Chemical Separations What is a chemical separation Examples

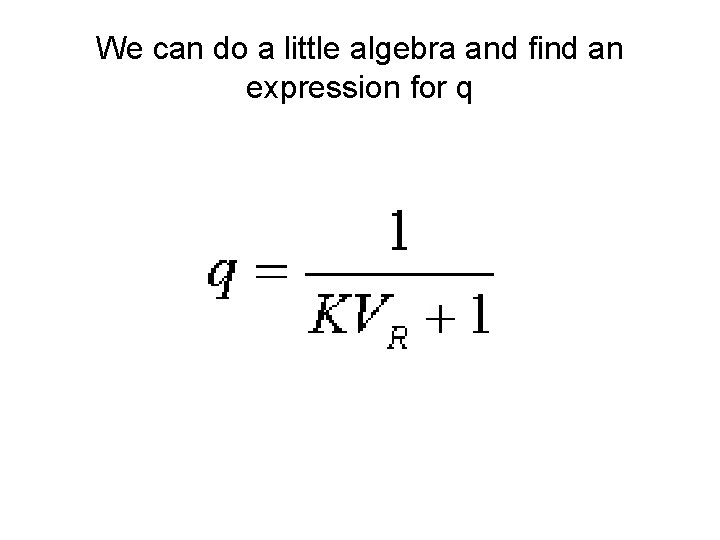
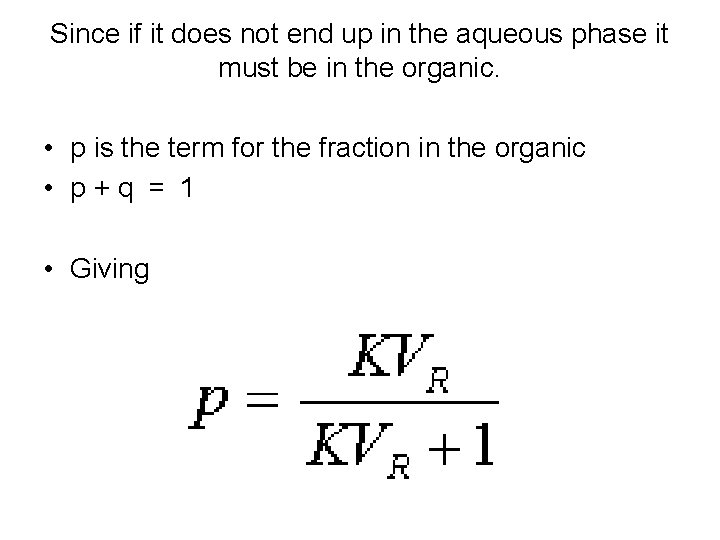
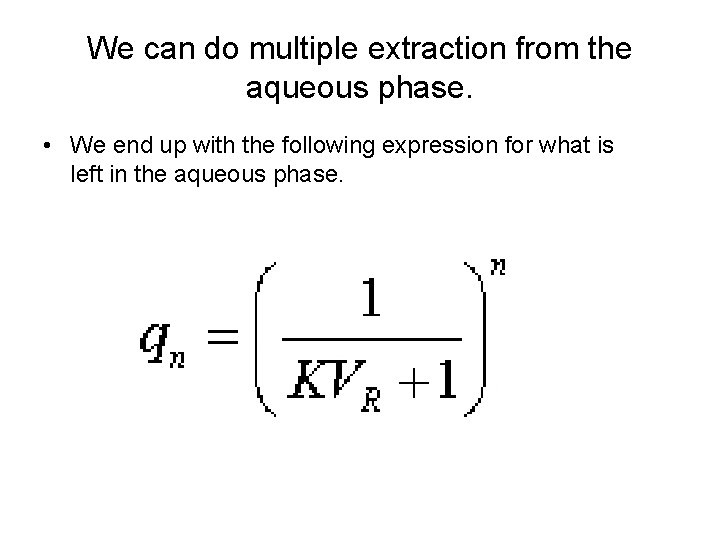

















- Slides: 57

Chemical Separations • What is a chemical separation? • Examples: • • • Filtration Precipitations Crystallizations Distillation HPLC GC Solvent Extraction Zone Melting Electrophoresis Mass Spectroscopy

Chemical Separations • What is the object of the separation. • Collection of a pure product • Isolation for subsequent analysis for either quantification or identification – Analysis » How Much? » What is it?

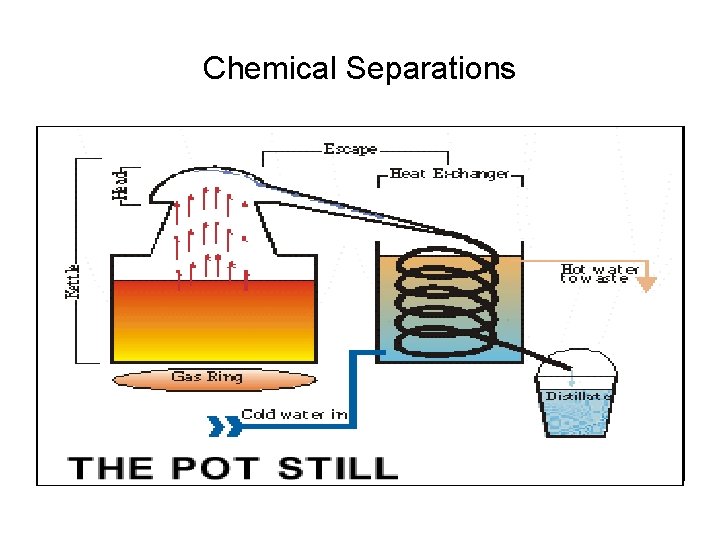
Chemical Separations • Major Industries – Petroleum Distillation – Distilled Spirits


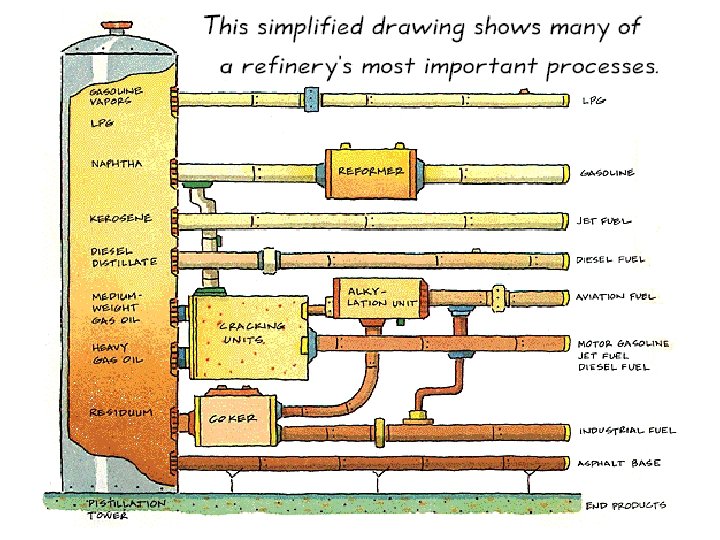
Chemical Separations • Petroleum is a mixture of hydrocarbons. – The larger the molecular weight the less volatile. – So we must separate into various molecular weight fractions (different boiling points) – The results are still complex mixtures

Chemical Separations •

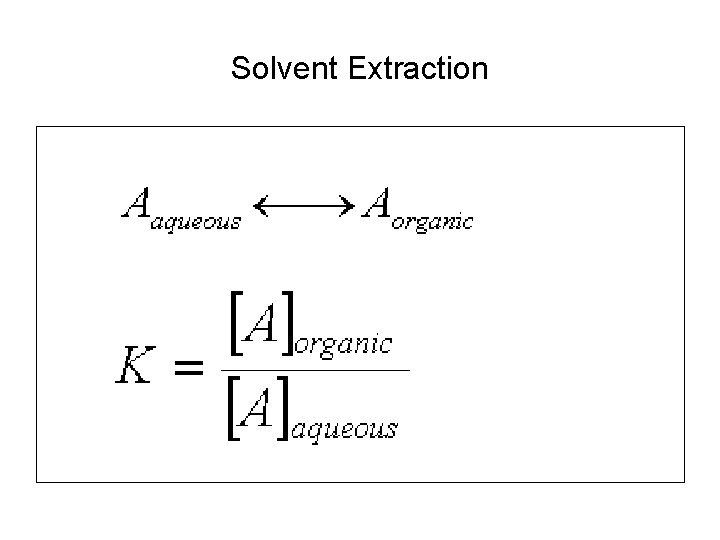
Solvent Extraction • http: //oceanexplorer. noaa. gov/explorations/02 sab/background/products. html

Solvent Extraction

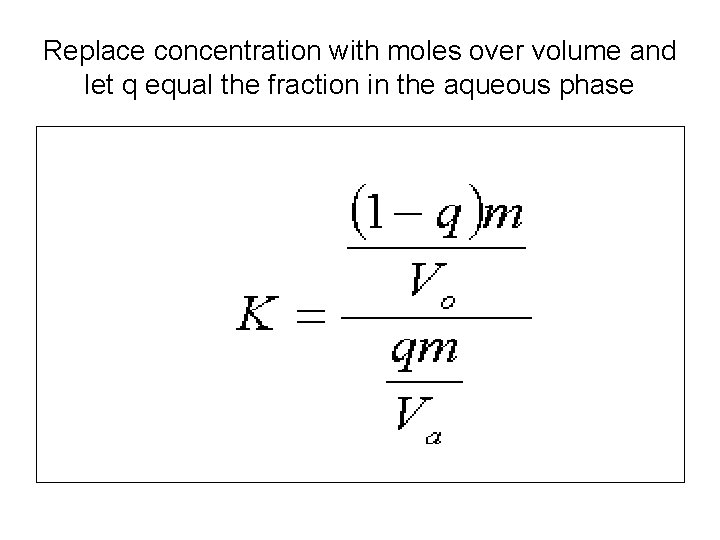
Replace concentration with moles over volume and let q equal the fraction in the aqueous phase

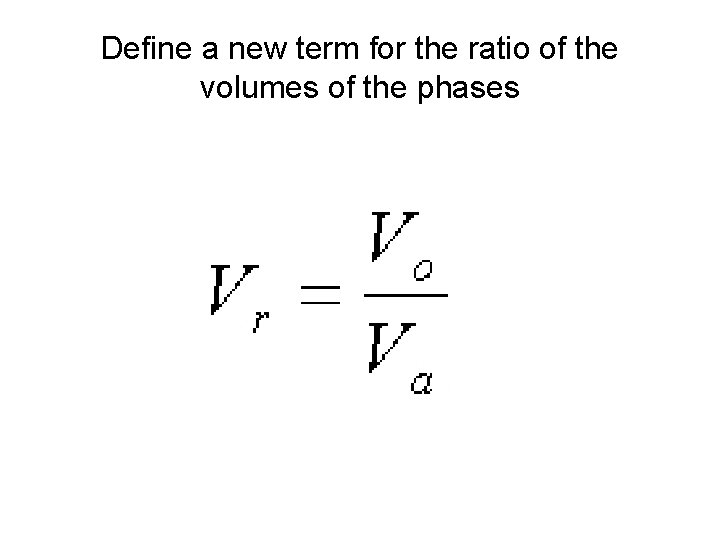
Define a new term for the ratio of the volumes of the phases
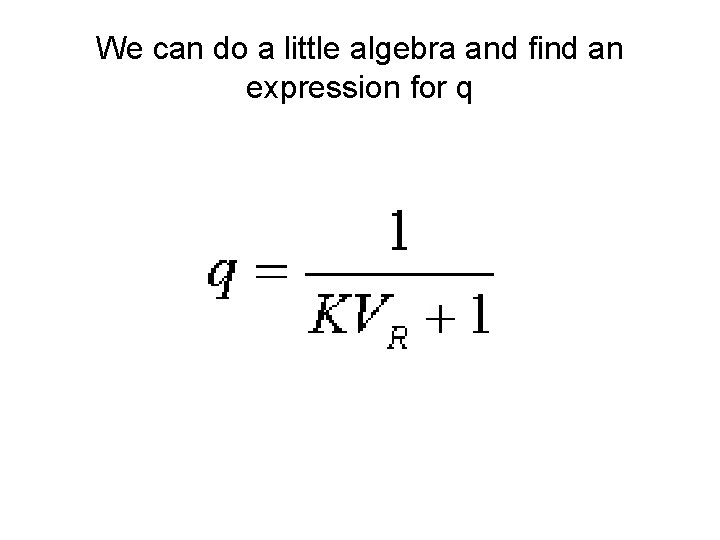
We can do a little algebra and find an expression for q
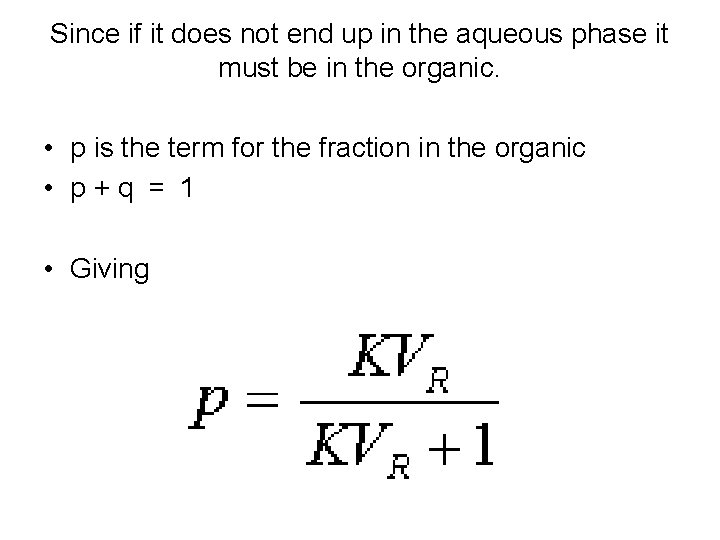
Since if it does not end up in the aqueous phase it must be in the organic. • p is the term for the fraction in the organic • p + q = 1 • Giving

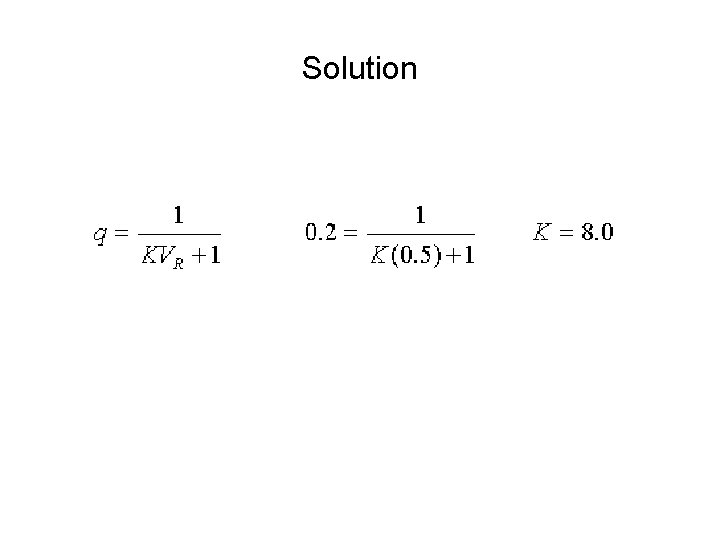
Sample Problem • You have 100. 0 m. L of an aqueous solution that is 100. 0 m. M in compound C. This solution is extracted with 50. 0 m. L of diethyl ether and the aqueous phase is assayed and it is found that the concentration of compound C that remains is 20. 0 m. M. What is the equilibrium constant for this extraction system.

Solution
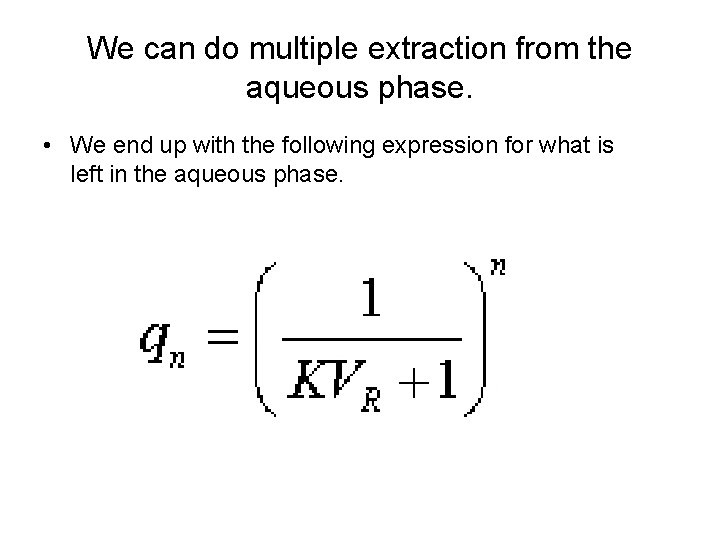
We can do multiple extraction from the aqueous phase. • We end up with the following expression for what is left in the aqueous phase.

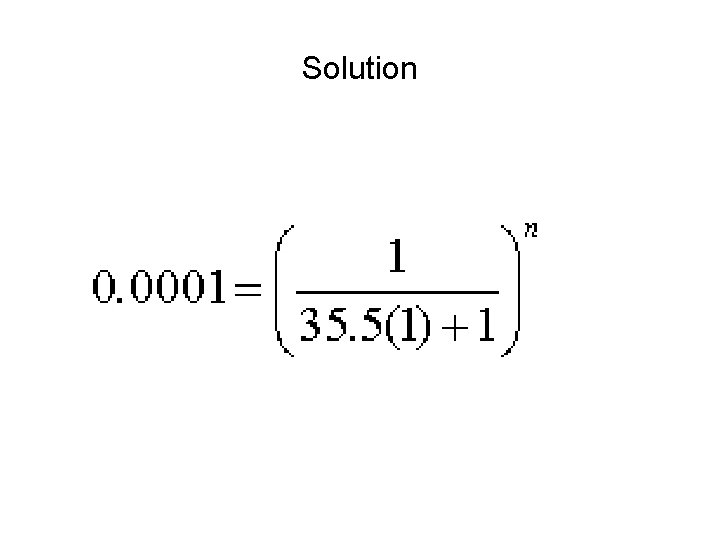
Example • How many extractions would be required to remove 99. 99% of aspirin from an aqueous solution with an equal volume of n-octanol? • Since 99. 99% must be removed the decimal fraction equivalent of this is 0. 9999. This leaves 0. 0001 in the aqueous phase. Since we have equal volumes then Vr is 1. 00. • We are able to find from the Interactive Analysis Web site that K for Aspirin is 35. 5. We plug these values into the q equation and the power is the unknown.

Solution

What if our compound can dissociate or participate in some other equilibrium? • A compound such as aspirin is a carboxylic acid. We can represent this as HA. • Do we expect the ion A- to be very soluble in the organic phase? ? ?

Dissociation • So if we have dissociation then less will go into the organic phase. • Kp is the ratio of concentration of aspirin (in the un-dissociated form) in each phase. This ratio will always be the same. • How do we account for the ion formation?

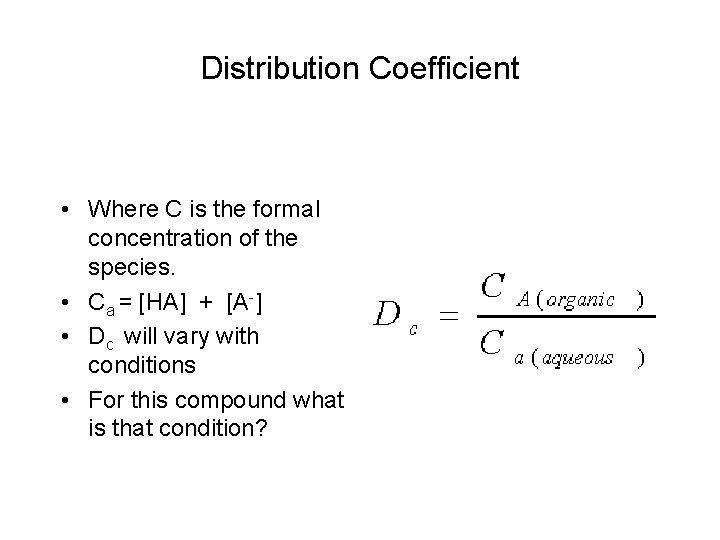
Distribution Coefficient • Where C is the formal concentration of the species. • Ca = [HA] + [A-] • Dc will vary with conditions • For this compound what is that condition?

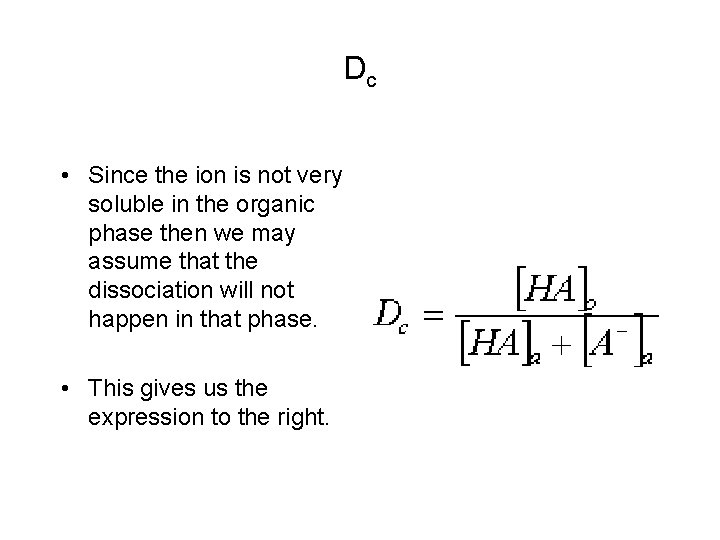
Dc • Since the ion is not very soluble in the organic phase then we may assume that the dissociation will not happen in that phase. • This gives us the expression to the right.

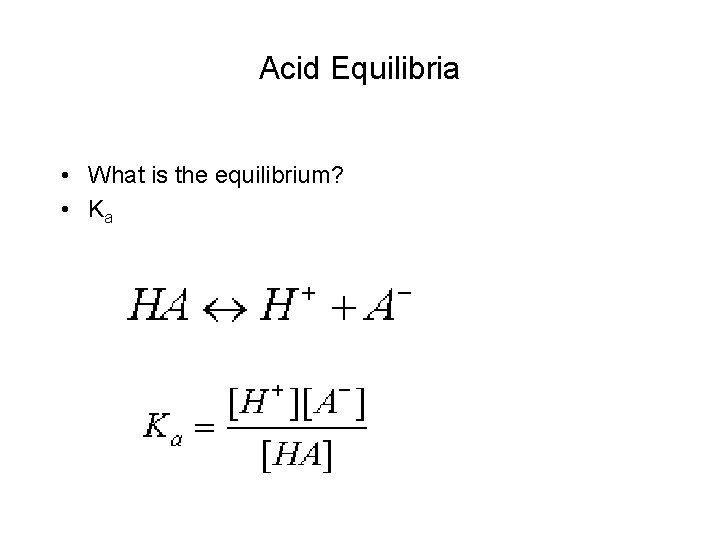
Acid Equilibria • What is the equilibrium? • Ka

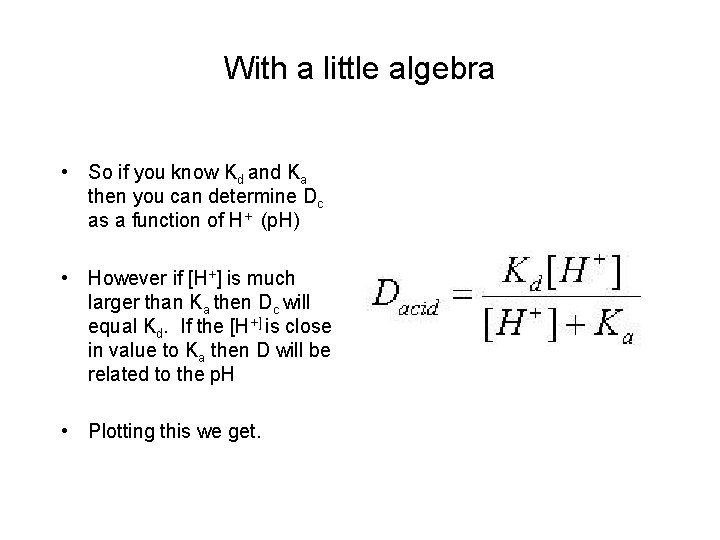
With a little algebra • So if you know Kd and Ka then you can determine Dc as a function of H+ (p. H) • However if [H+] is much larger than Ka then Dc will equal Kd. If the [H+] is close in value to Ka then D will be related to the p. H • Plotting this we get.


So What, Why is this useful. • Well we can now move a solute (analyte) from one phase to another. This can be very useful when extracting a compound that has significant chemical differences from other compounds in solution. As a matter of fact this has been used as an interview question for prospective co-ops when I worked in industry. • The question would go like this. You have carried out a series of reactions and it is now time to work up the product which currently sits in an organic solution (methylene chloride). Your expected product is a primary amine. Which of the following solutions would you extract this methylene chloride solution with to isolate your amine. Your choices are: A) Toluene. B) 0. 1 N Na. OH (aq) C) 0. 1 N HCl (aq) D) I never wanted to work here anyhow.

Separation • So far we can tell how one compound moves from one phase to another. What if we are try to separate two compounds, A and B • Well we might just suspect that if we find a solvent system that has different values of Dc for each compound we could end up with most of one compound in one phase and the other compound in the opposite phase. It is not that simple.

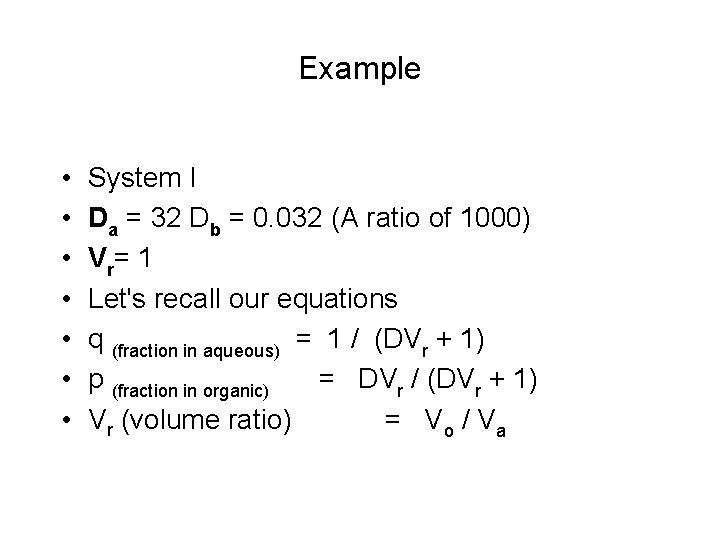
Example • • System I Da = 32 Db = 0. 032 (A ratio of 1000) Vr= 1 Let's recall our equations q (fraction in aqueous) = 1 / (DVr + 1) p (fraction in organic) = DVr / (DVr + 1) Vr (volume ratio) = Vo / Va

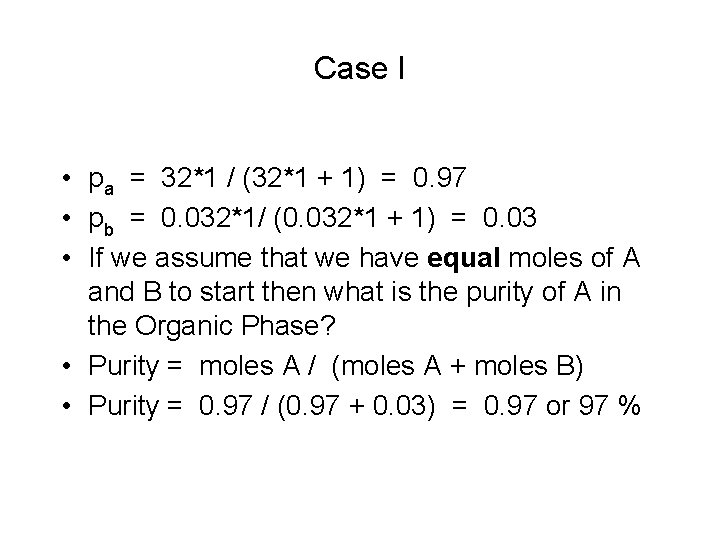
Case I • pa = 32*1 / (32*1 + 1) = 0. 97 • pb = 0. 032*1/ (0. 032*1 + 1) = 0. 03 • If we assume that we have equal moles of A and B to start then what is the purity of A in the Organic Phase? • Purity = moles A / (moles A + moles B) • Purity = 0. 97 / (0. 97 + 0. 03) = 0. 97 or 97 %

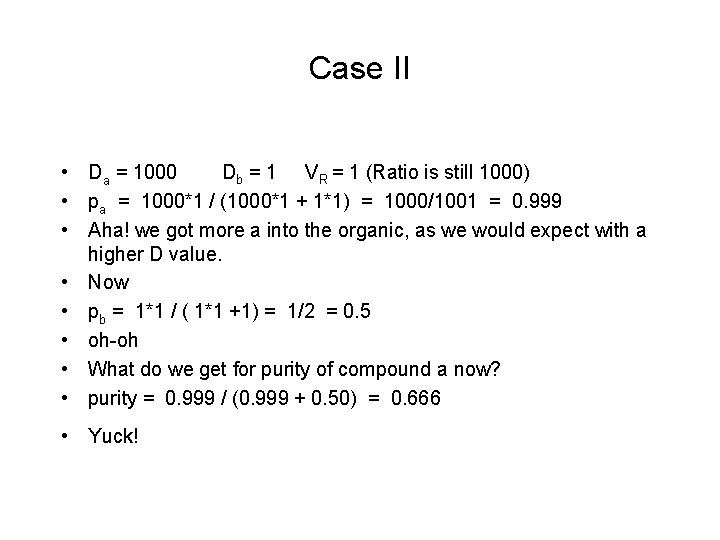
Case II • Da = 1000 Db = 1 VR = 1 (Ratio is still 1000) • pa = 1000*1 / (1000*1 + 1*1) = 1000/1001 = 0. 999 • Aha! we got more a into the organic, as we would expect with a higher D value. • Now • pb = 1*1 / ( 1*1 +1) = 1/2 = 0. 5 • oh-oh • What do we get for purity of compound a now? • purity = 0. 999 / (0. 999 + 0. 50) = 0. 666 • Yuck!

How can we get around this issue? • Once we have selected the solvent and p. H, then there is little that we can do to change D. What else do we have in our control? ? ? • Let's look • p = DVr / (DVr + 1) • Not much here except Vr and in fact that is the key to this problem. Is there an optimum Vr value for the values of D that we have? Yes! • Our equation for this is V r(opt) = (Da*Db)-0. 5

Revisit the two cases • So let us look at our two cases and see which will give us the optimum values. • Case I • Da = 32 and Db = 0. 032 • V r(opt) = (32 * 0. 032)-0. 5 = ( 1 )-0. 5 = 1 • So we were already at the optimum.

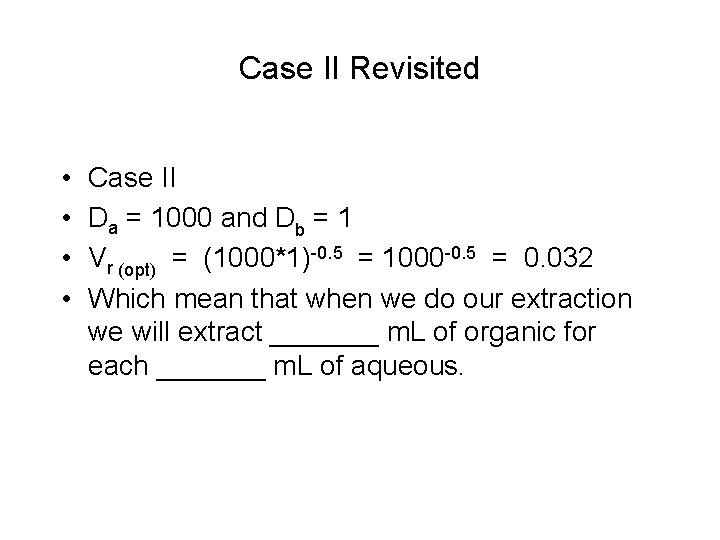
Case II Revisited • • Case II Da = 1000 and Db = 1 Vr (opt) = (1000*1)-0. 5 = 1000 -0. 5 = 0. 032 Which mean that when we do our extraction we will extract _______ m. L of organic for each _______ m. L of aqueous.

Purity for Case II • What is our purity for this system? • • • pa = 1000*0. 032 / (1000*0. 032 + 1) = 32/33 = 0. 97 and pb = 1*0. 032 / (1*0. 032 + 1) = 0. 032/1. 032 = 0. 03 • Purity of a then is 0. 97/ (0. 97 + 0. 03) • Which will give us the 97% purity we had for Case I with the Vr of 1.

Can we improve this purity? • If we were to extract again then we would just remove the same proportions. We would get more compound extracted but it would be the same purity. • What if we were to take the organic phase and extract it with fresh aqueous phase. We know that one of the two compounds will end up mostly in that aqueous phase so we should enhance the purity of the other compound in the organic phase.

Back Extraction • Called that since you are extracting back into the original phase.

Back Extraction Case I Example • Let's look at the numbers. • Da = 32 Db = 0. 032 Vr = 1 • pa = 0. 97 pb = 0. 03 • qa = 0. 03 qb = 0. 97 • Let’s prepare a table.

Initial conditions prior to starting back extraction . Before Shaking Amount A Amount B Organic Phase 0. 97 0. 03 Fresh Aqueous Phase 0 0

Now we extract – shake • How much goes to the Aqueous phase • q which is 0. 03 for A and 0. 97 for B • How much goes to the Organic phase • p which is 0. 97 for A and 0. 03 for B After Shaking Amount A Amount B Organic Phase (0. 97) (0. 03) Aqueous Phase (0. 97)(0. 03)(0. 97)

Now what is the purity for A in the organic phase? ? ? • Purity = Amount A / (Amount A + Amount B) = • 0. 97*0. 97 / (0. 97*0. 97 + 0. 03*0. 03) = • 0. 94/(0. 94 + 0. 0009) = 99. 9% • What is the yield of A (fraction of the total amount that we started with)

Let’s do it again – Can we improve purity even more? After second Back Extraction Amount A Amount B Organic Phase 0. 94*0. 97 0. 0009*0. 03 Aqueous Phase 0. 94*0. 03 0. 0009*0. 97 Purity A = 0. 913 / (0. 913 + 0. 000027) = 99. 997% But our yield has dropped to 91. 3%, there is a price to pay for the added purity.

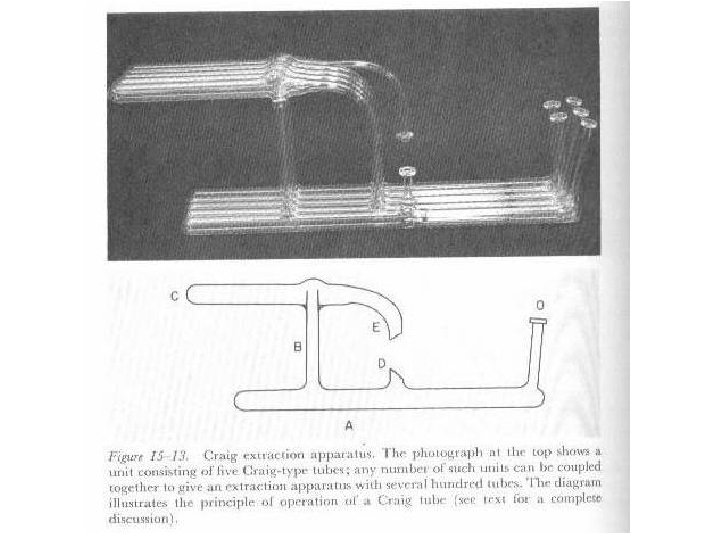
Can We Expand This? Why Would We Want to? • Such multiple extraction systems have been developed. • Still a viable option for preparative work. • For separations it has been replaced by HPLC • Called Craig Counter Current Extraction. • Special glassware is used.


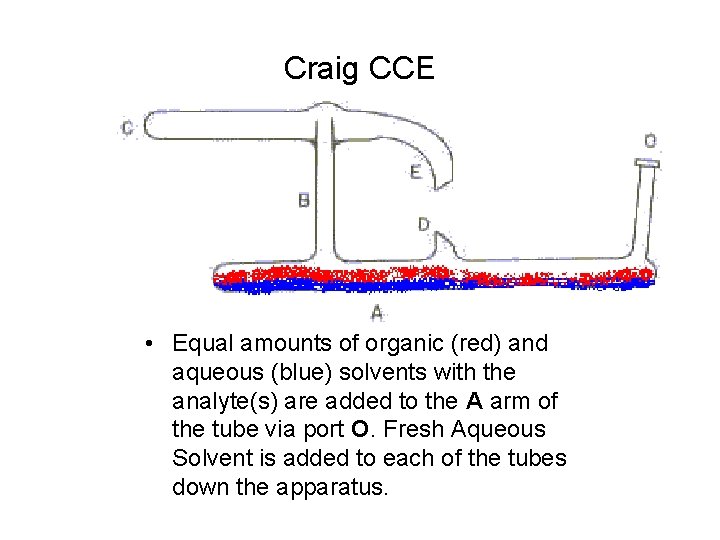
Craig CCE • Equal amounts of organic (red) and aqueous (blue) solvents with the analyte(s) are added to the A arm of the tube via port O. Fresh Aqueous Solvent is added to each of the tubes down the apparatus.

Craig CCE • Rock the system back and forth and to establish equilibrium. • Allow the system to stand for the layers to separate. • Rotate the apparatus counter clockwise about 90 o to 100 o.

Craig CCE • Rotate Back to Horizontal

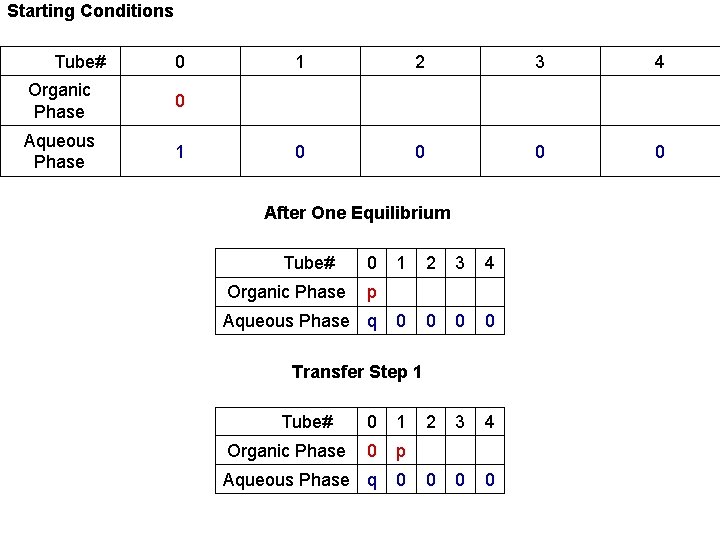
Starting Conditions Tube# 0 Organic Phase 0 Aqueous Phase 1 1 2 3 0 0 Tube# Organic Phase 0 1 2 3 4 p Aqueous Phase q 0 0 0 0 Transfer Step 1 Tube# Organic Phase 0 1 2 3 4 0 Aqueous Phase q 0 After One Equilibrium p 0 0 4 0

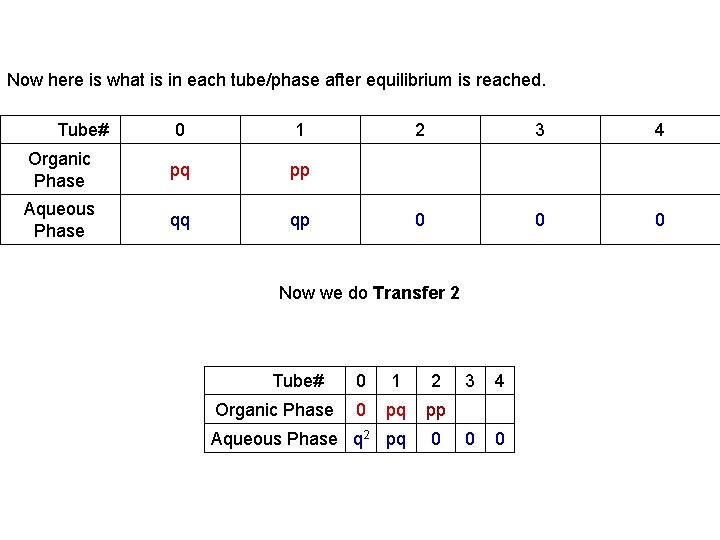
Now here is what is in each tube/phase after equilibrium is reached. Tube# 0 1 Organic Phase pq pp Aqueous Phase qq qp 2 3 0 0 Now we do Transfer 2 Tube# 0 1 Organic Phase 0 pq pp Aqueous Phase q 2 pq 2 0 3 4 0 0

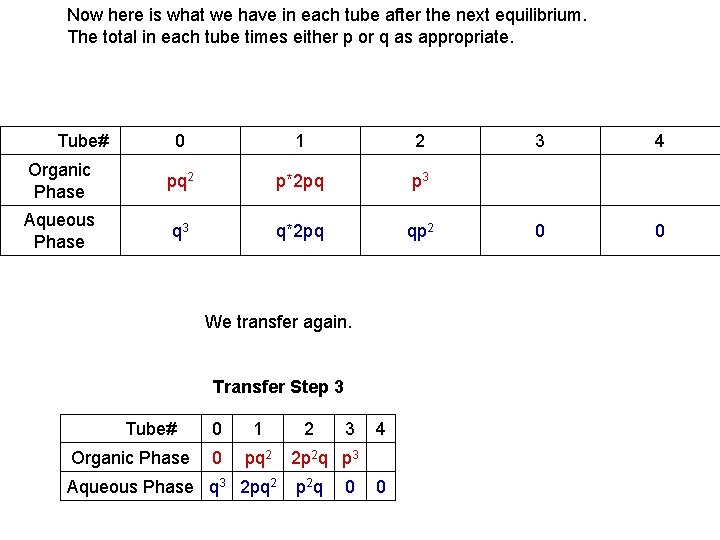
Now here is what we have in each tube after the next equilibrium. The total in each tube times either p or q as appropriate. Tube# 0 1 2 Organic Phase pq 2 p*2 pq p 3 Aqueous Phase q 3 q*2 pq qp 2 We transfer again. Tube# Organic Phase Transfer Step 3 0 1 2 3 4 0 pq 2 Aqueous Phase q 3 2 pq 2 2 p 2 q p 3 p 2 q 0 0 3 4 0 0

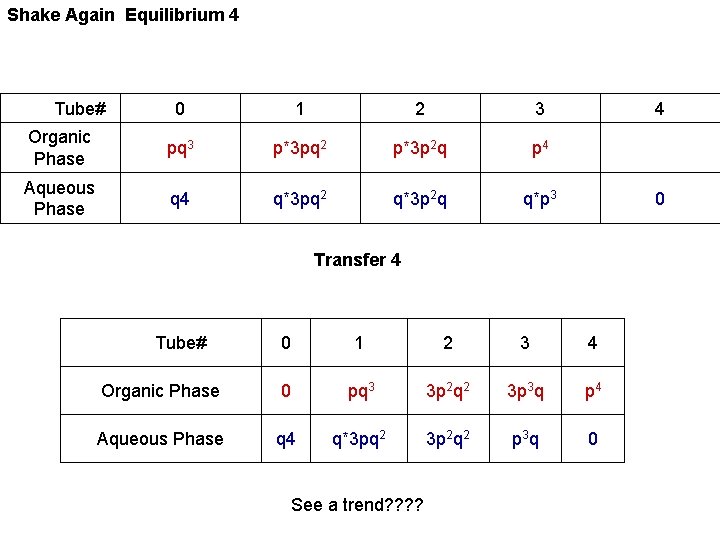
Shake Again Equilibrium 4 Tube# 0 1 2 3 Organic Phase pq 3 p*3 pq 2 p*3 p 2 q p 4 Aqueous Phase q 4 q*3 pq 2 q*3 p 2 q q*p 3 4 0 Transfer 4 Tube# 0 1 2 3 4 Organic Phase 0 pq 3 3 p 2 q 2 3 p 3 q p 4 Aqueous Phase q 4 q*3 pq 2 3 p 2 q 2 p 3 q 0 See a trend? ?

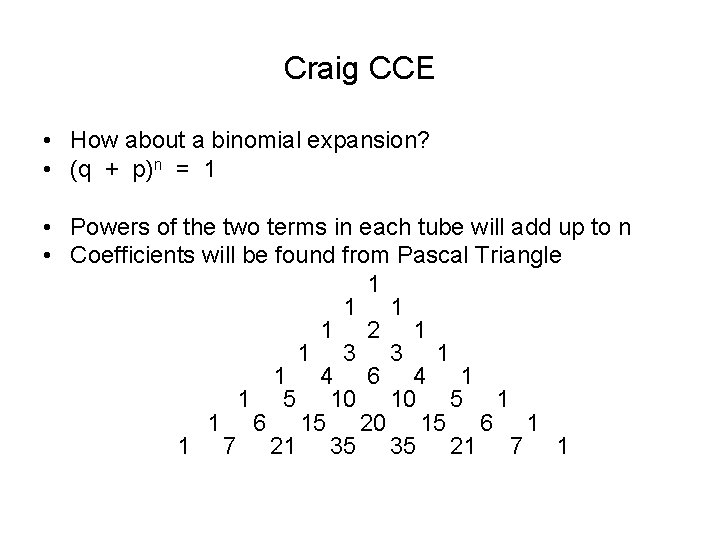
Craig CCE • How about a binomial expansion? • (q + p)n = 1 • Powers of the two terms in each tube will add up to n • Coefficients will be found from Pascal Triangle 1 1 2 1 1 3 3 1 1 4 6 4 1 1 5 10 10 5 1 1 6 15 20 15 6 1 1 7 21 35 35 21 7 1

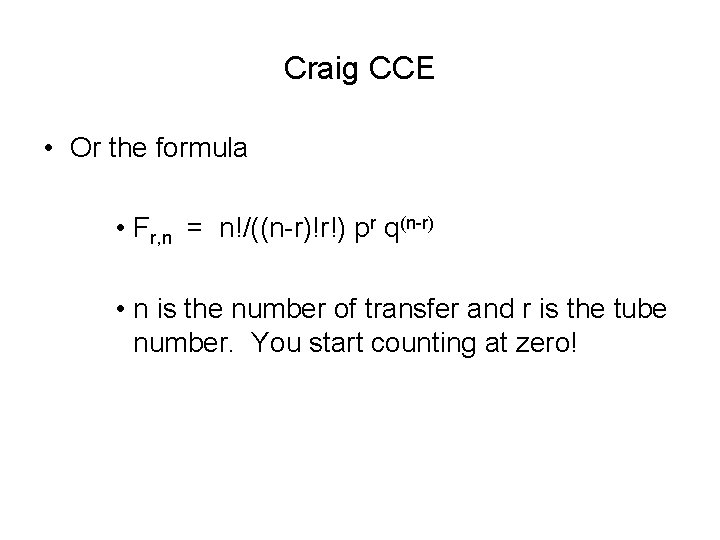
Craig CCE • Or the formula • Fr, n = n!/((n-r)!r!) pr q(n-r) • n is the number of transfer and r is the tube number. You start counting at zero!

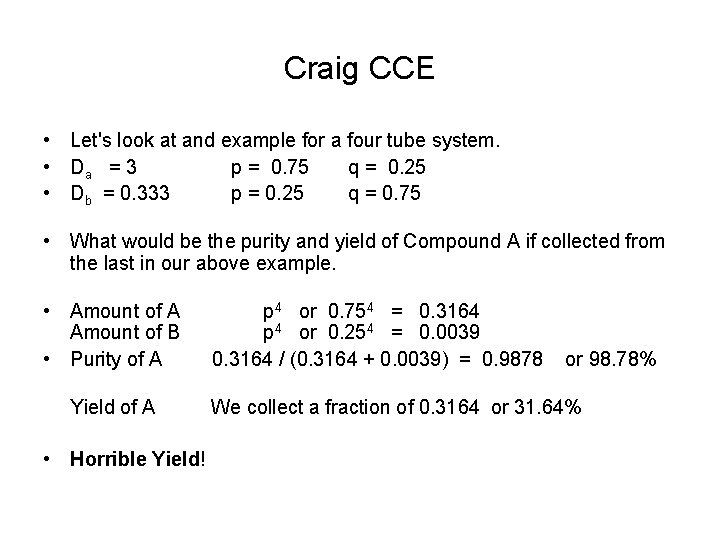
Craig CCE • Let's look at and example for a four tube system. • Da = 3 p = 0. 75 q = 0. 25 • Db = 0. 333 p = 0. 25 q = 0. 75 • What would be the purity and yield of Compound A if collected from the last in our above example. • Amount of A p 4 or 0. 754 = 0. 3164 Amount of B p 4 or 0. 254 = 0. 0039 • Purity of A 0. 3164 / (0. 3164 + 0. 0039) = 0. 9878 or 98. 78% Yield of A We collect a fraction of 0. 3164 or 31. 64% • Horrible Yield!

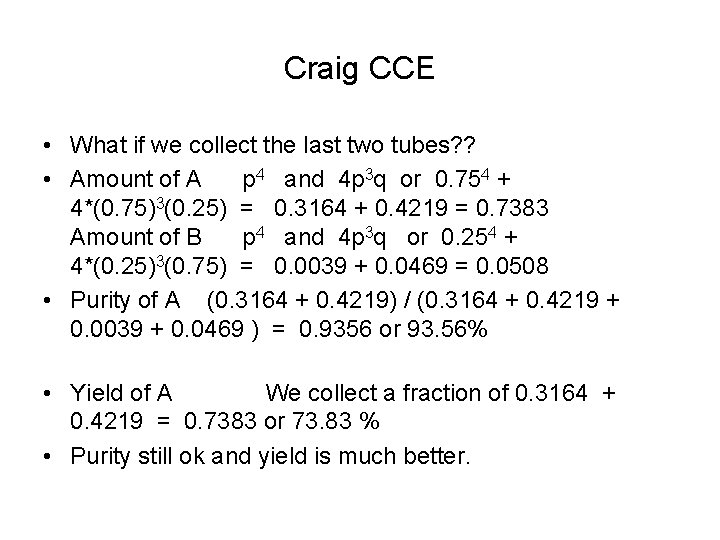
Craig CCE • What if we collect the last two tubes? ? • Amount of A p 4 and 4 p 3 q or 0. 754 + 4*(0. 75)3(0. 25) = 0. 3164 + 0. 4219 = 0. 7383 Amount of B p 4 and 4 p 3 q or 0. 254 + 4*(0. 25)3(0. 75) = 0. 0039 + 0. 0469 = 0. 0508 • Purity of A (0. 3164 + 0. 4219) / (0. 3164 + 0. 4219 + 0. 0039 + 0. 0469 ) = 0. 9356 or 93. 56% • Yield of A We collect a fraction of 0. 3164 + 0. 4219 = 0. 7383 or 73. 83 % • Purity still ok and yield is much better.

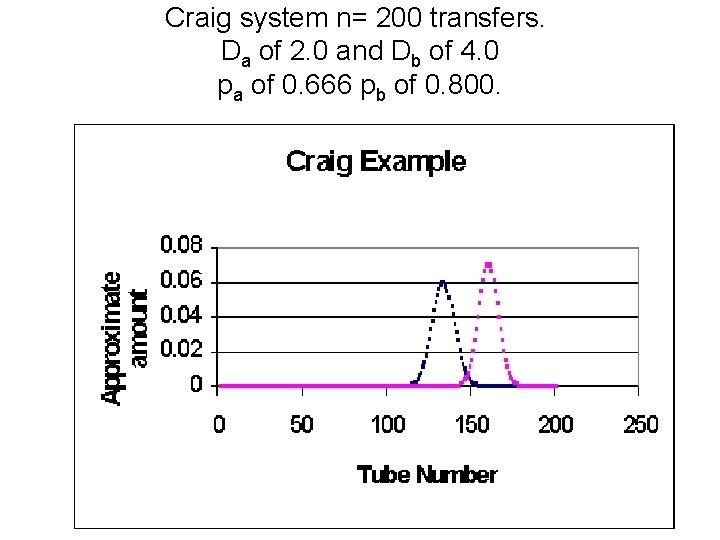
Craig system n= 200 transfers. Da of 2. 0 and Db of 4. 0 pa of 0. 666 pb of 0. 800.

Animation Link • http: //www. chem. uoa. gr/applets/Applet. Craig/Appl_Craig 2. html

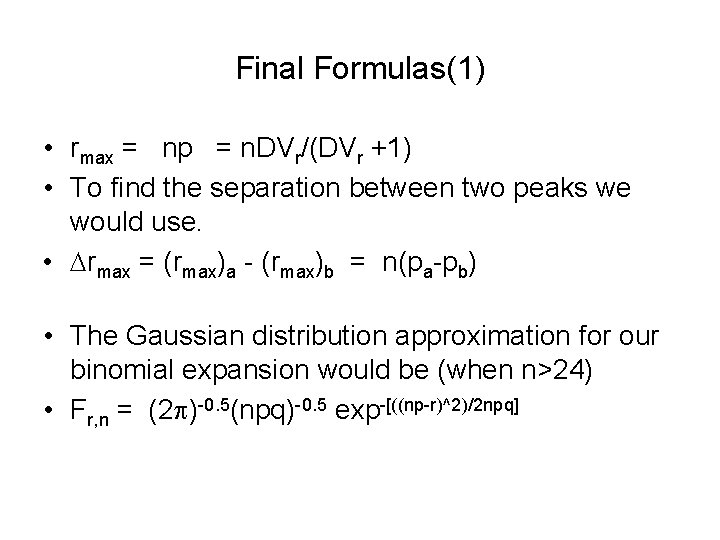
Final Formulas(1) • rmax = np = n. DVr/(DVr +1) • To find the separation between two peaks we would use. • Drmax = (rmax)a - (rmax)b = n(pa-pb) • The Gaussian distribution approximation for our binomial expansion would be (when n>24) • Fr, n = (2 p)-0. 5(npq)-0. 5 exp-[((np-r)^2)/2 npq]

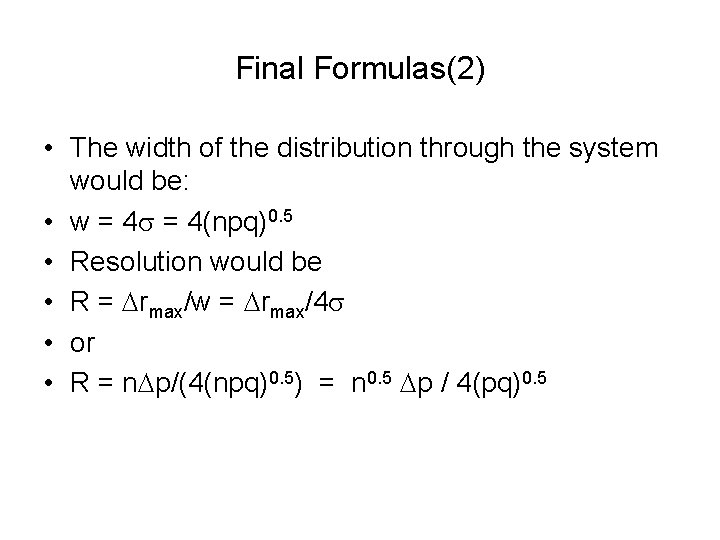
Final Formulas(2) • The width of the distribution through the system would be: • w = 4 s = 4(npq)0. 5 • Resolution would be • R = Drmax/w = Drmax/4 s • or • R = n. Dp/(4(npq)0. 5) = n 0. 5 Dp / 4(pq)0. 5