LIGHT and QUANTIZED ENERGY Much of our understanding
















- Slides: 60

LIGHT and QUANTIZED ENERGY

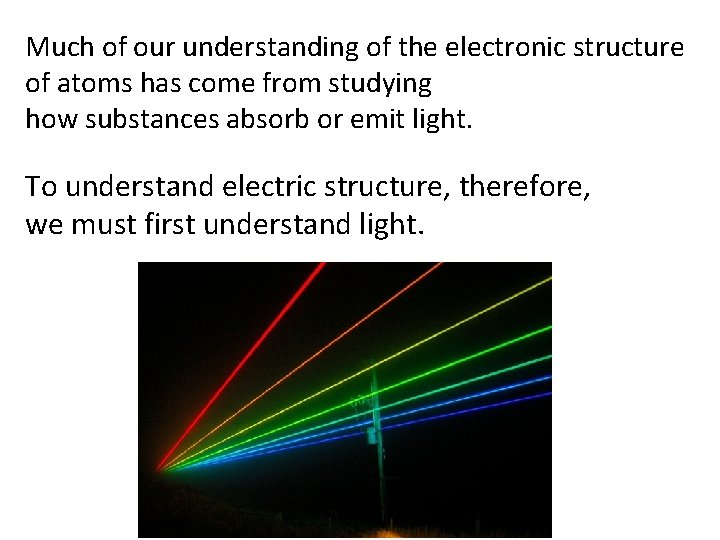
Much of our understanding of the electronic structure of atoms has come from studying how substances absorb or emit light. To understand electric structure, therefore, we must first understand light.

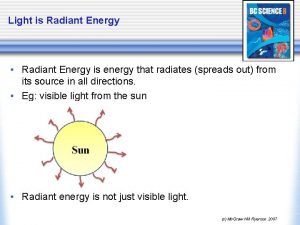
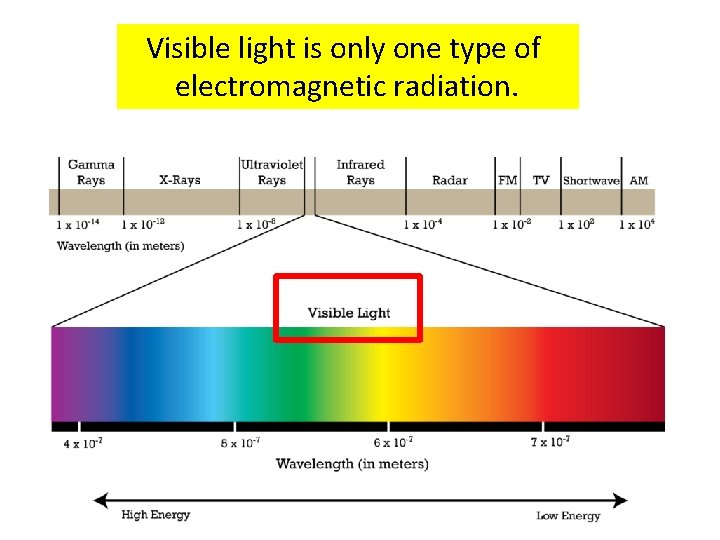
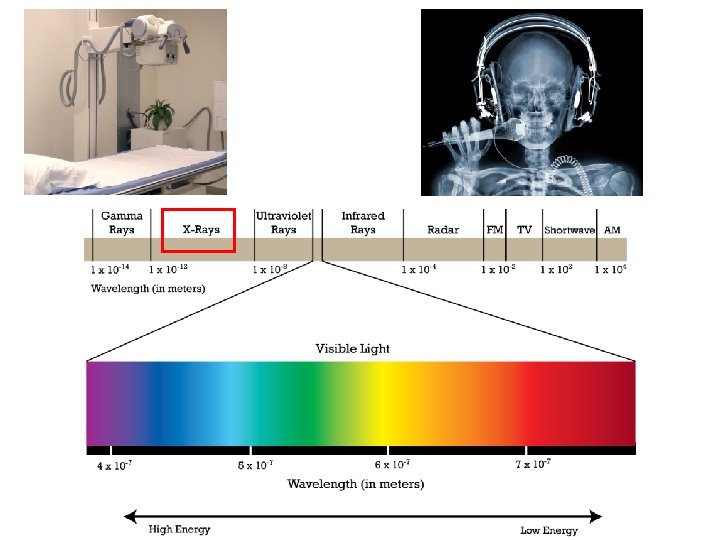
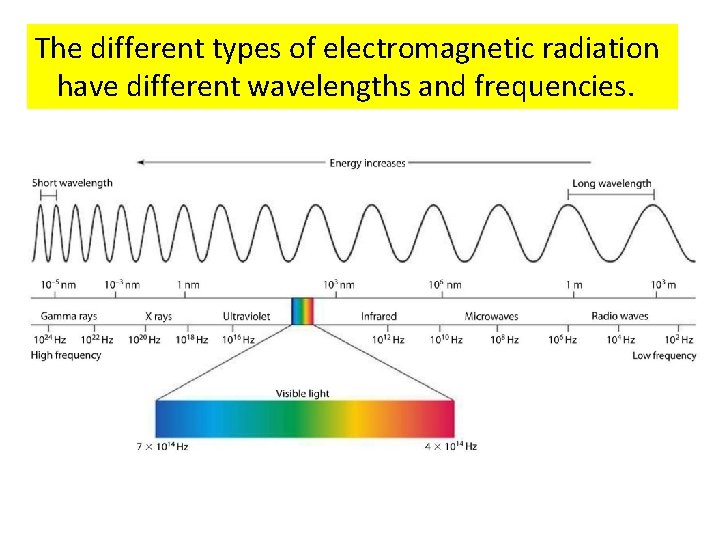
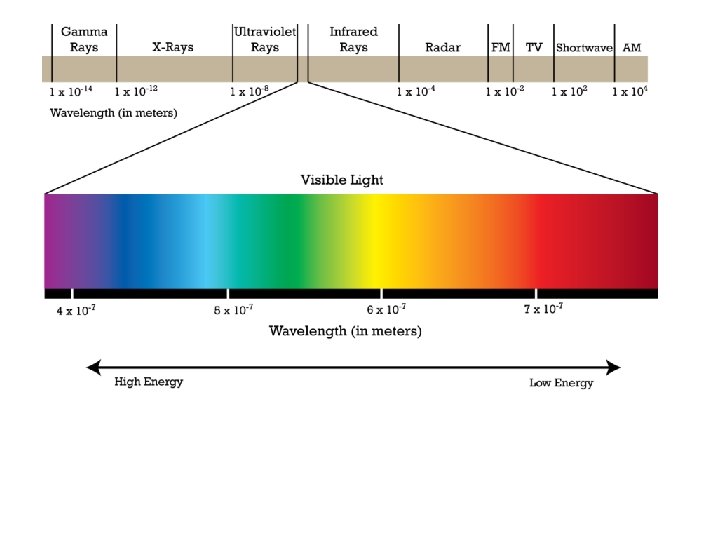
Visible light is only one type of electromagnetic radiation.

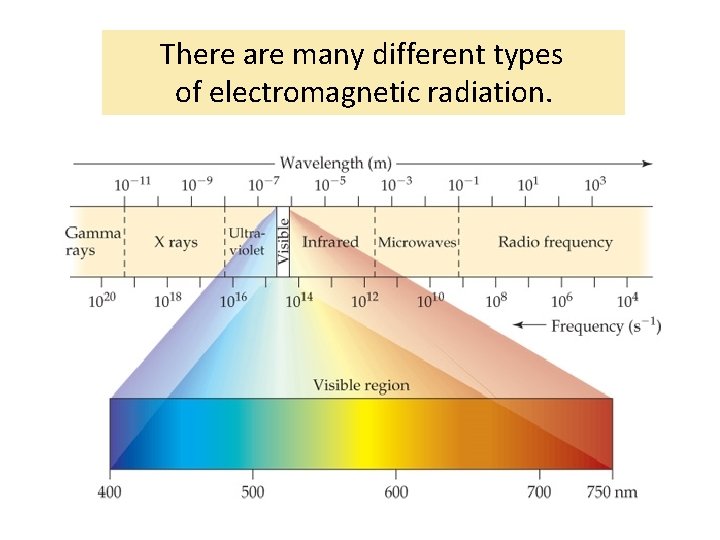
There are many different types of electromagnetic radiation.

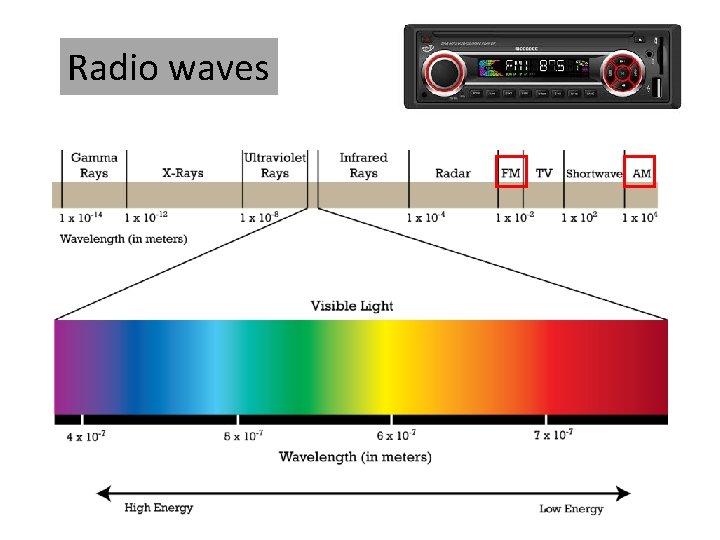
Radio waves



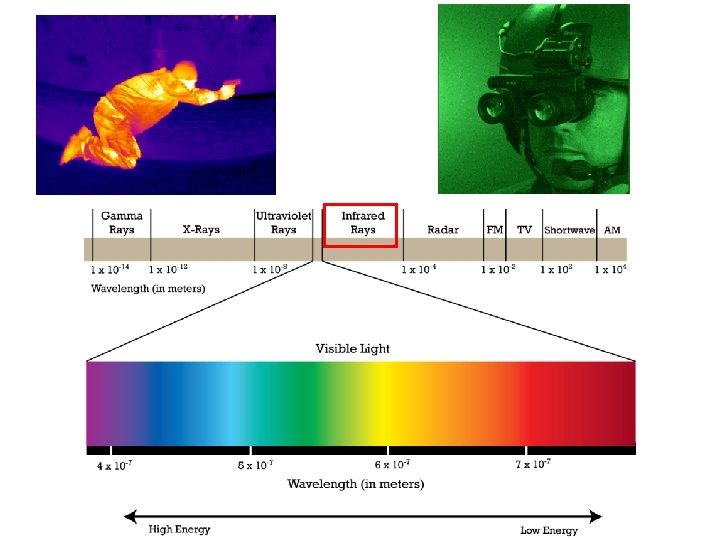
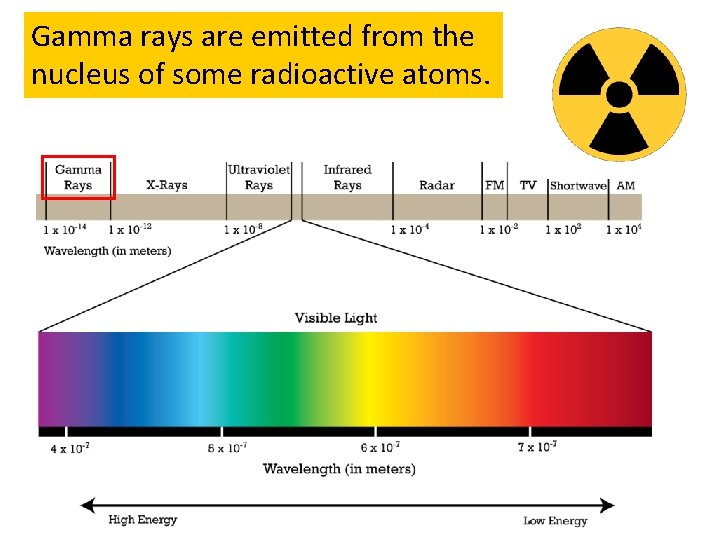
Gamma rays are emitted from the nucleus of some radioactive atoms.

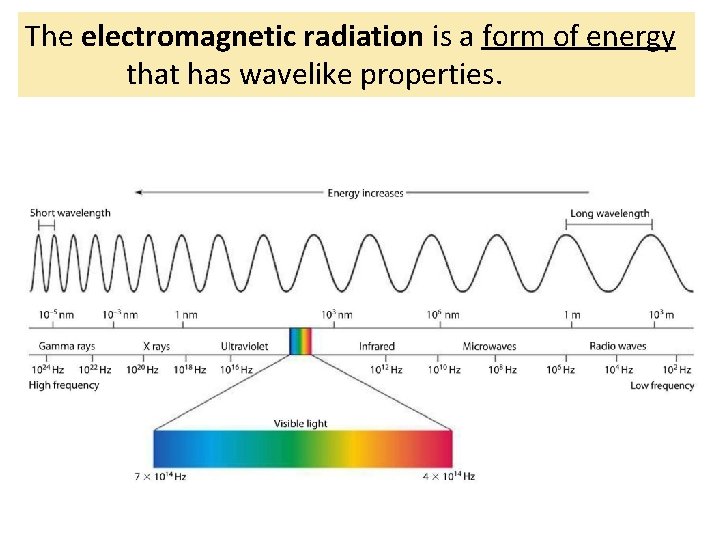
The electromagnetic radiation is a form of energy that has wavelike properties.

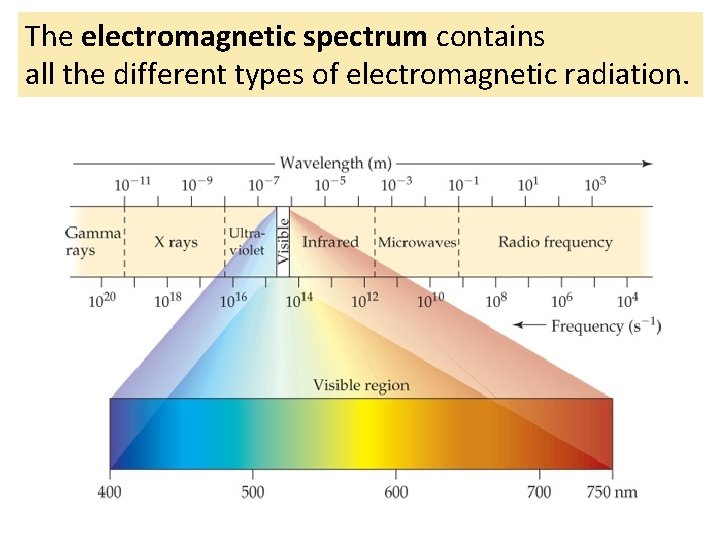
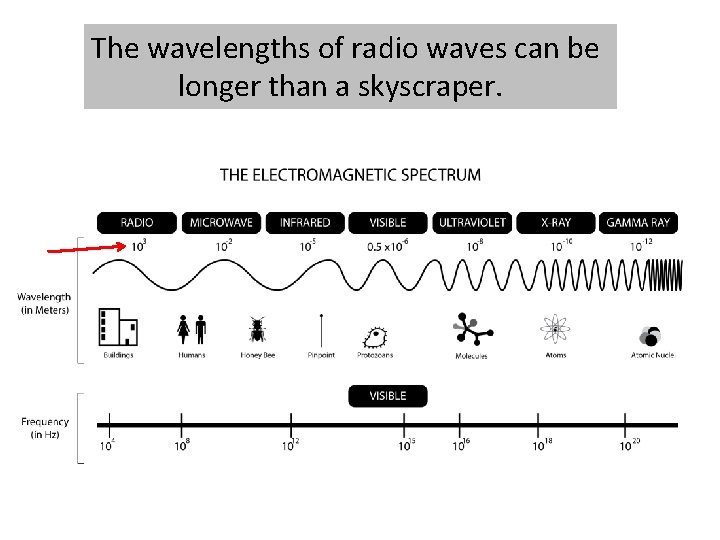
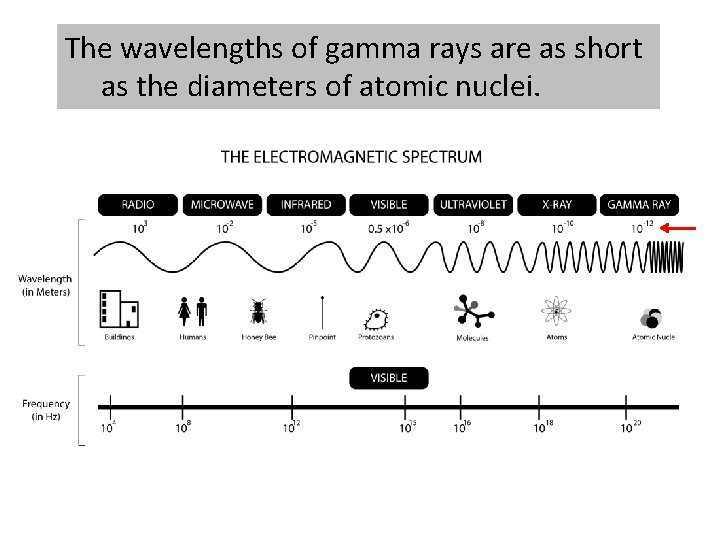
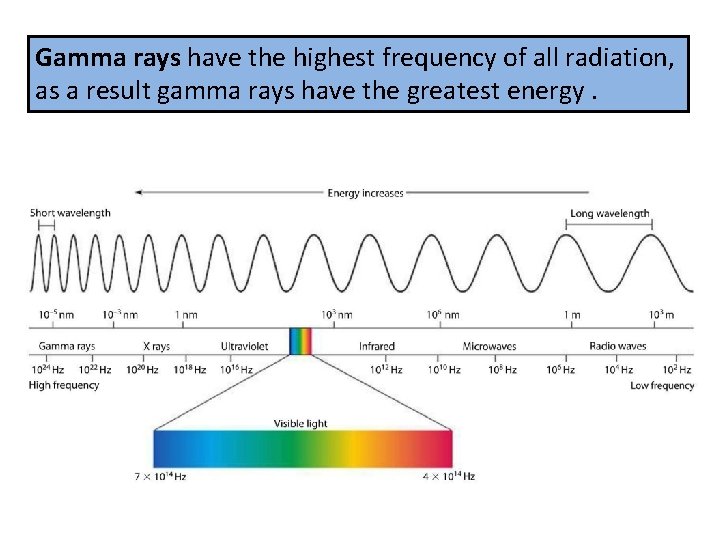
The electromagnetic spectrum contains all the different types of electromagnetic radiation.

The different types of electromagnetic radiation have different wavelengths and frequencies.

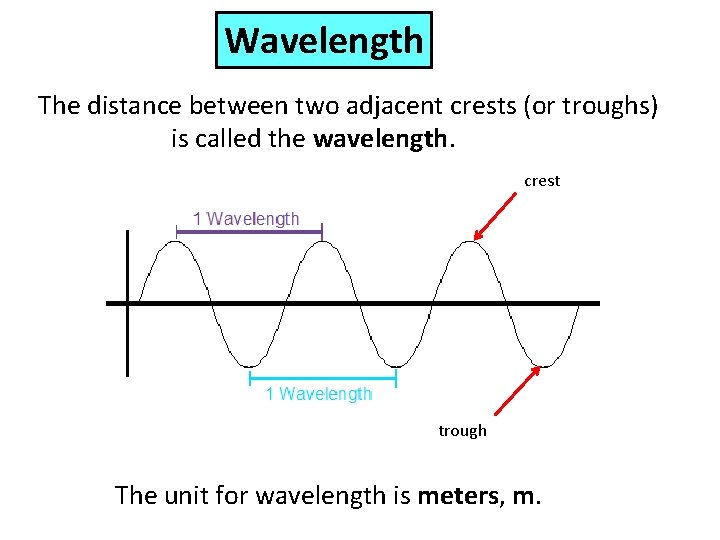
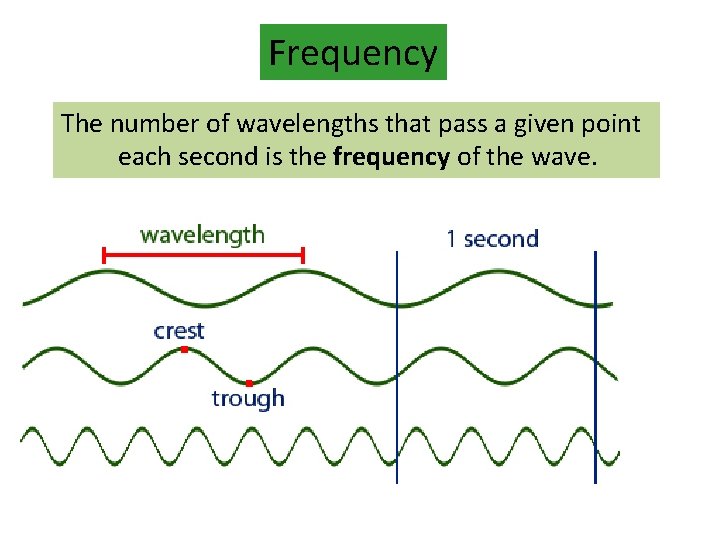
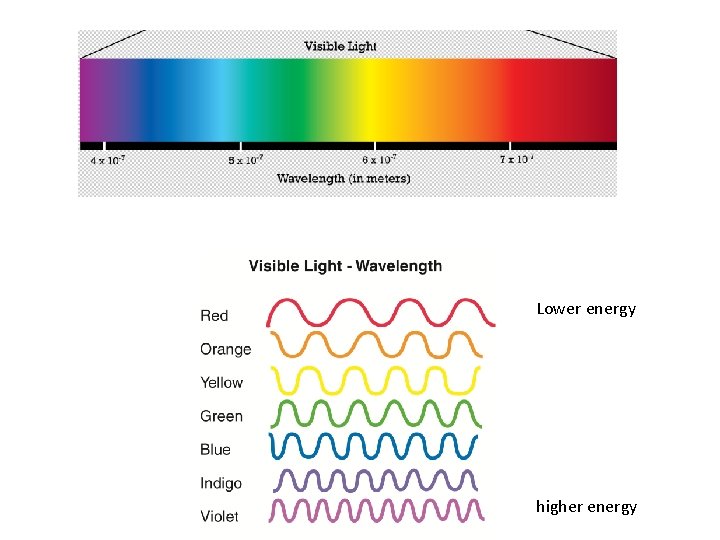
Wavelength The distance between two adjacent crests (or troughs) is called the wavelength. crest trough The unit for wavelength is meters, m.


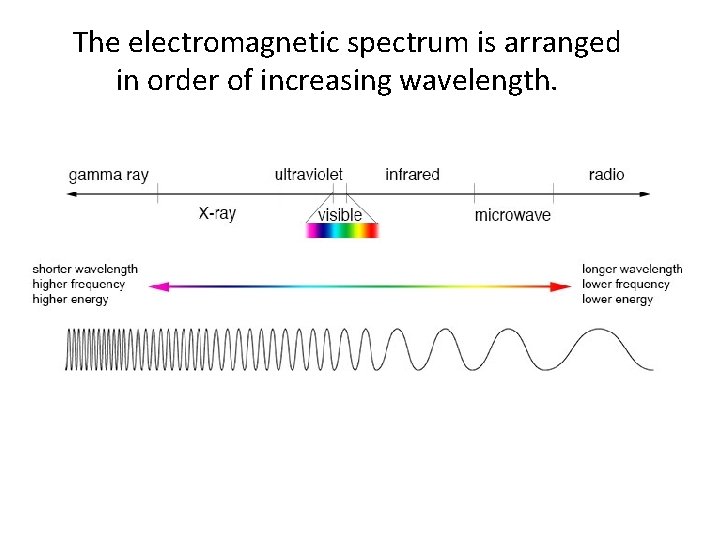
The electromagnetic spectrum is arranged in order of increasing wavelength.

The wavelengths of radio waves can be longer than a skyscraper.

The wavelengths of gamma rays are as short as the diameters of atomic nuclei.

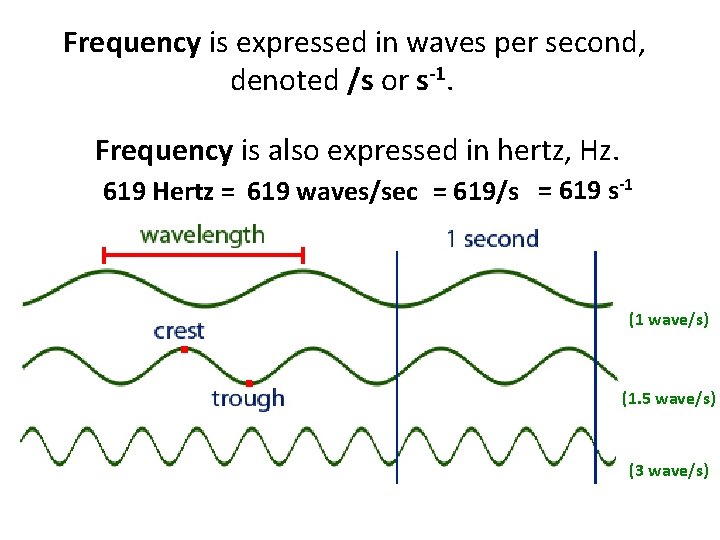
Frequency The number of wavelengths that pass a given point each second is the frequency of the wave. (1 wave/s) (1. 5 wave/s) (3 wave/s)

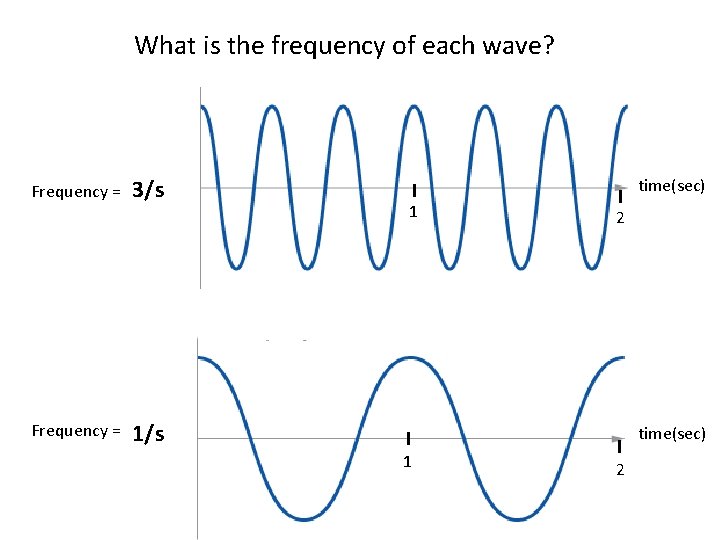
What is the frequency of each wave? Frequency = 3/s ? Frequency = time(sec) 1 2 ? 1/s time(sec) 1 2

Frequency is expressed in waves per second, denoted /s or s-1. Frequency is also expressed in hertz, Hz. 619 Hertz = 619 waves/sec = 619/s = 619 s-1 (1 wave/s) (1. 5 wave/s) (3 wave/s)

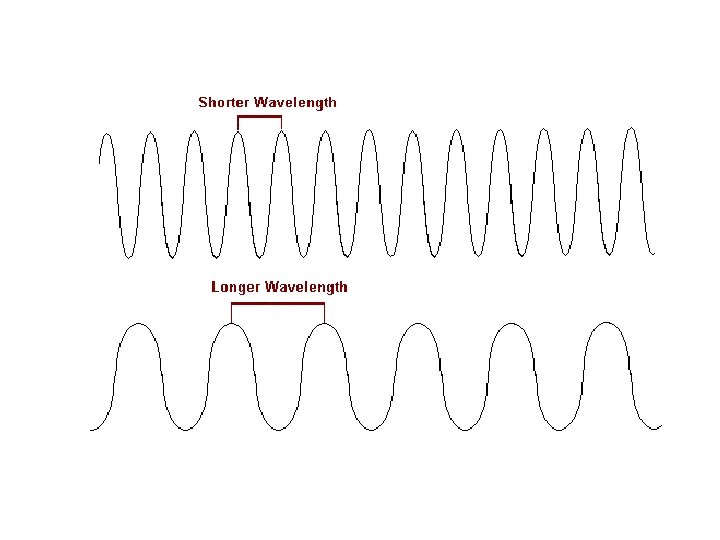
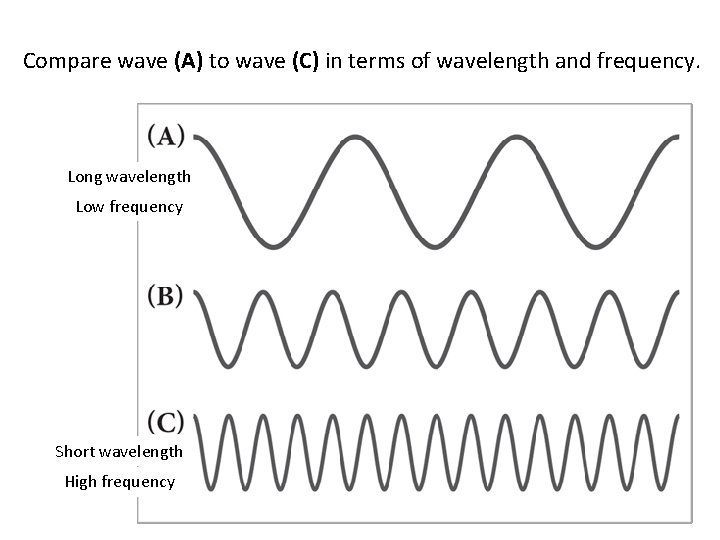
Compare wave (A) to wave (C) in terms of wavelength and frequency. Long wavelength Low frequency Short wavelength High frequency

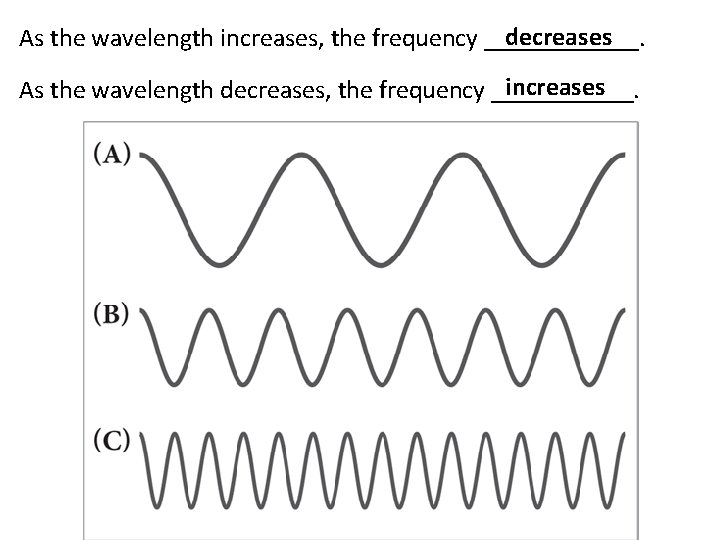
decreases As the wavelength increases, the frequency ______. increases As the wavelength decreases, the frequency ______.

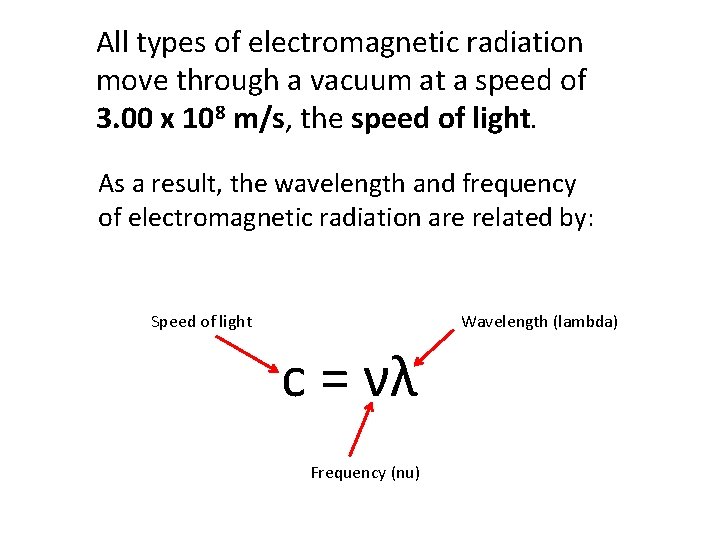
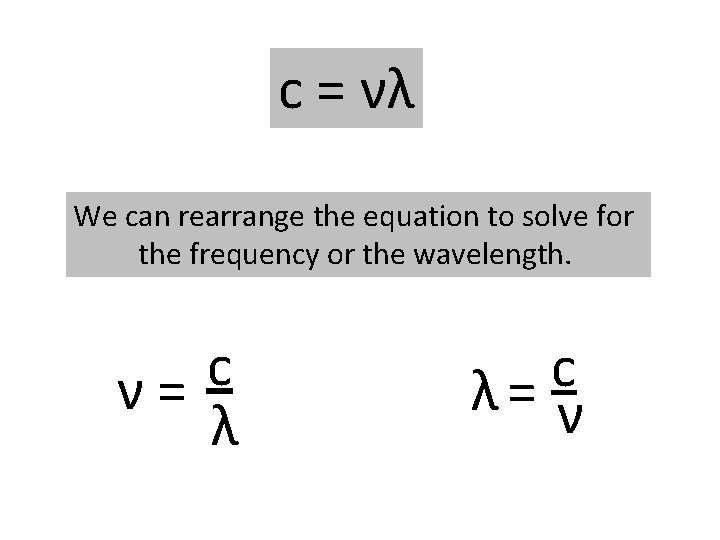
All types of electromagnetic radiation move through a vacuum at a speed of 3. 00 x 108 m/s, the speed of light. As a result, the wavelength and frequency of electromagnetic radiation are related by: Speed of light Wavelength (lambda) c = νλ Frequency (nu)

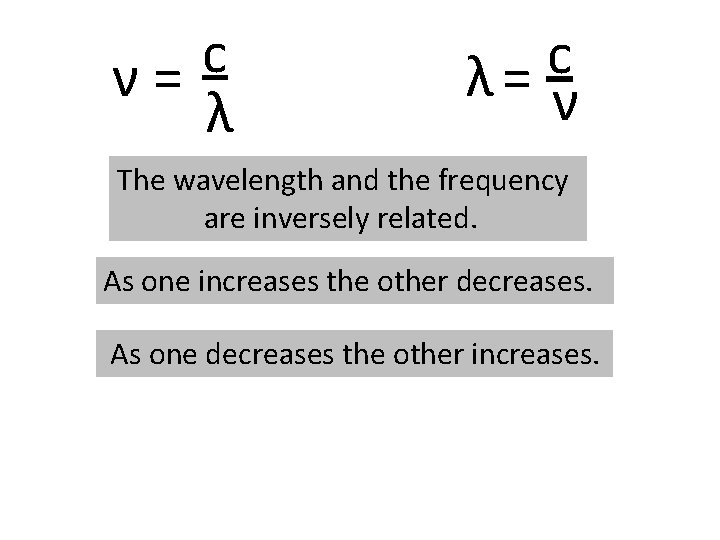
c = νλ We can rearrange the equation to solve for the frequency or the wavelength. c ν= λ c λ= ν

c ν= λ c λ= ν The wavelength and the frequency are inversely related. As one increases the other decreases. As one decreases the other increases.

c = νλ We can rearrange the equation to solve for the frequency or the wavelength.

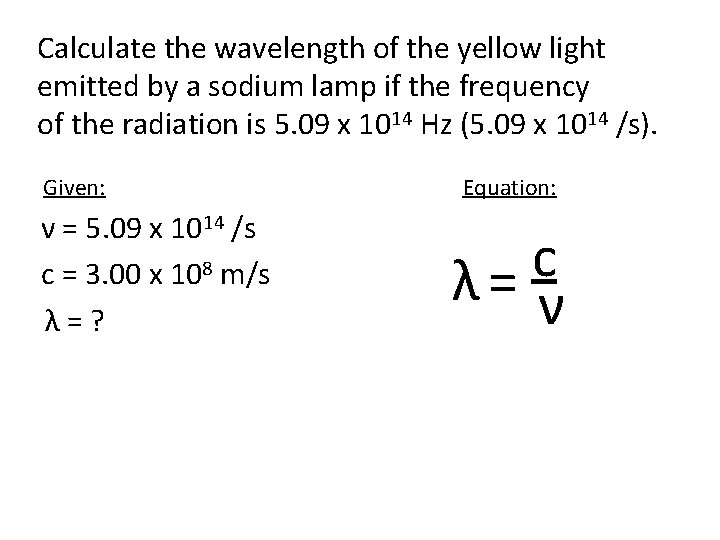
Calculate the wavelength of the yellow light emitted by a sodium lamp if the frequency of the radiation is 5. 09 x 1014 Hz (5. 09 x 1014 /s). Given: ν = 5. 09 x 1014 /s c = 3. 00 x 108 m/s λ=? Equation: c λ= ν

An gamma ray has a wavelength of 4. 1 x 10 -12 m. What is the frequency? Given: Equation:

A radio station broadcasts at a frequency of 590 k. Hz. What is the wavelength of the radio waves? Given: Equation:

WHITEBOARD PRACTICE

1. What is the frequency of orange light, which has a wavelength of 6. 09 x 10 -7 m? Given: Equation:

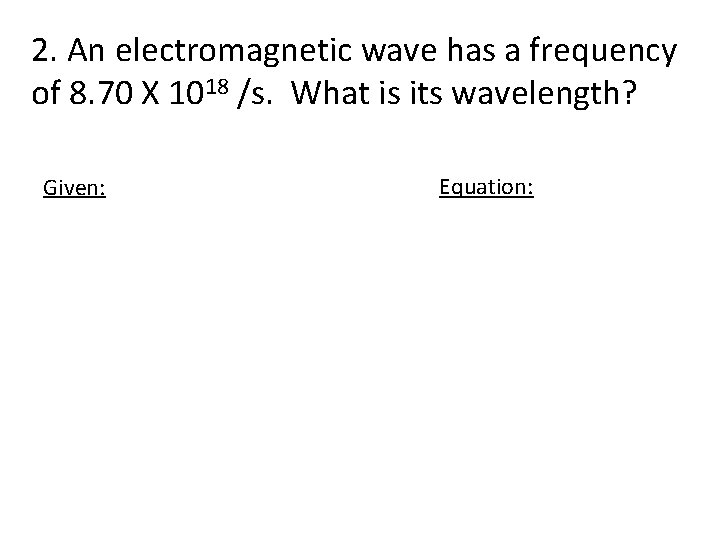
2. An electromagnetic wave has a frequency of 8. 70 X 1018 /s. What is its wavelength? Given: Equation:

3. The yellow light given off by a sodium vapor lamp used for public lighting has a wavelength of 589 nm. What is the frequency of this radiation? Given: Equation:

The Particle Nature of Light Thus far, we have learned that light and other radiation behave like waves. But light and other radiation also behave as if composed of particles or rather packets of energy. ed b r bso ion. a t no s fash s i gy inue r e s e En cont u tin in a con , d unts. e rb mo tum o s ab ific a quan s i y pec led g r s l e En mall ts ca s in acke p in stepwise

ed b r so on. b a t hi o s n a is sf y e g u r n s Ene conti e u a in tin con wavelike , d unts. e rb mo tum o s b fic a uan a s i eci d q y g er all sp calle n E m ts s e k in ac p in stepwise particle-like

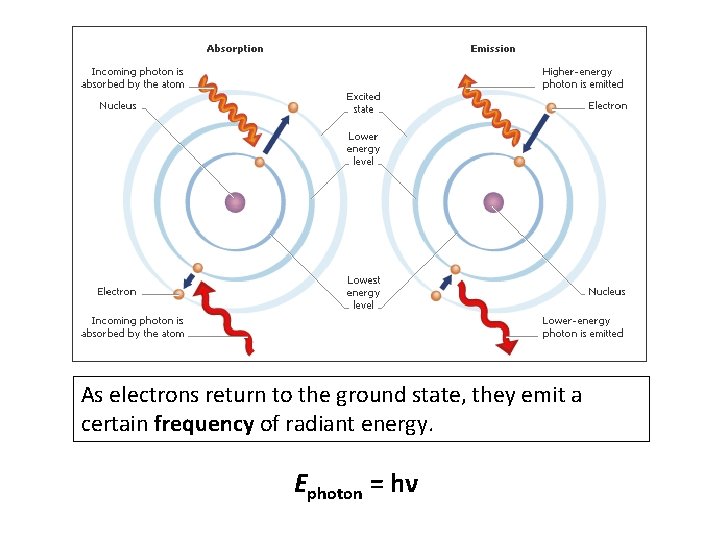
Matter can gain or lose energy only in small, specific amounts called quanta (quantum). That is, a quantum is the minimum amount of energy that can be gained or lost by an atom. Radiant energy is quantized.

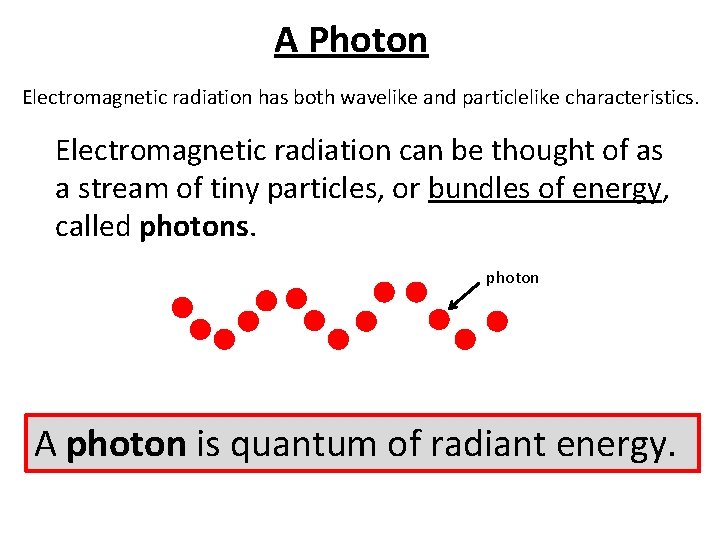
A Photon Electromagnetic radiation has both wavelike and particlelike characteristics. Electromagnetic radiation can be thought of as a stream of tiny particles, or bundles of energy, called photons. photon A photon is quantum of radiant energy.

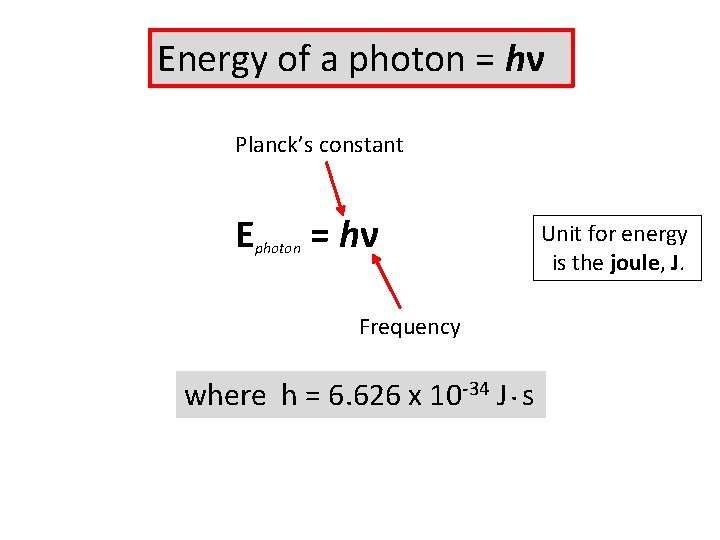
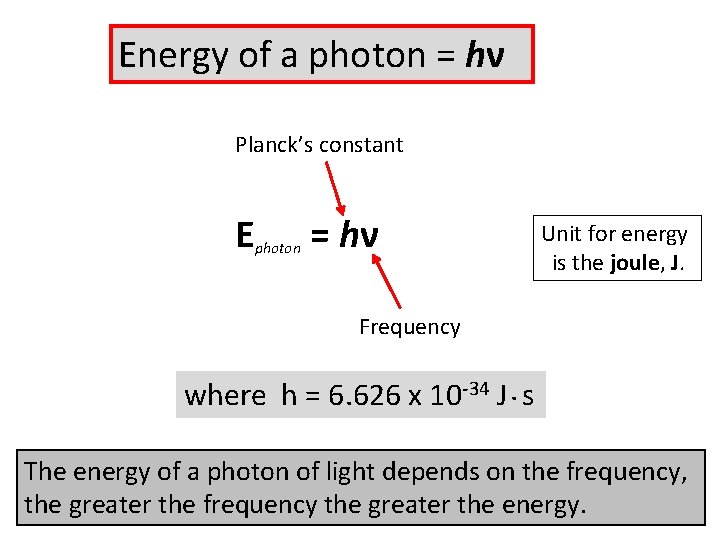
Energy of a photon = hν Planck’s constant E photon = hν Frequency where h = 6. 626 x 10 -34 J. s Unit for energy is the joule, J.

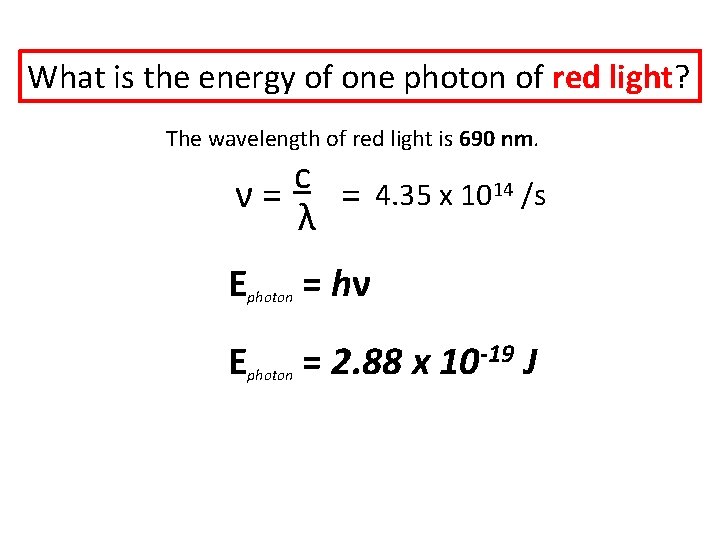
What is the energy of one photon of red light? The wavelength of red light is 690 nm. c ν= = λ E E 4. 35 x 1014 /s photon = hν photon = 2. 88 x 10 -19 J

Energy of a photon = hν Planck’s constant E photon = hν Unit for energy is the joule, J. Frequency where h = 6. 626 x 10 -34 J. s The energy of a photon of light depends on the frequency, the greater the frequency the greater the energy.

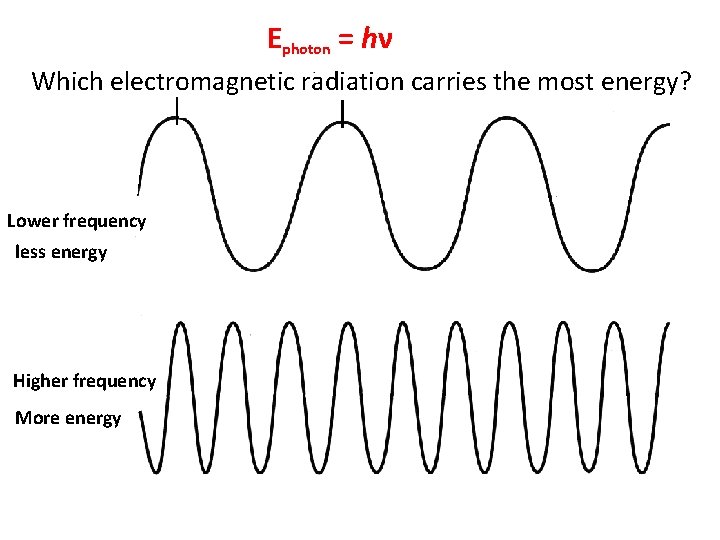
Ephoton = hν Which electromagnetic radiation carries the most energy? Lower frequency less energy Higher frequency More energy

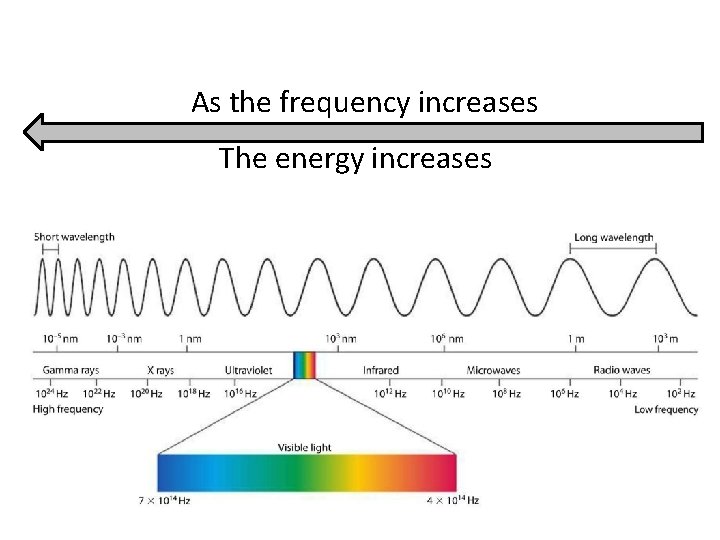
As the frequency increases The energy increases

Gamma rays have the highest frequency of all radiation, as a result gamma rays have the greatest energy.

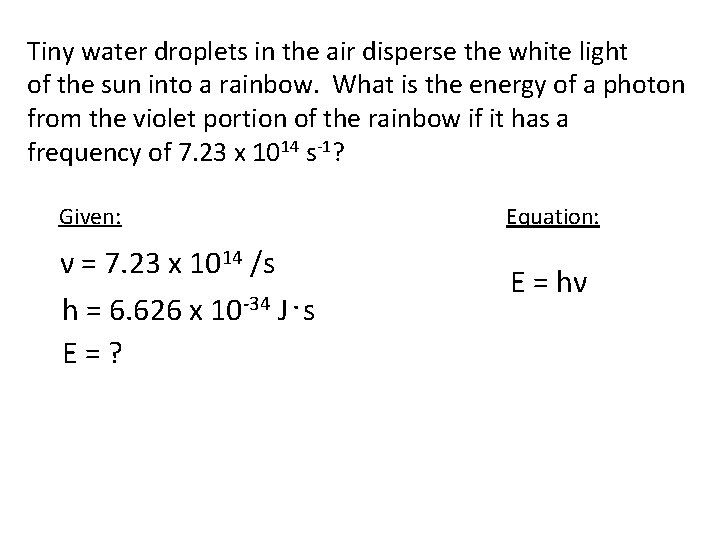
Tiny water droplets in the air disperse the white light of the sun into a rainbow. What is the energy of a photon from the violet portion of the rainbow if it has a frequency of 7. 23 x 1014 s-1? Given: ν = 7. 23 x 1014 /s h = 6. 626 x 10 -34 J. s E=? Equation: E = hν

Microwave ovens emit microwave energy with a wavelength of 12. 9 cm. What is the energy of exactly one photon of this microwave radiation? Given: Equation:

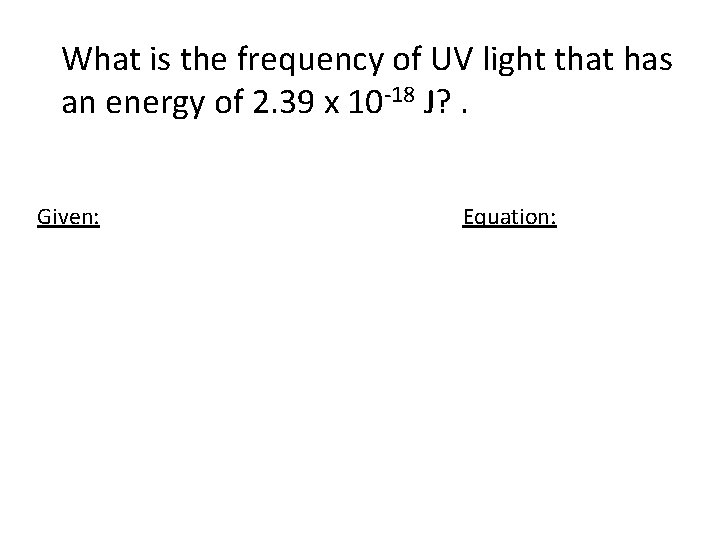
What is the frequency of UV light that has an energy of 2. 39 x 10 -18 J? . Given: Equation:

WHITEBOARD PRACTICE

4. What is the energy of radiation that has a frequency of 4. 2 X 1017 /s? Given: Equation:

5. Calculate the energy of one photon of orange light that has a wavelength of 605 nm. Given: Equation:

6. Calculate the energy and frequency of red light having a wavelength of 6. 80 x 10 -5 cm. Given: Equation:

7. A ruby laser produces red light that has a wavelength of 715 nm. Calculate its energy. Given: Equation:

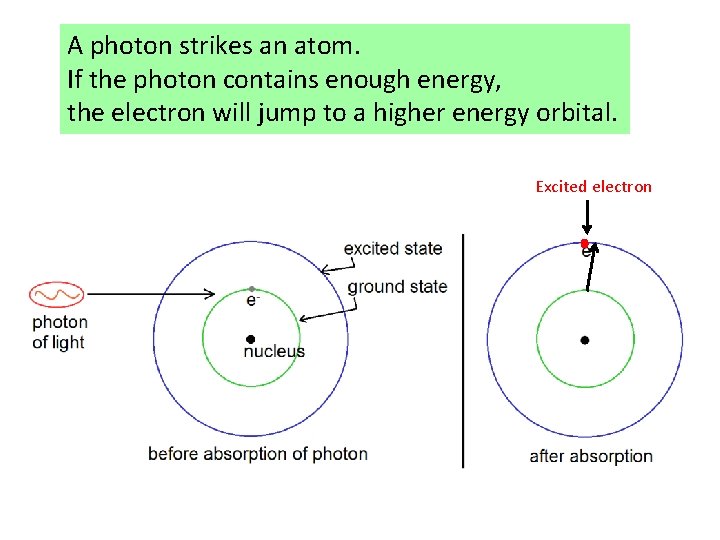
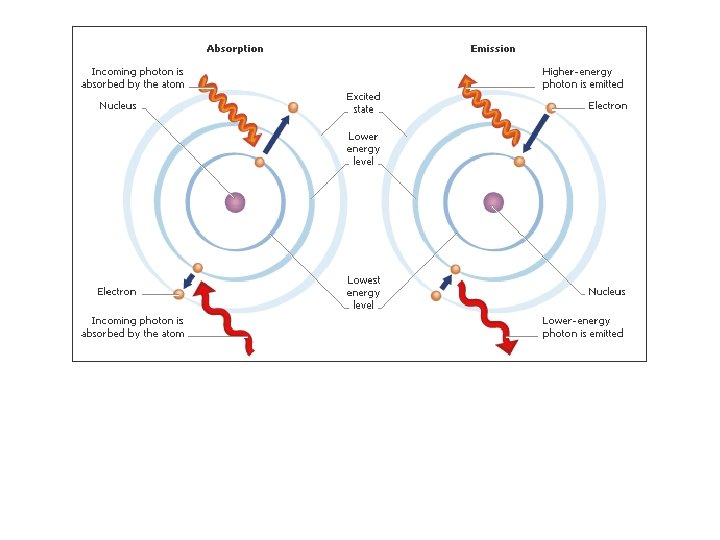
A photon strikes an atom. If the photon contains enough energy, the electron will jump to a higher energy orbital. Excited electron

If the photon doesn’t contain enough energy, the electron will remain in the ground state.


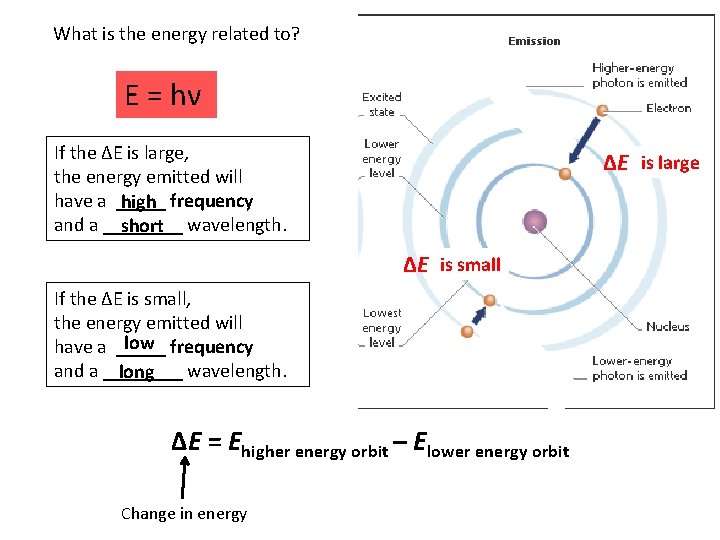
What is the energy related to? E = hν If the ∆E is large, the energy emitted will have a _____ high frequency and a ____ short wavelength. ∆E =is? large ∆E =is? small If the ∆E is small, the energy emitted will low frequency have a _____ and a ____ wavelength. long ∆E = Ehigher energy orbit – Elower energy orbit Change in energy

As electrons return to the ground state, they emit a certain frequency of radiant energy. Ephoton = hν


LAB: FLAME TEST

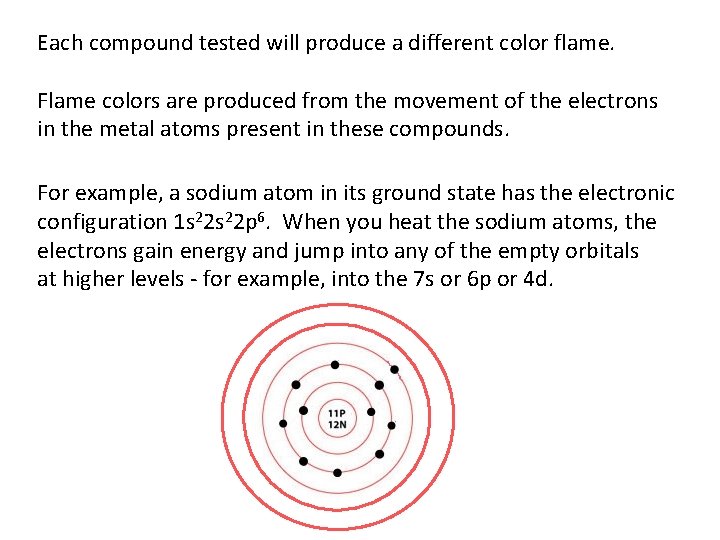
Each compound tested will produce a different color flame. Flame colors are produced from the movement of the electrons in the metal atoms present in these compounds. For example, a sodium atom in its ground state has the electronic configuration 1 s 22 p 6. When you heat the sodium atoms, the electrons gain energy and jump into any of the empty orbitals at higher levels - for example, into the 7 s or 6 p or 4 d.

Because the electrons are now at a higher and more energetically unstable level, they tend to fall back down to the ground state. As they return to the ground state, they emit photons of a specific energy. This energy corresponds to a particular wavelength of light, and so produces particular colors of light. Each metal has a unique electron configuration. The exact sizes of the possible jumps in energy terms vary from one metal to another. That means that each different metal will produce a different flame color.

Lower energy higher energy
 Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Is light quantized
Is light quantized Section 1 light and quantized energy
Section 1 light and quantized energy Christ be our light bernadette farrell
Christ be our light bernadette farrell Quantized inertia
Quantized inertia Light light light chapter 23
Light light light chapter 23 Light light light chapter 22
Light light light chapter 22 Light light light chapter 22
Light light light chapter 22 How much caffeine is too much
How much caffeine is too much To whom much is given much is expected meaning
To whom much is given much is expected meaning How much is too much plagiarism
How much is too much plagiarism ________ converts light energy into chemical energy. *
________ converts light energy into chemical energy. * Photosynthesis transforms light energy into chemical energy
Photosynthesis transforms light energy into chemical energy Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Awareness of ourselves and our environment is
Awareness of ourselves and our environment is Our awareness of ourselves and our environment.
Our awareness of ourselves and our environment. Awareness of ourselves and our environment is
Awareness of ourselves and our environment is Awareness of ourselves and our environment is
Awareness of ourselves and our environment is Chapter 1 section 1 understanding our environment answers
Chapter 1 section 1 understanding our environment answers Section 1 understanding our environment answer key
Section 1 understanding our environment answer key Cec
Cec Thinking affects our language, which then affects our:
Thinking affects our language, which then affects our: Our census our future
Our census our future Our life is what our thoughts make it
Our life is what our thoughts make it We bow our hearts we bend our knees
We bow our hearts we bend our knees Our census our future
Our census our future Our life is what our thoughts make it
Our life is what our thoughts make it Poem money madness
Poem money madness God our father christ our brother
God our father christ our brother Our future is in our hands quotes
Our future is in our hands quotes Put out that light
Put out that light Bacteria double membrane
Bacteria double membrane The bouncing off of light
The bouncing off of light The weight of glory summary
The weight of glory summary The visible light we see from our sun comes from which part
The visible light we see from our sun comes from which part What is the primary source of light on earth
What is the primary source of light on earth Christ be our light
Christ be our light Our lady of the light convent kerala
Our lady of the light convent kerala Trophic level energy transfer calculator
Trophic level energy transfer calculator How much energy do consumers obtain when they eat
How much energy do consumers obtain when they eat Materials that do not block light
Materials that do not block light Solar energy is radiant light and heat from the sun
Solar energy is radiant light and heat from the sun Heat light and sound energy
Heat light and sound energy Energy in our daily lives
Energy in our daily lives How is mechanical energy formed
How is mechanical energy formed What is the energy transformation in a light bulb
What is the energy transformation in a light bulb Light is form of energy
Light is form of energy Radiant energy
Radiant energy Form of energy that travels in straight lines
Form of energy that travels in straight lines Light energy definition
Light energy definition Sankey diagram energy efficient light bulb
Sankey diagram energy efficient light bulb Infrared light is also known as bill nye
Infrared light is also known as bill nye Igcse physics energy transfer questions
Igcse physics energy transfer questions Energy is the ability to do
Energy is the ability to do Light energy
Light energy Different wavelengths
Different wavelengths Light energy
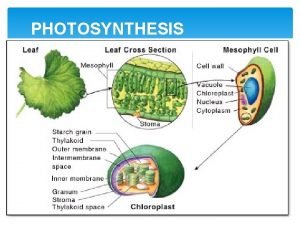
Light energy Plants gather energy with light-absorbing pigments called *
Plants gather energy with light-absorbing pigments called * Do light waves transfer energy
Do light waves transfer energy