Chapter 5 1 Light and Quantized Energy u























![Practice u Ba [Xe]6 s 2 u u Mg [Ne]3 s 2 u u Practice u Ba [Xe]6 s 2 u u Mg [Ne]3 s 2 u u](https://slidetodoc.com/presentation_image_h2/fad64c1e2862551e9c190a3bc9bb54cb/image-55.jpg)

- Slides: 60

Chapter 5. 1 Light and Quantized Energy u Wave-Particle Nature of Light • Electrons and light have a dual waveparticle nature. • Light which are particles behave as waves. • Electromagnetic Radiation (EMR) u Form of energy that exhibits wavelike behavior and travels at the speed of light. • Speed of Light (C) = 3 x 10 8 m/s (in a vacuum)

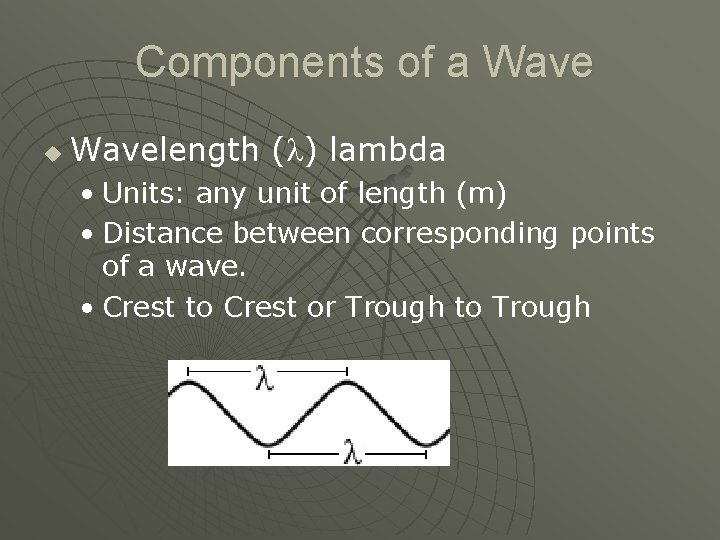
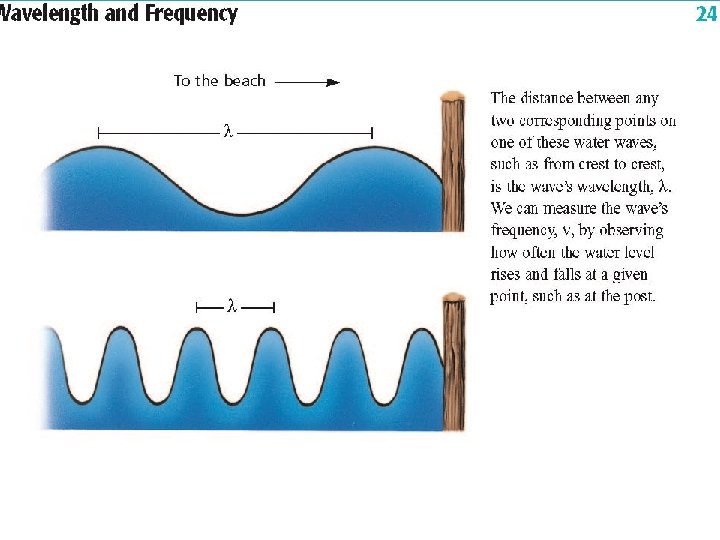
Components of a Wave u Wavelength ( ) lambda • Units: any unit of length (m) • Distance between corresponding points of a wave. • Crest to Crest or Trough to Trough


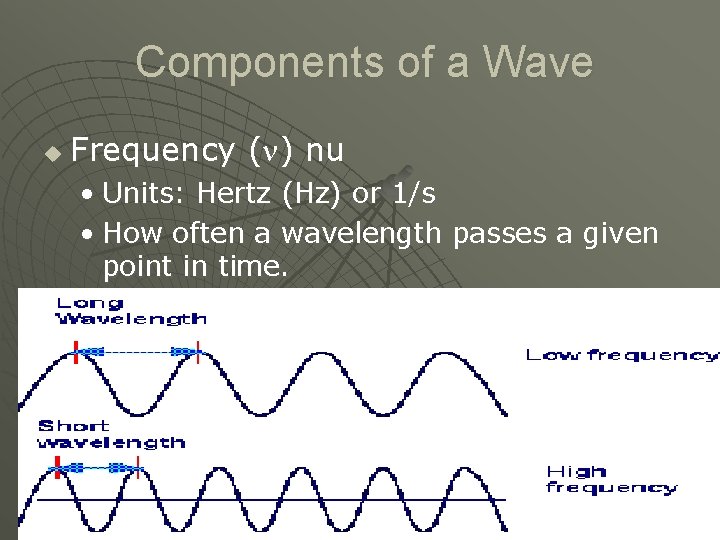
Components of a Wave u Frequency ( ) nu • Units: Hertz (Hz) or 1/s • How often a wavelength passes a given point in time.

Components of a Wave u Amplitude • Height of the wavelength. • Measured from the origin to crest or origin to trough. • Brightness of light.

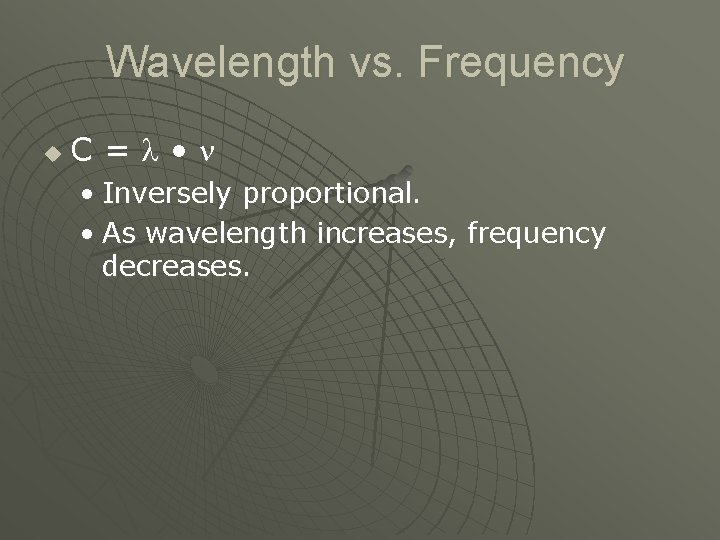
Wavelength vs. Frequency u C= • • Inversely proportional. • As wavelength increases, frequency decreases.

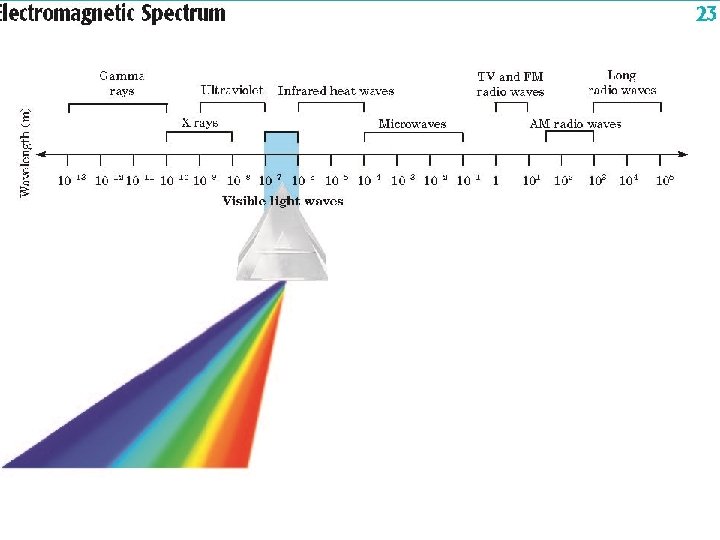
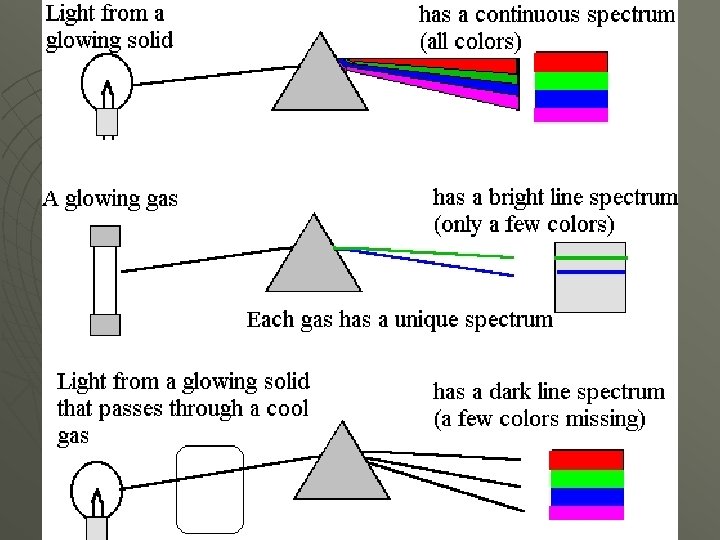
u Spectrums • Range of wavelengths for a series of waves. u Electromagnetic Spectrum • Consist of all electromagnetic radiation. u Continuous Spectrum • Spectrum where all wavelengths within a given range are together. u Examples: Visible Light, X-Rays, U. V. Light, etc


u 7 Parts EMR Spectrum • Longest wavelength to Shortest: Radio u Microwaves u Infrared u Visible Light u U. V. Light u X-Rays u Gamma-Rays u

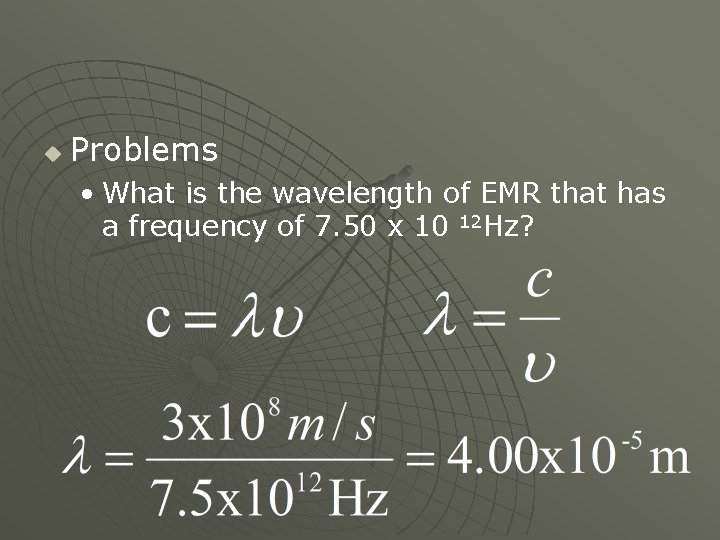
u Problems • What is the wavelength of EMR that has a frequency of 7. 50 x 10 12 Hz?

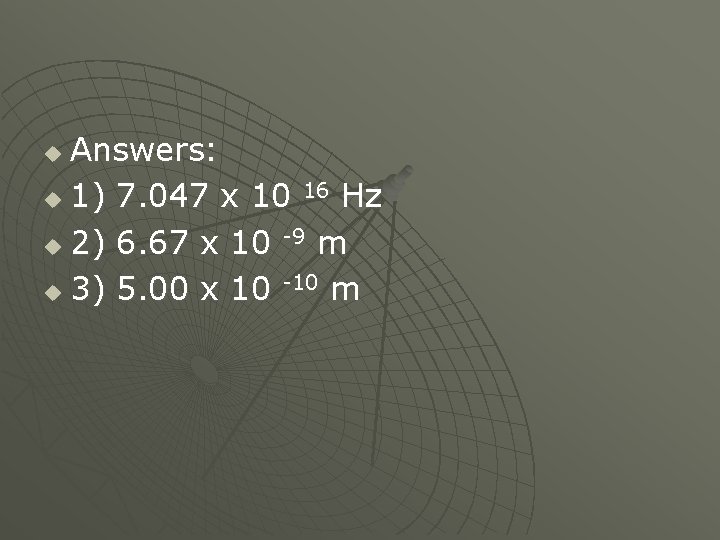
Problems: u 1. Determine the frequency of light with a wavelength of 4. 257 x 10 -7 cm. u 2. What is the wavelength of U. V light that has a frequency of 4. 50 x 10 16 Hz? u 3. What is the wavelength and color of light, that has a frequency of 6. 00 x 10 14 k. Hz? u

Answers: u 1) 7. 047 x 10 16 Hz u 2) 6. 67 x 10 -9 m u 3) 5. 00 x 10 -10 m u

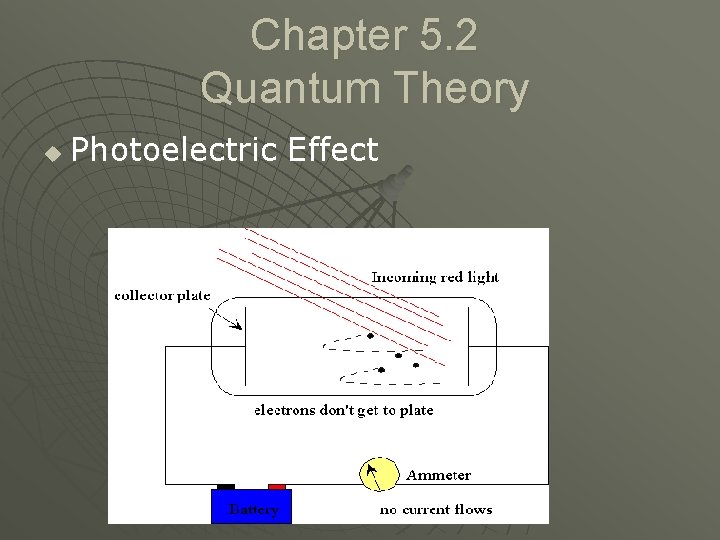
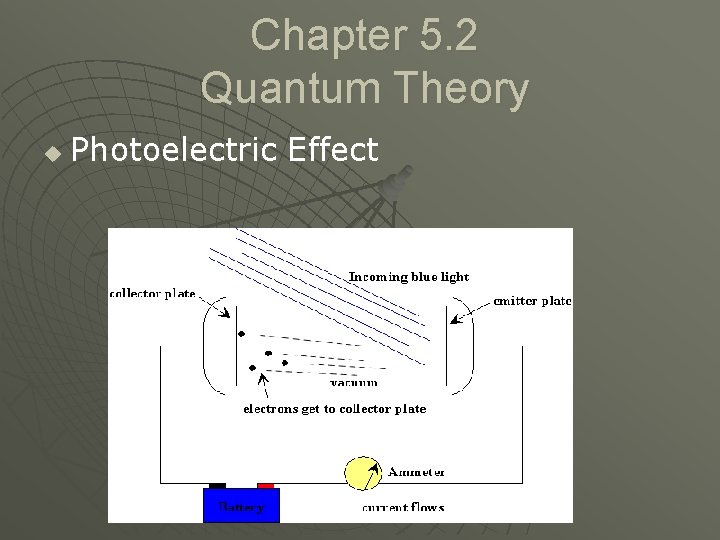
Chapter 5. 2 Quantum Theory u Photoelectric Effect • Emission of electrons by certain metals when sufficient light shines on them. u Photoelectric effect

Chapter 5. 2 Quantum Theory u Photoelectric Effect

Chapter 5. 2 Quantum Theory u Photoelectric Effect

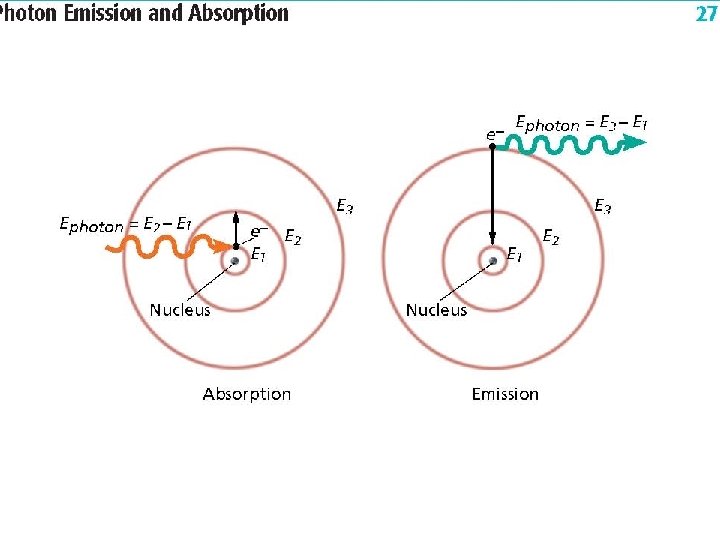
Chapter 5. 2 Quantum Theory u Quantum • Finite quantity of energy that can be gained or lost by an atom. • Planck’s Equation: E = h h u. E u u = = 6. 63 x 10 – 34 Js quantum of energy Photon • An individual quantum of light, caused by electrons losing quanta of energy.

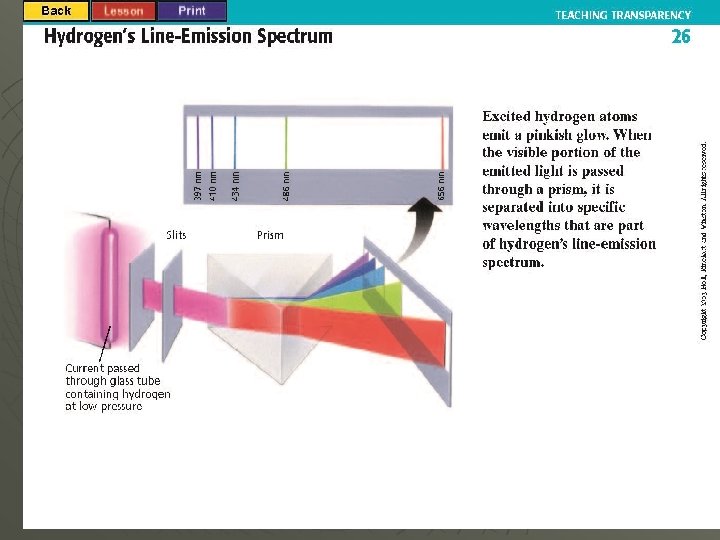
Chapter 5. 2 Quantum Theory u Visible Light Emissions • As electrons gain quanta of energy they release it in the form of photons. • Energy States of an Atom Ground State- an atoms lowest energy level. u Excited State- an atoms highest energy level. u , is produced when electrons Line from Spectrum drop the excited to the ground states. u


Chapter 4. 2 Quantum Theory

Chapter 5. 2 Quantum Theory u Problems: • What is the energy of U. V. light with a frequency of 4. 50 x 10 16 Hz? u E = h , h = 6. 63 x 10 – 34 Js

Chapter 5. 2 Quantum Theory u Problems: • Determine the energy of light that has a wavelength of 450 nm.

Problems: u 1. What is the energy of a photon of green light with a frequency of 5. 80 x 1014 1/s? u 2. What is the energy, in joules, of a quantum of radiant energy whose wavelength is 6. 82 x 10 – 6 cm? u 3. Determine the wavelength of a photon that has 3. 11 x 10 – 19 J of energy. u 4. Determine the frequency, in MHz, of a photon that has wavelength of 1. 36 x 1010 nm. u

Summary u u u Planck – restricted the amount of energy that an object emits or absorbs as a quantum. (E = h ) Einstein – used Planck’s theory and explained the photoelectric effect. Compton – light travels as tiny particles, photons.


Bohr’s Model u u u The Line Spectra demonstrates that the energy levels of an electron in an atom are quantized Similar to the rungs of a ladder, nothing exist in between. (For Hydrogen (1 p+ & 1 e- ) • 1 st Energy Level • 2 nd & so on u u n=1 n = 2, 3, 4, 5, 6, … ∞ Only electrons dropping from a Higher Level to a Lower one emit EMR A Number of Possibilities for electron drops

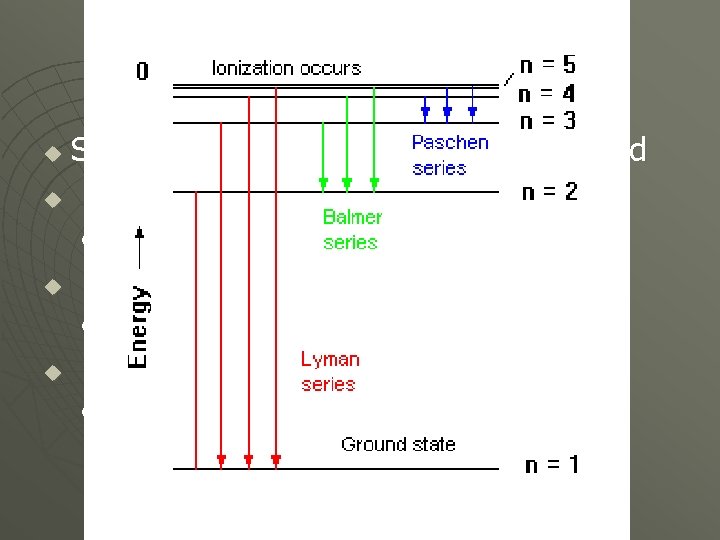
Hydrogen’s Line Spectrum Several Series of lines are observed u Electron Drops to the n = 1 Level u • Lyman Series (U. V. Range) u Electron Drops to the n = 2 Level • Balmer Series (Visible Range) u Electron Drops to the n = 3 Level • Paschen Series (Infrared Range)

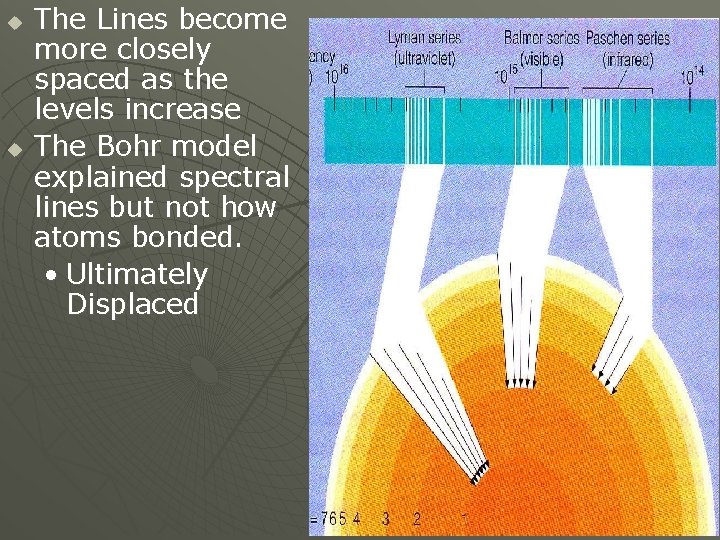
u u The Lines become more closely spaced as the levels increase The Bohr model explained spectral lines but not how atoms bonded. • Ultimately Displaced

1924 Louis de Brogile u French Graduate Student (asked an important question) • If light behaves as waves & particles, can particles of matter behave as waves? • Derived an Equation u Predicts that all matter exhibits h – Planck’s Con. wavelike motions. m – mass v - velocity

Dual Slit Dr. Quantum u Large Objects – Small Wavelengths • 200 g Baseball @ 30 m/s = 10 -32 cm • Undetectable u Small Objects – Large Wavelengths • 9. 11 x 10 -28 g @ 30 m/s = 10 -3 cm • Very Detectable w/ proper instruments u New Ballgame • Classical Mechanics vs. Quantum Mechanics

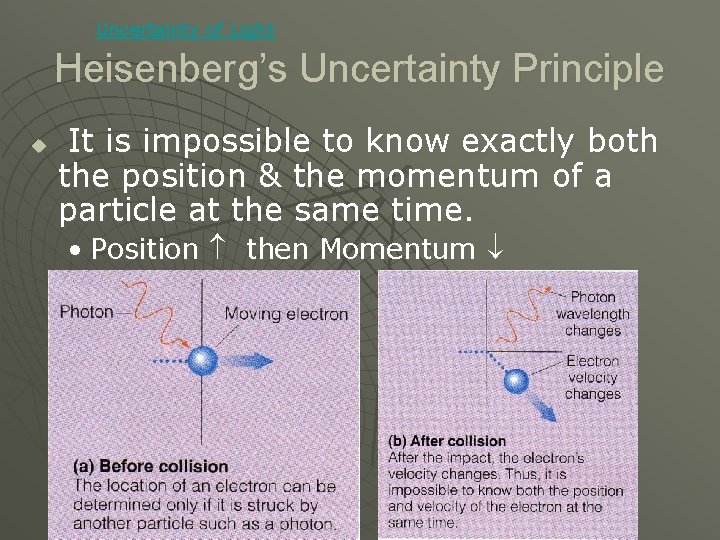
Uncertainty of Light Heisenberg’s Uncertainty Principle u It is impossible to know exactly both the position & the momentum of a particle at the same time. • Position then Momentum

Classical Vs Quantum 1. 2. Classical adequately describes the motions of bodies much larger than the atoms of which they are composed. It appears that such a body loses energy in any amount. Quantum describes the motions of subatomic particles and atoms as waves. These particles gain or lose energy in packages called quanta.

Quantum Mechanical Model u u u Max Born - Modern description of the electrons derived from the mathematical solution to the Schrodinger equation. Erwin Schrodinger - used wave mechanics to show the electrons about the nucleus emit vibration frequencies that were constant. Quantum Numbers - specify the properties of atomic orbital and their electrons. distance from the nucleus. Atomic Model Simulation Quantum Mechanics Video

Chapter 5. 2 Quantum Numbers u Principal Quantum Number (n) • Main energy level surrounding the nucleus. • Size of each orbital. • Primary distance from the nucleus. • Has values of n =1 to 7, 1 is the closest 7 is the farthest from the nucleus.

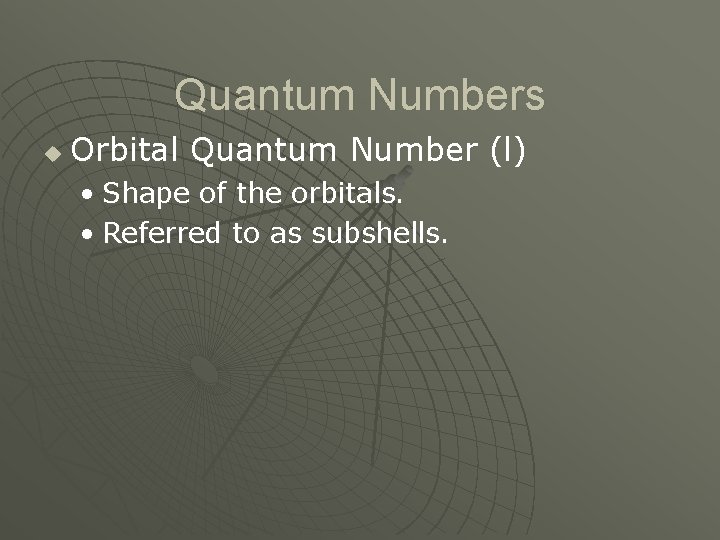
Quantum Numbers u Orbital Quantum Number (l) • Shape of the orbitals. • Referred to as subshells.

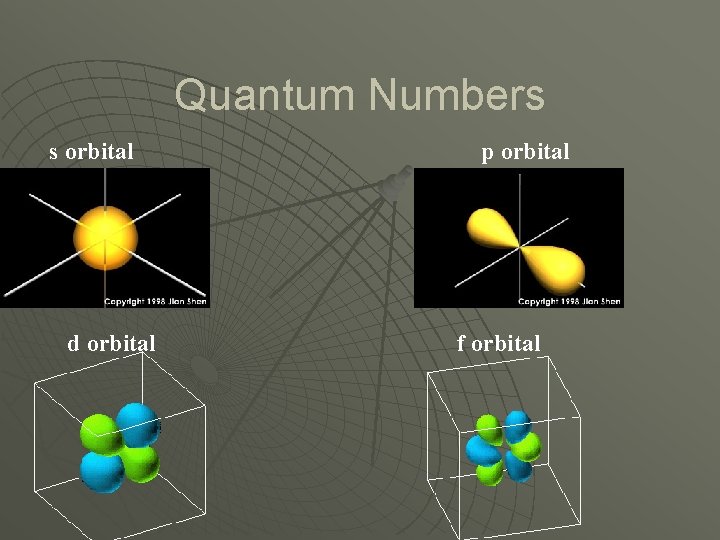
Quantum Numbers s orbital d orbital p orbital f orbital

Quantum Numbers u Magnetic Quantum Number (m) • Orientation of an orbital about the nucleus. • l = s u m=0 • l = p u m = -1, 0, 1 • l = d u m = -2, -1, 0, 1, 2 • l = f u m = -3, -2, -1, 0, 1, 2, 3

Quantum Numbers u s orbital, 1 orientation.

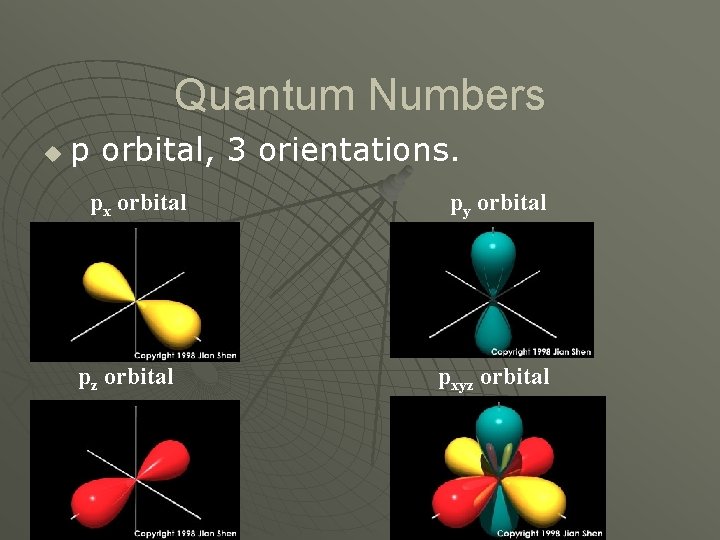
Quantum Numbers u p orbital, 3 orientations. px orbital pz orbital py orbital pxyz orbital

Chapter 4. 3 Quantum Numbers u d orbital, 5 orientations.

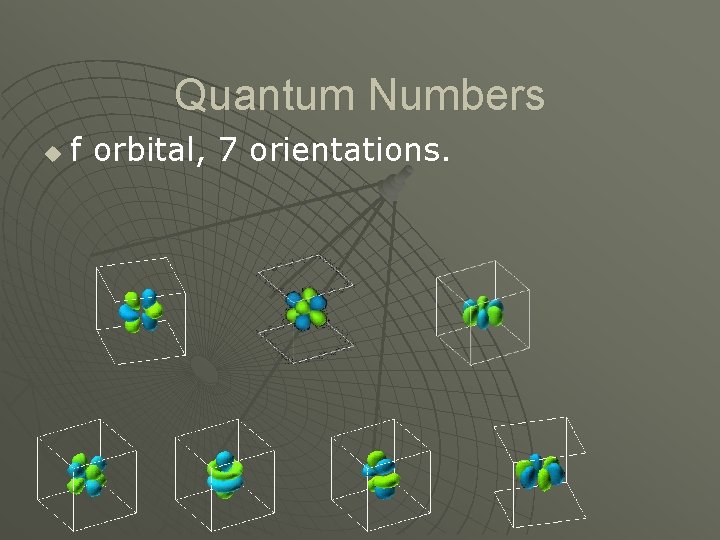
Quantum Numbers u f orbital, 7 orientations.

Quantum Numbers u Spin Quantum Number(+1/2 , -1/2) • Indicates two possible states on an electron in an orbital.

Quantum Numbers u Magnetism • Caused by the motion of electrons about the nuclei of atoms. • Diamagnetism – substance is weakly repelled by a magnetic force. • Paramagnetism – substance is weakly attracted by a magnetic force. • Ferromagnetism – Strong attraction by a magnetic force.

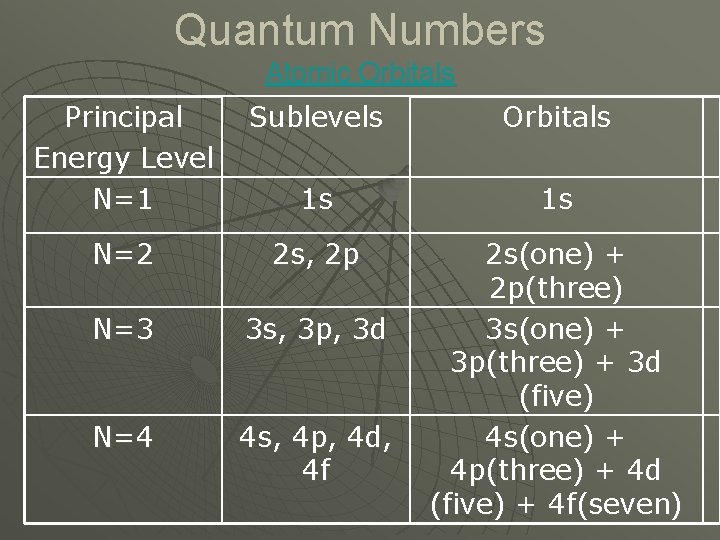
Quantum Numbers Atomic Orbitals Principal Energy Level N=1 Sublevels Orbitals 1 s 1 s N=2 2 s, 2 p N=3 3 s, 3 p, 3 d N=4 4 s, 4 p, 4 d, 4 f 2 s(one) + 2 p(three) 3 s(one) + 3 p(three) + 3 d (five) 4 s(one) + 4 p(three) + 4 d (five) + 4 f(seven)

Chapter 5. 2 Quantum Numbers Principal Q. N. 1 # Orbitals per # Electrons Main level per main level 2) 2 (2 n (n ) 1 2 2 4 8 3 9 18 4 16 32

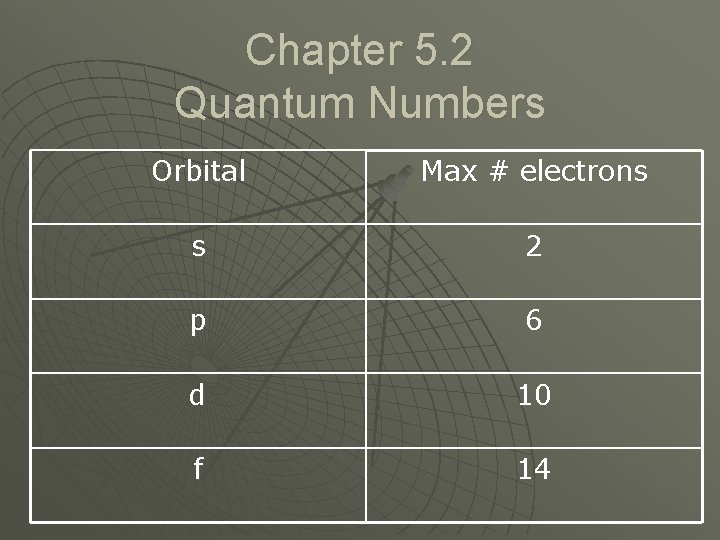
Chapter 5. 2 Quantum Numbers Orbital Max # electrons s 2 p 6 d 10 f 14

5. 3 Rules Governing Electron Configurations Electron Configuration – arrangement of electrons in the atom Rules u Aufbau Rule – electron occupies the lowest energy level that will receive it. u Hund’s Rule – orbitals of equal energy each receive one electron (equal spin) before any receive two. u Pauli’s Exclusion Principle – no two electrons can have the same set of 4 quantum numbers (n, l, m, s)

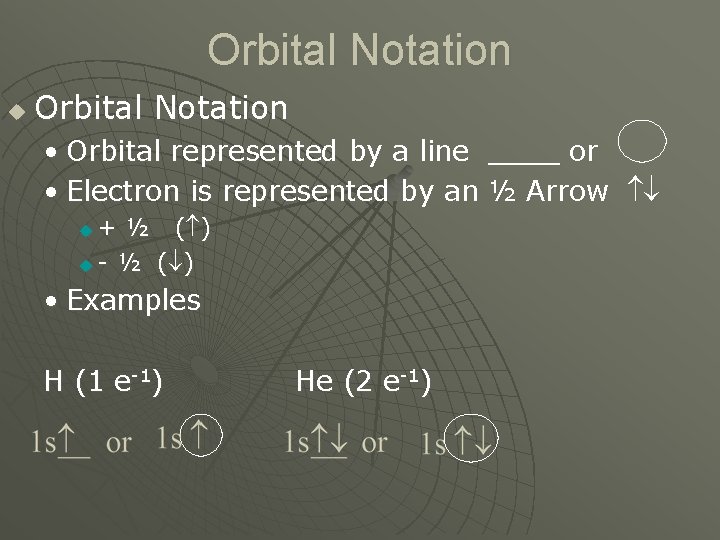
Orbital Notation u Orbital Notation • Orbital represented by a line ____ or • Electron is represented by an ½ Arrow + ½ ( ) u - ½ ( ) u • Examples H (1 e-1) He (2 e-1)

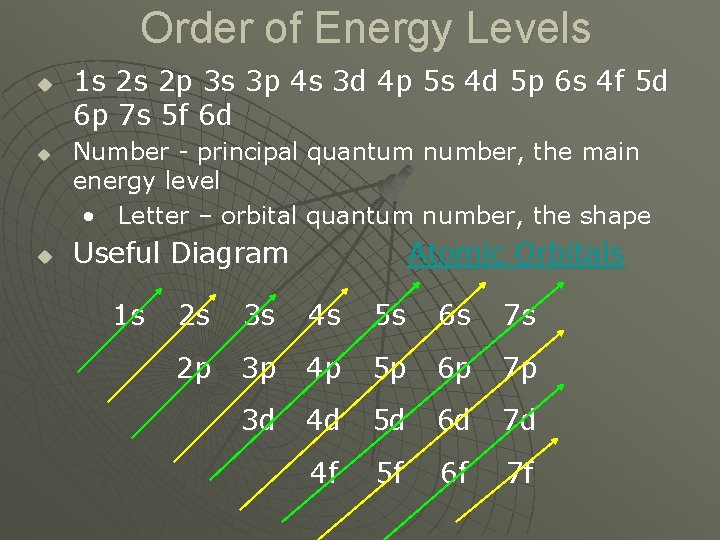
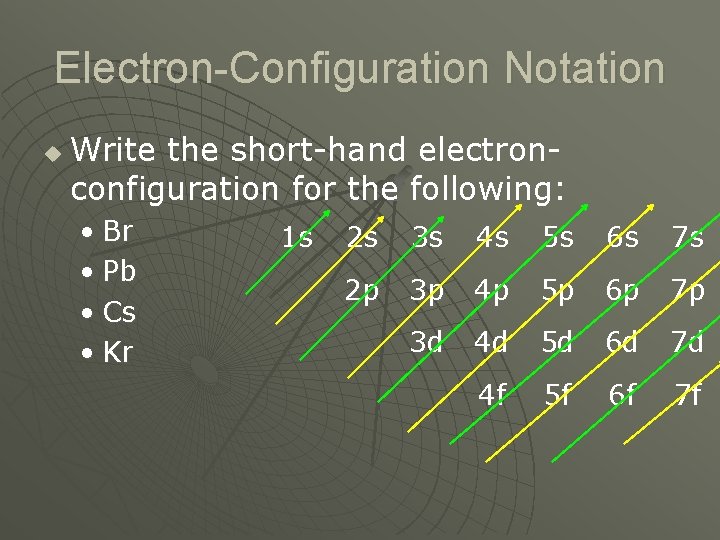
Order of Energy Levels u u u 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 s 4 f 5 d 6 p 7 s 5 f 6 d Number - principal quantum number, the main energy level • Letter – orbital quantum number, the shape Useful Diagram 1 s Atomic Orbitals 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 7 d 4 f 5 f 6 f 7 f

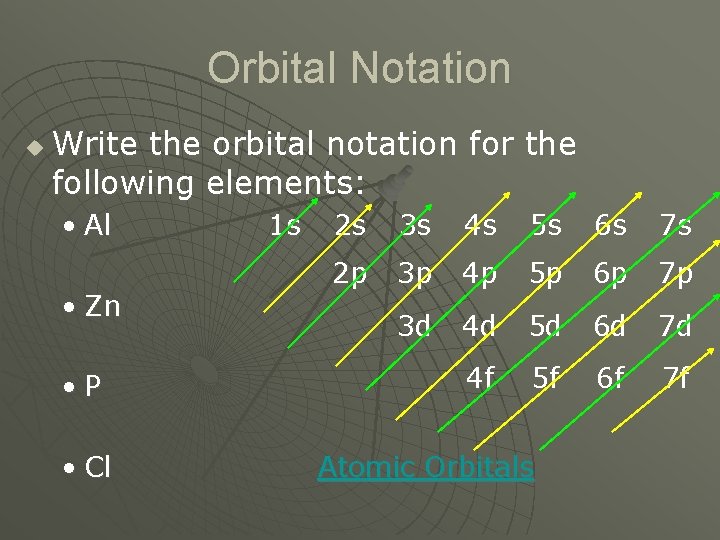
Orbital Notation u Write the orbital notation for the following elements: • Al • Zn • P • Cl 1 s 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 7 d 4 f 5 f 6 f 7 f Atomic Orbitals

Electron-Configuration Notation Eliminates the lines & arrows u Superscripts are used to illustrate the number of electrons in the sublevel u Same order of sublevels u Examples u • H (1 e-1) - 1 s 1 • He (2 e-1) - 1 s 2 • Li (3 e-1) - 1 s 22 s 1

Electron-Configuration Notation u Write the short-hand electronconfiguration for the following: • Br • Pb • Cs • Kr 1 s 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 7 d 4 f 5 f 6 f 7 f

Noble Gas Configuration Shortest method of writing the electron configurations. u Use the last noble gas to occur prior to the element that is being configured. u Start at the n. S where n equals the period in which the element being configured can be found. u • Example: Zr u Noble gas would be Kr and start configuration at 5 s.

Writing in noble gas configuration u u Al Write the noble gas configuration for the following: • V • Rb • I • Hg • U • W

Exceptions to Aufbau All elements prefer a more stable configuration of electrons. u Fully filled and ½ filled orbitals are more stable than others. u Elements that are 1 shy of a full or ½ filled d orbital configuration will have electrons transfer from the s to the d to reach this stable state. u Example if you have a 4 s 2 and 3 d 4 the actual configuration should be 4 s 1 and 3 d 5. u
![Practice u Ba Xe6 s 2 u u Mg Ne3 s 2 u u Practice u Ba [Xe]6 s 2 u u Mg [Ne]3 s 2 u u](https://slidetodoc.com/presentation_image_h2/fad64c1e2862551e9c190a3bc9bb54cb/image-55.jpg)
Practice u Ba [Xe]6 s 2 u u Mg [Ne]3 s 2 u u W [Xe]6 s 14 f 145 d 5 u u Ag [Kr]5 s 14 d 10 u u Sb [Kr]5 s 24 d 105 p 3 u

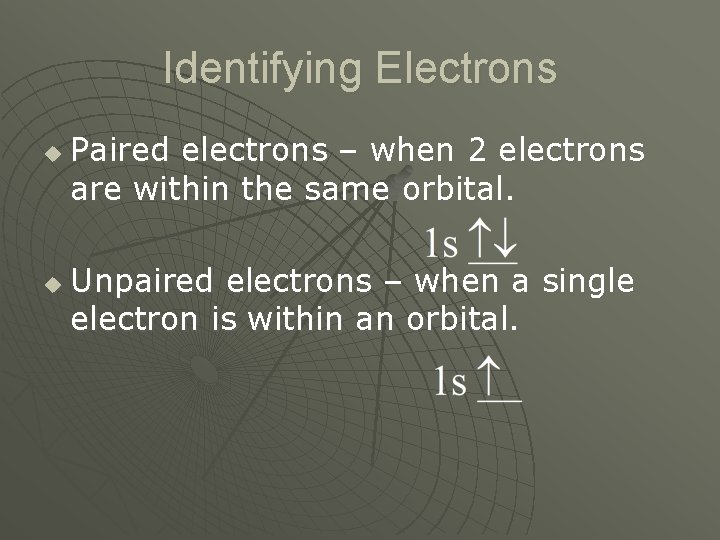
Identifying Electrons u u Paired electrons – when 2 electrons are within the same orbital. Unpaired electrons – when a single electron is within an orbital.

Identifying Electrons u How many unpaired electrons does the following elements have? • Na • O • B

Electron Dot Notation Describes the number of electrons in an atom’s outer electron cloud, or highest energy level, as dots around the symbol. u Valence electron - refers to the highest energy level electron within an atom. u

Electron Dot Notation Draw the electron-configuration and electron-dot notation for Nitrogen. u 1 s 2 2 p 3 u The highest energy level is 2. u There are 5 valence electrons in Nitrogen. u

Electron Dot Notation u Write the noble gas-configuration and electron dot notation for the following. • Al • As • I • Sr