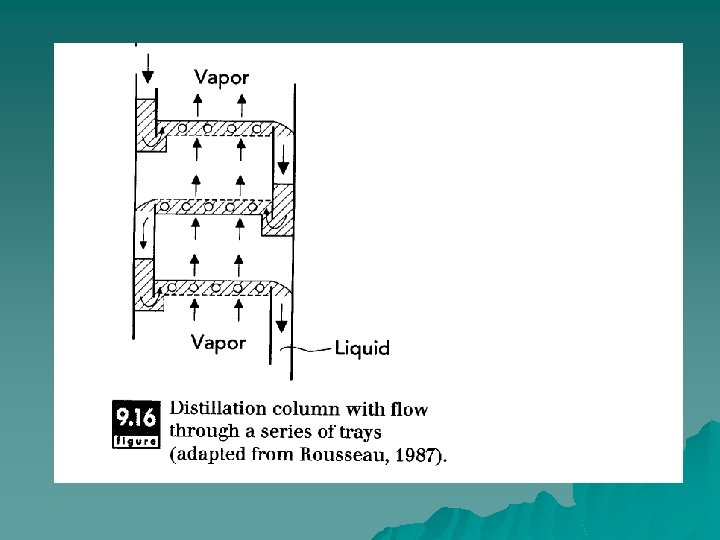
Packing & tray columns u Distillation columns are either filled with packing material to enhance contact between vapor and liquid phases or have distinct trays that allow intimate contact between phases. In tray columns, flow of liquid is down at one side of the tray, whereas flow of vapor is upwards through the tray Trays are designed to allow optimal flow of both vapor and liquid phases but still allow sufficient mixing of the two phases, so that equilibration of volatile components occurs between phases.

Tray columns u In tray columns, flow of liquid is down at one side of the tray, whereas flow of vapor is upwards through the tray. Trays are designed to allow optimal flow of both vapor and liquid phases but still allow sufficient mixing of the two phases, so that equilibration of volatile components occurs between phases.

Packing columns u In columns filled with packing material, flow in and around the packing material allows intimate contact between vapor and liquid phases. Packing material is often shaped like a saddle or a ring and made of either ceramics, plastics or metals. Packing materials range in size from 15 to 90 mm, and are typically randomly oriented within the column to allow uniform flow of both phases.

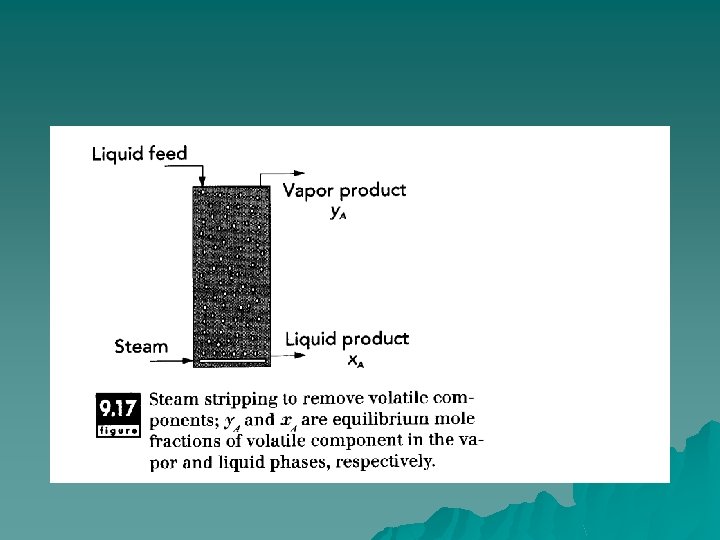
Absorption/Stripping Removal of small amounts of impurities in a vapor phase by selectively absorbing or dissolving a component into a liquid is called absorption. It is commonly used, for example, to remove ammonia or hydrogen sulfide from air. u The reverse process, removal of impurities from a liquid by selective absorption into a gas stream, is called stripping. In the food industry, steam stripping is used for deodorization of vegetable oils. u

u In oil processing, undesired flavors and aromas are caused by the presence of small amounts of free fatty acids and other organic material (aldehydes, ketones). Many applications of vegetable oils in the food and cosmetic industries require that these oils be free from aromas characteristic of their origin. basically, a steam stripping process is used to selectively remove them from the oil

u Steam stripping is based on the differences in concentration between free fatty acids and the main triglycerides of vegetable oils. That is, free fatty acids have some finite solubility in steam, whereas triglycerides are essentially insoluble. Thus, when steam contacts the vegetable oil, free fatty acids (and other volatiles) are selectively removed, leaving essentially pure triglycerides in the liquid oil.

Principles of Operation u The driving force for either absorption or stripping is the difference in concentration of the impurity compound in the liquid and gas phases. In absorption, the impurity migrates to the liquid phase until equilibrium is attained, whereas in stripping, the impurity migrates to the vapor phase to reach equilibrium. In stripping, differences in vapor pressure between components provide the driving force for selective removal of volatile components (fatty acids, etc. ). In deodorization of vegetable oils, steam stripping is accomplished under vacuum conditions and elevated temperatures.

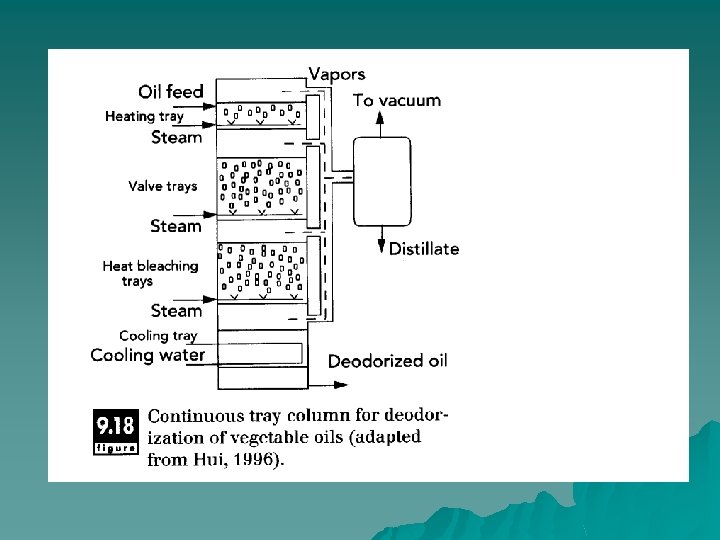
Batch Deodorization Process u The oil is filled into the stripping column and steam introduced at the bottom of the vessel. As the bubbles of steam rise in the column, fatty acids are removed from the oil and absorbed into the steam. At the top of the column is a separator, followed by a condenser that cools the steam to condense the fatty acids, and an exhaust to the vacuum system. Distillate, which contains the free fatty acid volatiles, is removed from the vapor condensate, and the deodorized oil is removed from the bottom of the chamber.

Continuous Deodorization Process u Steam rises upwards through a column as the liquid oil migrates to the bottom. To provide adequate contact area between the vapor and liquid in either absorption or stripping: (1) the vapor can be broken into small bubbles within a continuous liquid (tray column); (2) the liquid stream can be broken into multiple thin films that flow across the continuous vapor phase packed columns); or (3) the liquid can be dispersed into small droplets within the vapor phase (spray contactors).



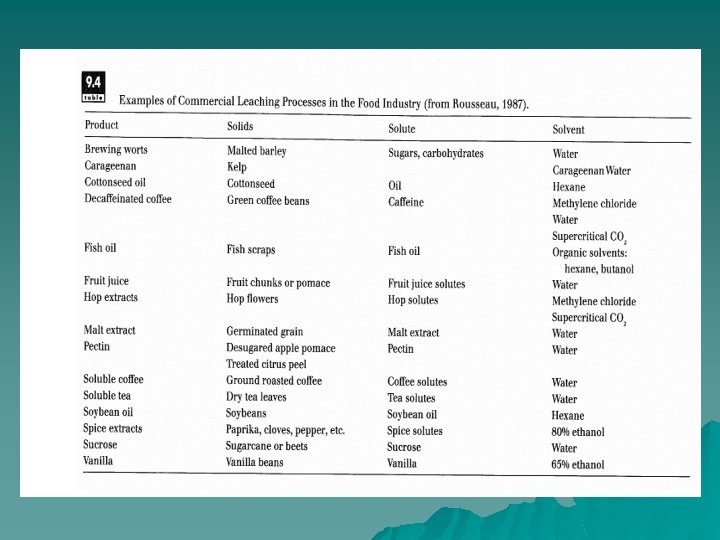
Extraction / Leaching u u One component contained in a solid raw material is extracted by dissolving it in a liquid solvent. Brewing of coffee or tea from ground coffee beans or tea leaves is a form of leaching. Here, the soluble components (organic acids, volatile flavors and aromas, etc. ) contained in coffee grounds or tea leaves are extracted by passing hot water over the solid material. The components in the grounds or leaves that are soluble in hot water diffuse into the hot water, based on the difference in concentration.

u the water-soluble compounds in ground sugarcane or beet are extracted by soaking them in hot water prior to sugar refining. The dilute aqueous solution after leaching contains the soluble sugars, as well as all other water-soluble components. To refine the sugar from this dilute juice, the nonsugar components must be removed and the juice concentrated.


extraction of vegetable oils by organic solvents u Organic solvents such as hexane, acetone, or alcohol come into contact with ground or flaked seeds to extract the oils contained within. Pretreatment of the raw material (flaking or grinding) is necessary in this case in order to break the cell walls and free the oil entrapped within. Sometimes the residual oil in a press cake after mechanical expression is removed by solvent extraction. Residual solvents in both the extracted phase and the remaining solid phase must be removed by some evaporation technique.

Supercritical Fluid Extraction u By raising the pressure and temperature of CO 2 gas above its critical point (T = 31. 2"C; P = 7. 38 Mpa), it becomes a supercritical fluid. Under these conditions, CO 2 has densities near that of a liquid but still maintains gas like qualities of penetration. This makes it an excellent solvent for certain types of compounds. In addition, removal of CO 2 after extraction is easily accomplished by simply lowering pressure and causing the CO 2 to convert to the gaseous phase. supercritical fluid extraction using CO 2 has found commercial application in decaffeination of coffee beans.


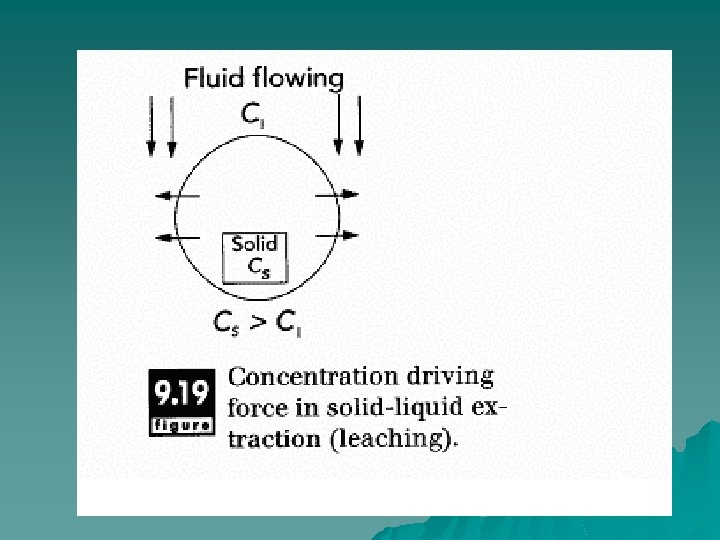
Principles of Operation u The basic principle underlying solid-liquid extraction is that there is a concentration difference between the solid and liquid that causes molecules to diffuse from one to the other. Actually, diffusion of solute within the solids may not be the only mechanism involved in leaching. Washing of solutes off the solid surface, displacement of extract from inter particle pores, and solubilization (or reationinduced creation of soluble solutes from insoluble precursors) may also occur during solid--liquid extraction.

u In the case of sugar refining, the concentration of sucrose in the sugarcane is fairly small (only a few percent). But since the initial concentration of sugar in the hot water is zero, there is a concentration driving force for the sugar to diffuse into the hot water. This mass transfer continues until the concentrations are identical in the solid and liquid phases.


pretreat u In order to ensure efficient and rapid removal of desired compounds in food raw materials, it is often necessary to pretreat the material in some way. For example, nuts and seeds are ground or flaked to ensure rupture of cell walls and allow efficient mass transfer of desired compounds into the extraction liquid. Extraction of coffee from ground beans and tea from tea leaves is best accomplished after drying and grinding the raw materials. In addition to rupturing cell walls, drying and grinding provide a large surface area for extraction to take place.

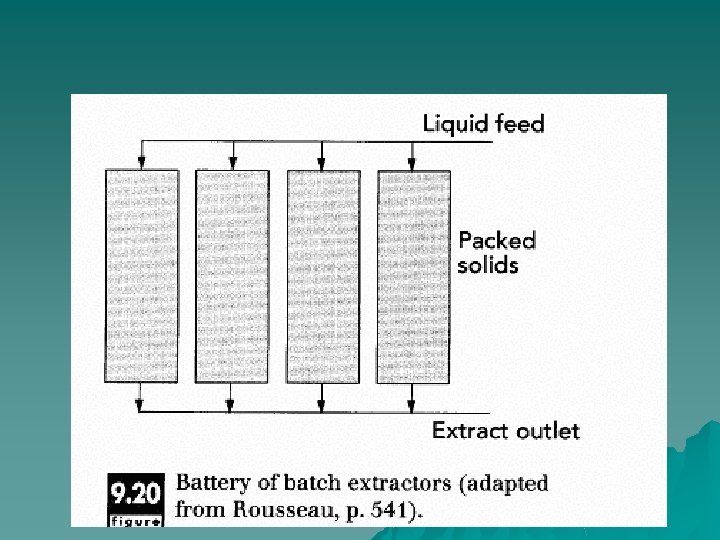
Batch processes u Solvent is allowed to come into contact with the prepared solids, and batch time is determined by rates of diffusion of the soluble components out of the solids. Once a batch of solids has been extracted, the vessel is emptied, cleaned, and refilled with a new charge of solids. A battery of batch extractors is shown in Figure 9. 20.

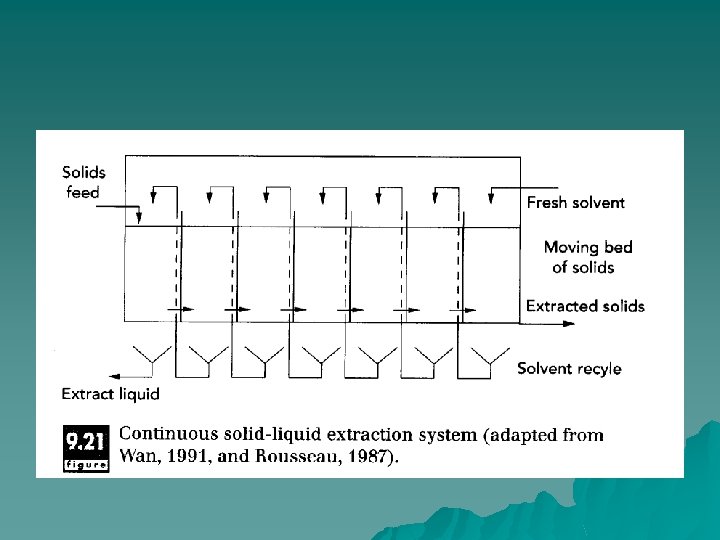
Continuous leaching processes u percolation extractors. u screw-conveyor extractors.

percolation extractors u In this case, the flakes are filled into discrete hoppers, which move along the extractor. Solvent is fed to the top of each bed and collected as it drains through at the bottom. Solvent is fed countercurrent to the movement of the bed of flakes. A moving conveyor also can be used to transport solids along the length of the extractor, with solvent fed countercurrent in a similar fashion.



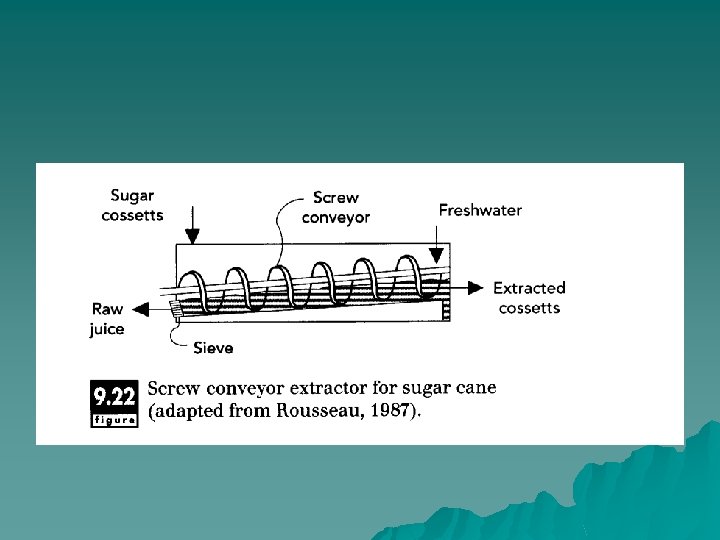
screw-conveyor extractors u In the sugar-refining industry, screw conveyors are often used to transport the sliced sugarcane or beet for contact with hot water. In these systems, the screw conveyor provides a moving bed of cossettes for countercurrent extraction, as shown in Figure 9. 22. Having the cossette move up a slope allows the hot water to drain at the bottom.




