Separation processes general Mechanical separations e g filtration




















- Slides: 34

Separation processes - general • Mechanical separations e. g. filtration of a solid from a suspension in a liquid, centrifugation, screening etc • Mass transfer operations e. g. distillation, extraction etc Paul Ashall 2007

Mass transfer operations – nature of interface between phases • Gas-liquid contact e. g. absorption, evaporation, distillation etc • Liquid-liquid contact e. g. extraction • Liquid-solid contact e. g. crystallisation, adsorption • Gas-solid contact e. g. adsorption, drying etc Paul Ashall 2007

Mass transfer operations – controlling transport phenomenon • Mass transfer controlling e. g. distillation, absorption, extraction, adsorption etc • Mass transfer and heat transfer controlling e. g. drying, crystallisation • Heat transfer controlling e. g. evaporation Paul Ashall 2007

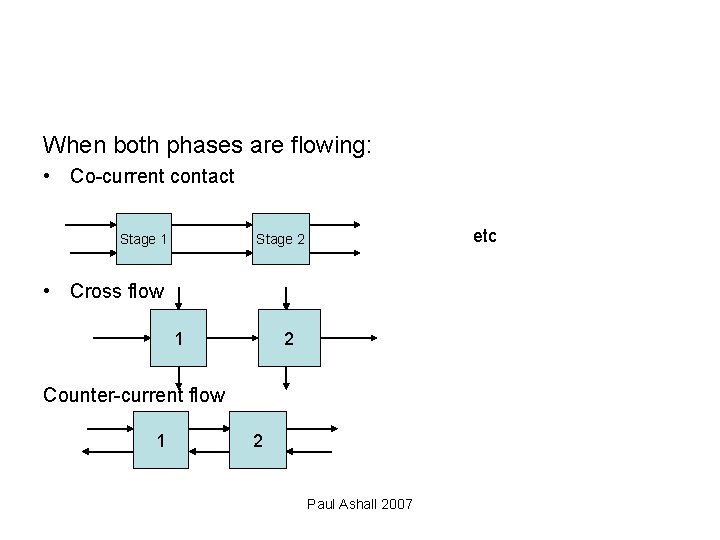
Methods of operation • Non steady state – concentration changes with time e. g. batch processes • Steady state • Stage • Differential contact Paul Ashall 2007

When both phases are flowing: • Co-current contact Stage 1 etc Stage 2 • Cross flow 1 2 Counter-current flow 1 2 Paul Ashall 2007

Choice of separation process Factors to be considered: • Feasibility • Product value • Cost • Product quality • selectivity Paul Ashall 2007

Liquid-liquid extraction principles Feed phase contains a component, i, which is to be removed. Addition of a second phase (solvent phase) which is immiscible with feed phase but component i is soluble in both phases. Some of component i (solute) is transferred from the feed phase to the solvent phase. After extraction the feed and solvent phases are called the raffinate (R) and extract (E) phases respectively. Paul Ashall 2007

continued Normally one of the two phases is an organic phase while the other is an aqueous phase. Under equilibrium conditions the distribution of solute i over the two phases is determined by the distribution law. After the extraction the two phases can be separated because of their immiscibility. Component i is then separated from the extract phase by a technique such as distillation and the solvent is regenerated. Further extractions may be carried out to remove more component i. Liquid liquid extraction can also be used to remove a component from an organic phase by adding an aqueous phase. Paul Ashall 2007

Example - Penicillin G 6 -aminopenicillanic acid (6 -APA) is manufactured by GSK in Irvine. It is used to manufacture amoxicillin and ‘Augmentin’. Fermentation products (penicillin G broth) are filtered (microfiltration) and extracted at low p. H with amyl acetate or methyl isobutyl ketone. The penicillin G is then extracted further at a higher p. H into an aqueous phosphate buffer. Paul Ashall 2007

Extractants The efficiency of a liquid extraction can be enhanced by adding one or more extractants to the solvent phase. The extractant interacts with component i increasing the capacity of the solvent for i. To recover the solute from the extract phase the extractant-solute complex has to be degraded. Paul Ashall 2007

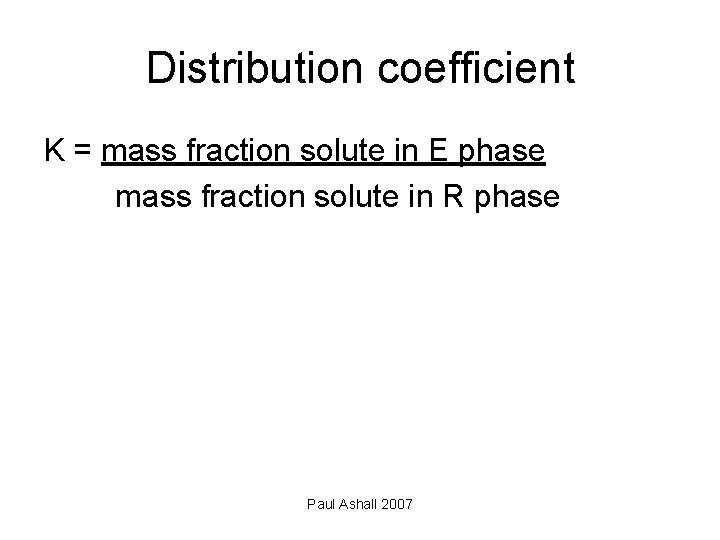
Distribution coefficient K = mass fraction solute in E phase mass fraction solute in R phase Paul Ashall 2007

Immiscible liquids e. g. water – chloroform Consider a feed of water/acetone(solute). K = mass fraction acetone in chloroform phase mass fraction acetone in water phase K = kg acetone/kg chloroform = y/x kg acetone/kg water K = 1. 72 i. e. acetone is preferentially soluble in the chloroform phase Paul Ashall 2007

Partially miscible liquids E. g. water – MIBK Consider a solute acetone. Need to use a triangular phase diagram to show equilibrium compositions of MIBKacetone-water mixtures. Characteristics are single phase and two phase regions, tie lines connecting equilibrium phase compositions in two phase region. Paul Ashall 2007

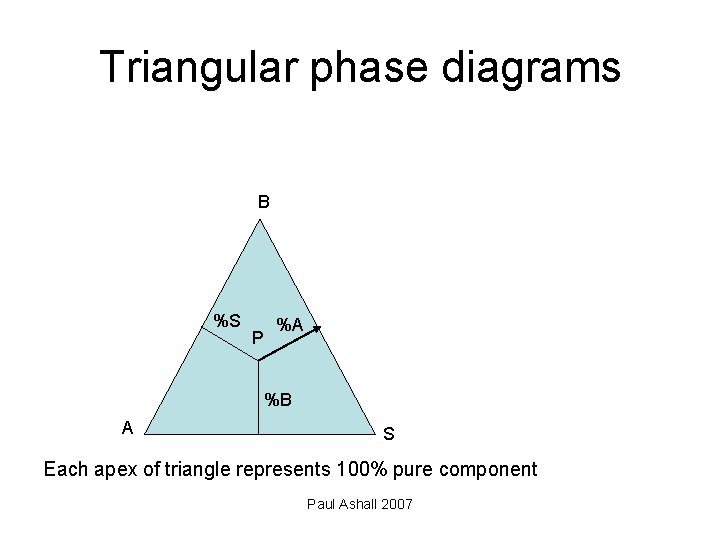
Triangular phase diagrams B %S P %A %B A S Each apex of triangle represents 100% pure component Paul Ashall 2007

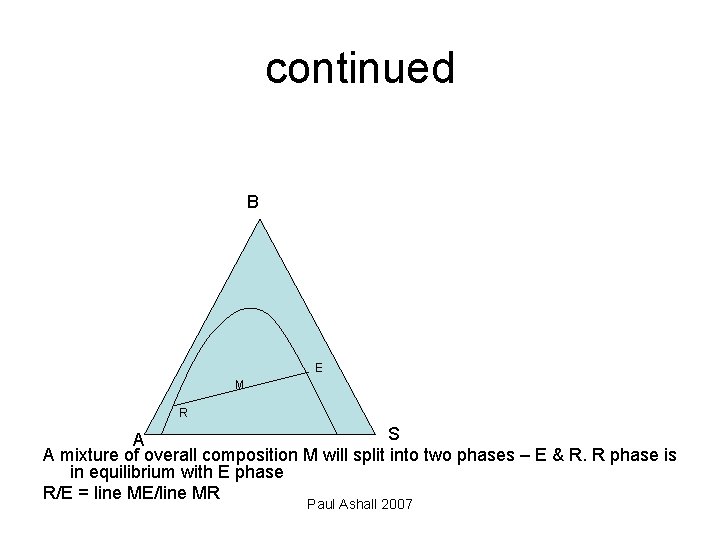
continued B E M R S A A mixture of overall composition M will split into two phases – E & R. R phase is in equilibrium with E phase R/E = line ME/line MR Paul Ashall 2007

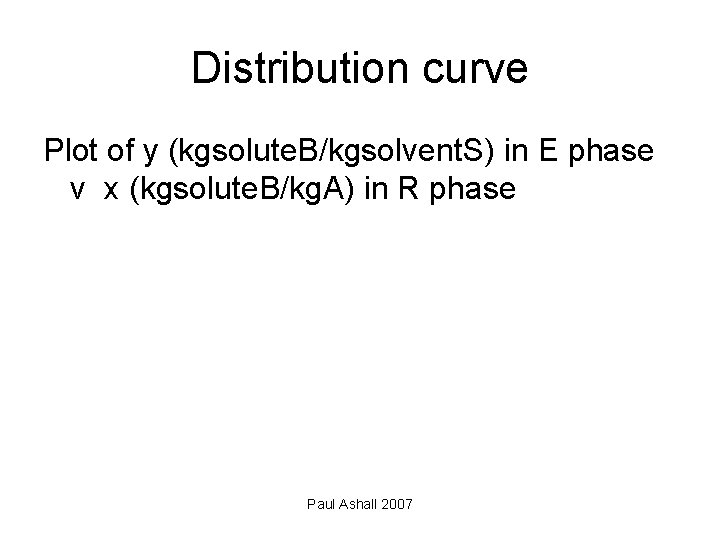
Distribution curve Plot of y (kgsolute. B/kgsolvent. S) in E phase v x (kgsolute. B/kg. A) in R phase Paul Ashall 2007

Example Paul Ashall 2007

Choice of solvent Factors to be considered: • Selectivity • Distribution coefficient • Insolubility of solvent • Recoverability of solute from solvent • Density difference between liquid phases • Interfacial tension • Chemical reactivity • Cost • Viscosity, vapour pressure • Flammability, toxicity Paul Ashall 2007

Selectivity β = (mass fraction B in E)/(mass fraction A in E) (mass fraction B in R)/(mass fraction A in R) β>1 Paul Ashall 2007

Distribution coefficient K = y/x Large values are desirable since less solvent is required for a given degree of extraction Paul Ashall 2007

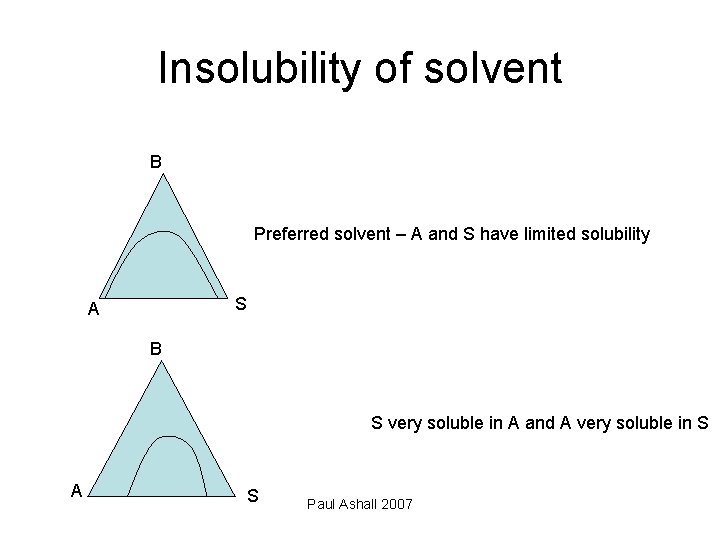
Insolubility of solvent B Preferred solvent – A and S have limited solubility S A B S very soluble in A and A very soluble in S A S Paul Ashall 2007

Recoverability of solvent and solute • No azeotrope formed between solvent and solute • Mixtures should have a high relative volatility • Solvent should have a small latent heat of vapourisation Paul Ashall 2007

Density A density difference is required between the two phases. Paul Ashall 2007

Interfacial tension The larger the interfacial tension between the two phases the more readily coalescence of emulsions will occur to give two distinct liquid phases but the more difficult will be the dispersion of one liquid in the other to give efficient solute extraction. Paul Ashall 2007

Chemical reactivity Solvent should be stable and inert. Paul Ashall 2007

Physical properties For material handling: • Low viscosity • Low vapour pressure • Non-flammable (high flash point) • Non-toxic Paul Ashall 2007

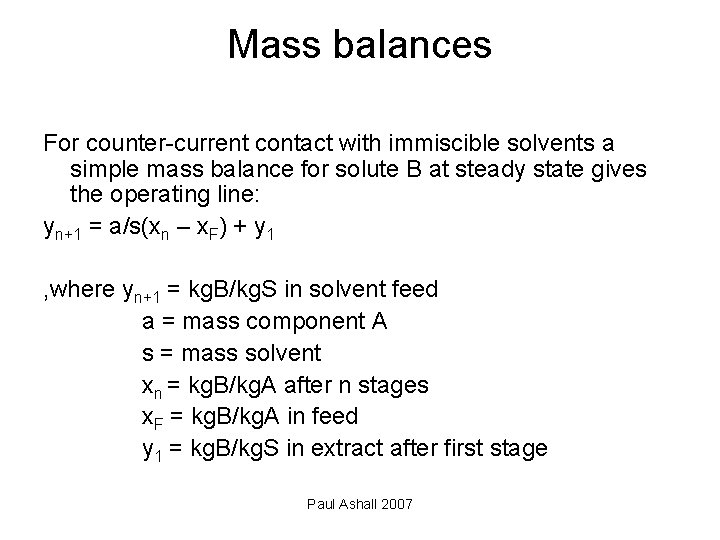
Mass balances For counter-current contact with immiscible solvents a simple mass balance for solute B at steady state gives the operating line: yn+1 = a/s(xn – x. F) + y 1 , where yn+1 = kg. B/kg. S in solvent feed a = mass component A s = mass solvent xn = kg. B/kg. A after n stages x. F = kg. B/kg. A in feed y 1 = kg. B/kg. S in extract after first stage Paul Ashall 2007

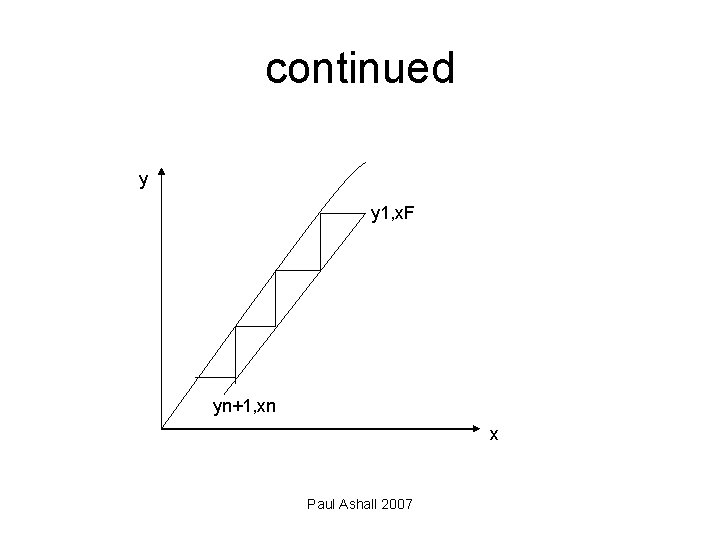
continued A graphical procedure may be used to analyse these systems. The number of theoretical stages (n) required to pass from x. F to xn is found by drawing in ‘steps’ between the operating line and the equilibrium curve (yn, xn). In practice equilibrium conditions may not be attained and extraction efficiency will be less than 100% thus requiring more stages in practice than the above analysis would suggest. Also partial miscibility of the solvents has to be considered in the separation process. Paul Ashall 2007

continued y y 1, x. F yn+1, xn x Paul Ashall 2007

Operation • Batch • Continuous • Single/multi stage contact Paul Ashall 2007

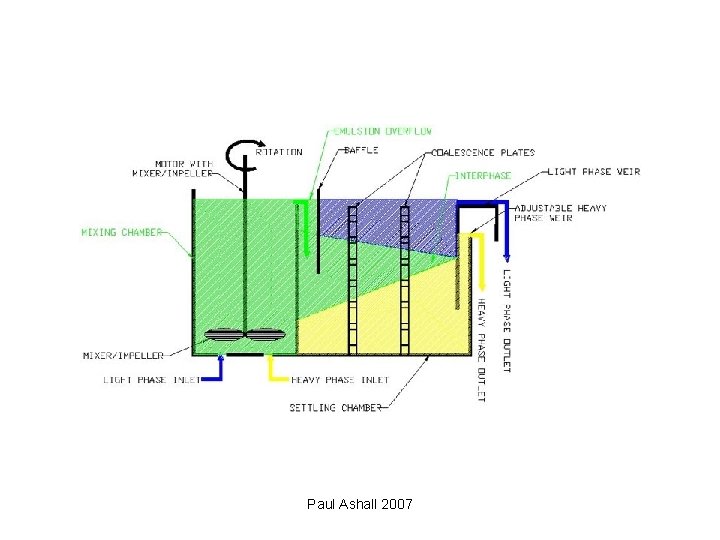
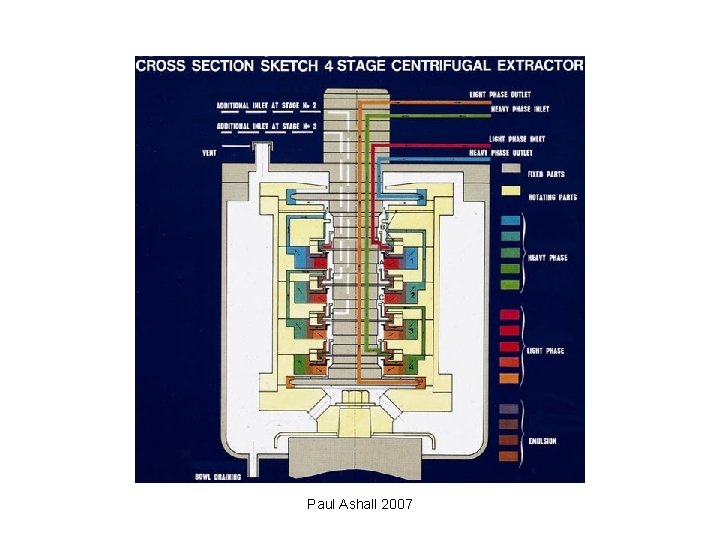
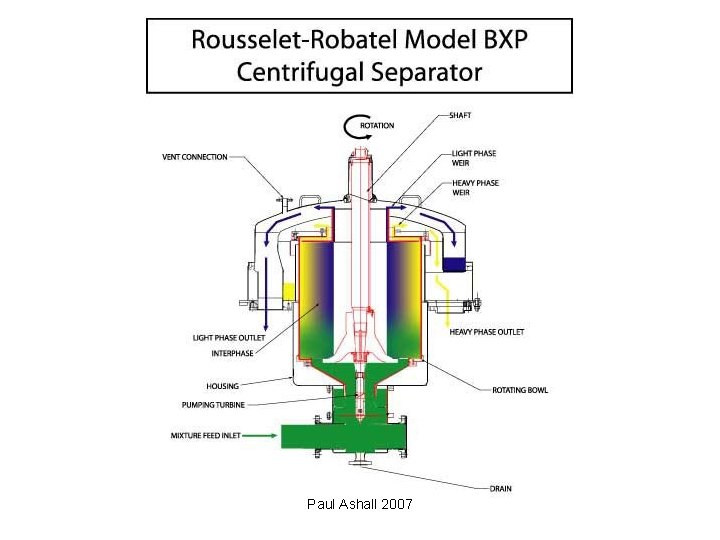
Equipment • Mixer-settler units • Columns • Centrifugal contactors Paul Ashall 2007

Paul Ashall 2007

Paul Ashall 2007

Paul Ashall 2007