The allowed energy levels are quantized much like



- Slides: 28


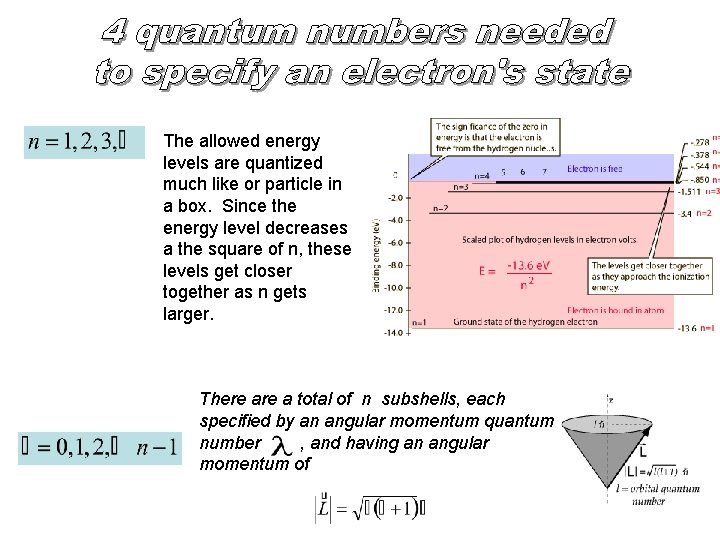
The allowed energy levels are quantized much like or particle in a box. Since the energy level decreases a the square of n, these levels get closer together as n gets larger. There a total of n subshells, each specified by an angular momentum quantum number , and having an angular momentum of

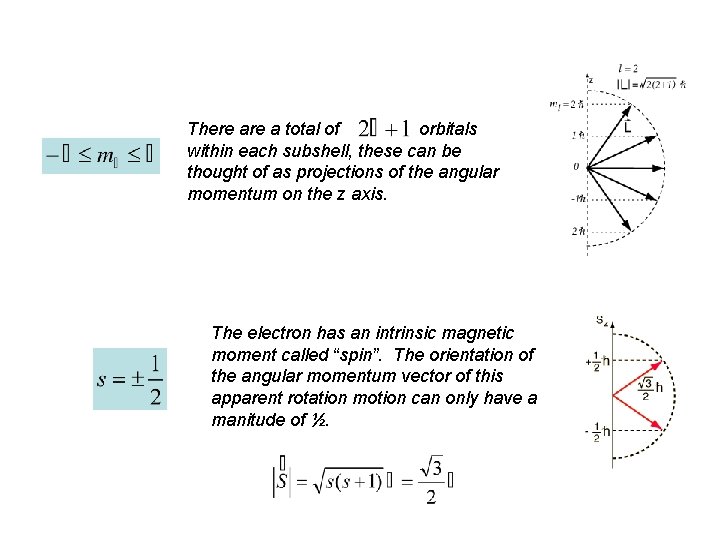
There a total of orbitals within each subshell, these can be thought of as projections of the angular momentum on the z axis. The electron has an intrinsic magnetic moment called “spin”. The orientation of the angular momentum vector of this apparent rotation motion can only have a manitude of ½.

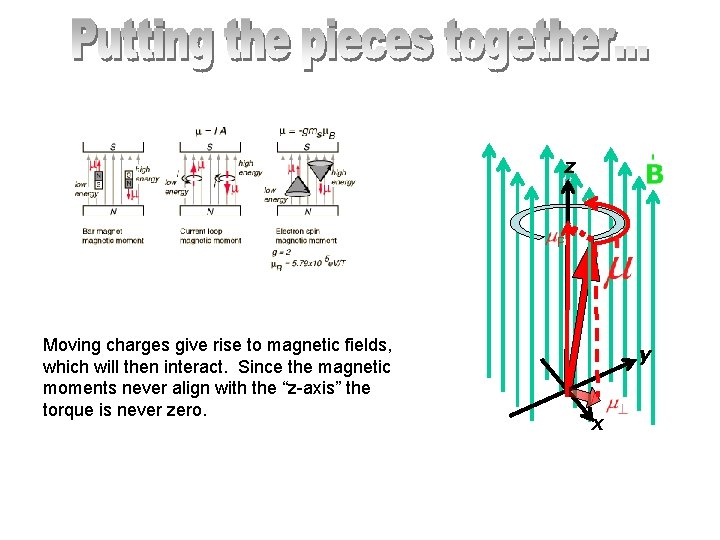
z Moving charges give rise to magnetic fields, which will then interact. Since the magnetic moments never align with the “z-axis” the torque is never zero. y x

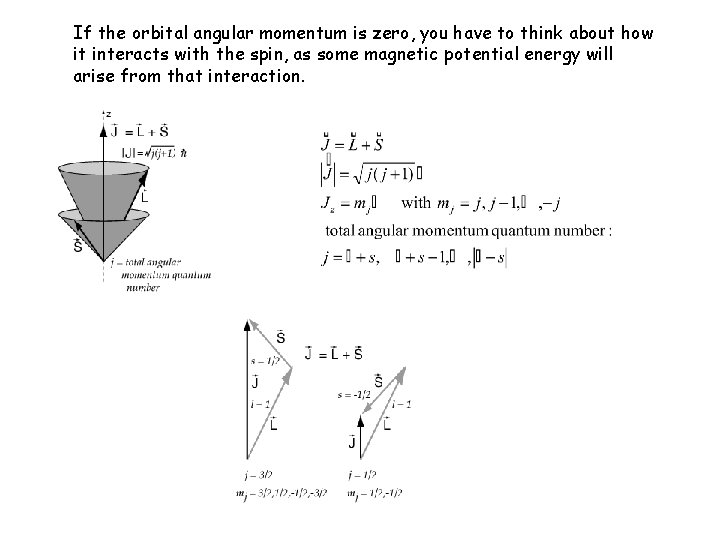
If the orbital angular momentum is zero, you have to think about how it interacts with the spin, as some magnetic potential energy will arise from that interaction.


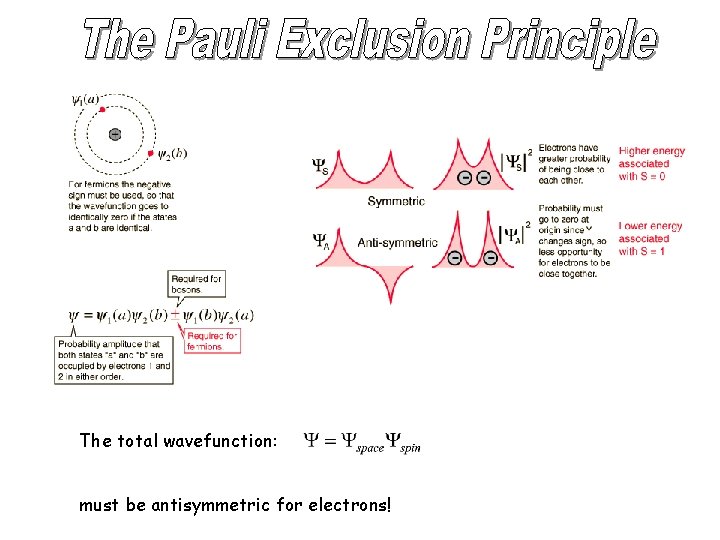
The total wavefunction: must be antisymmetric for electrons!

Chemical properties of an atom are determined by the least tightly bound electrons. Factors: • Occupancy of subshell • Energy separation between the subshell and the next higher subshell.

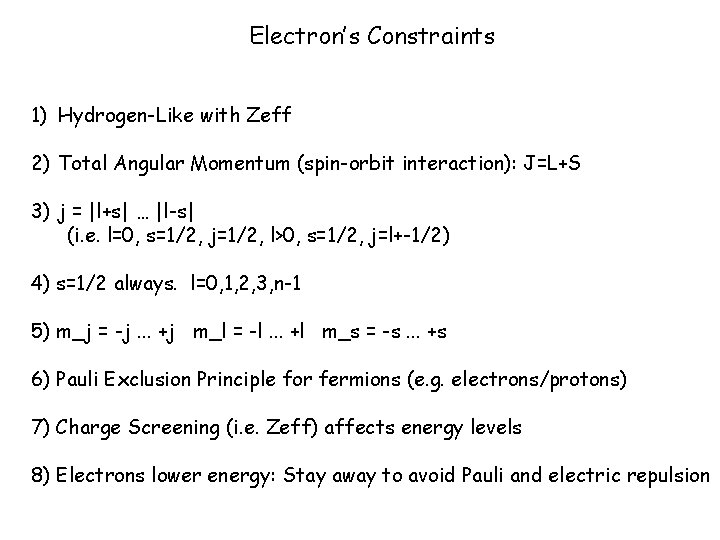
Electron’s Constraints 1) Hydrogen-Like with Zeff 2) Total Angular Momentum (spin-orbit interaction): J=L+S 3) j = |l+s| … |l-s| (i. e. l=0, s=1/2, j=1/2, l>0, s=1/2, j=l+-1/2) 4) s=1/2 always. l=0, 1, 2, 3, n-1 5) m_j = -j. . . +j m_l = -l. . . +l m_s = -s. . . +s 6) Pauli Exclusion Principle for fermions (e. g. electrons/protons) 7) Charge Screening (i. e. Zeff) affects energy levels 8) Electrons lower energy: Stay away to avoid Pauli and electric repulsion

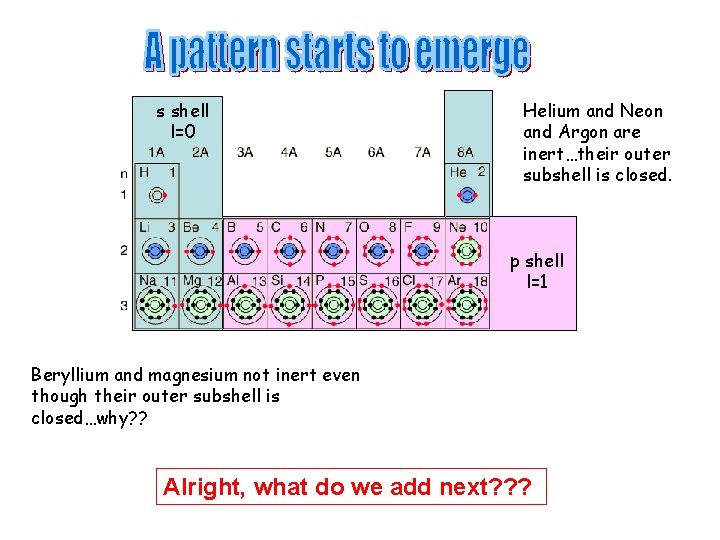
s shell l=0 Helium and Neon and Argon are inert…their outer subshell is closed. p shell l=1 Beryllium and magnesium not inert even though their outer subshell is closed…why? ? Alright, what do we add next? ? ?

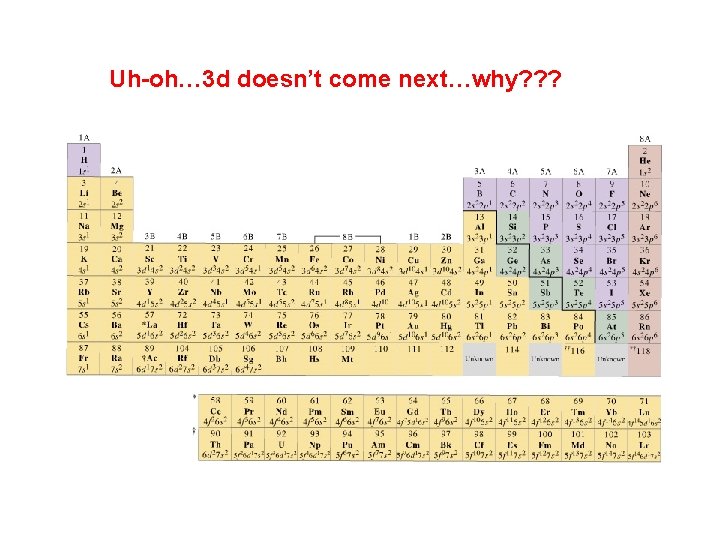
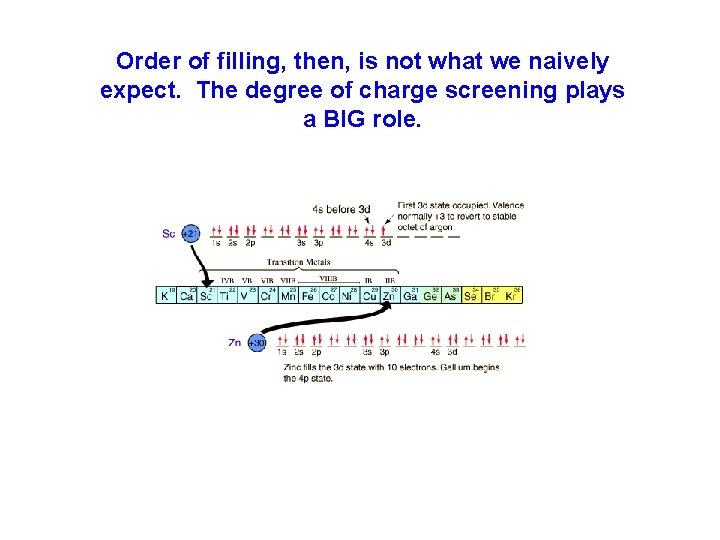
Uh-oh… 3 d doesn’t come next…why? ? ?

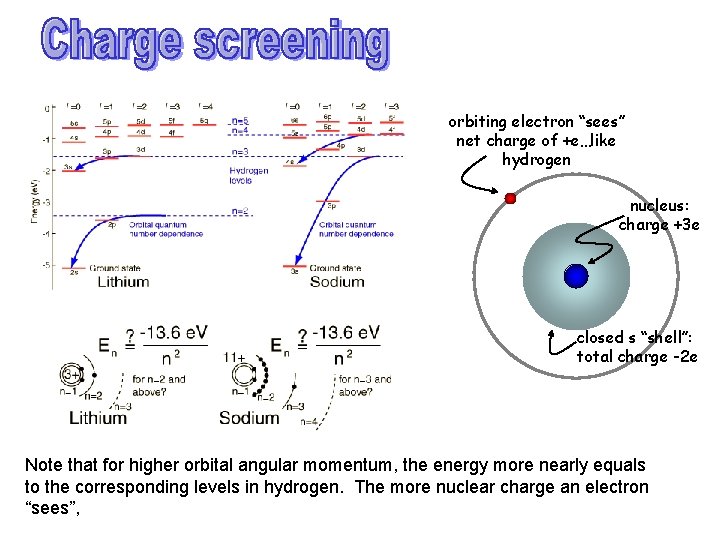
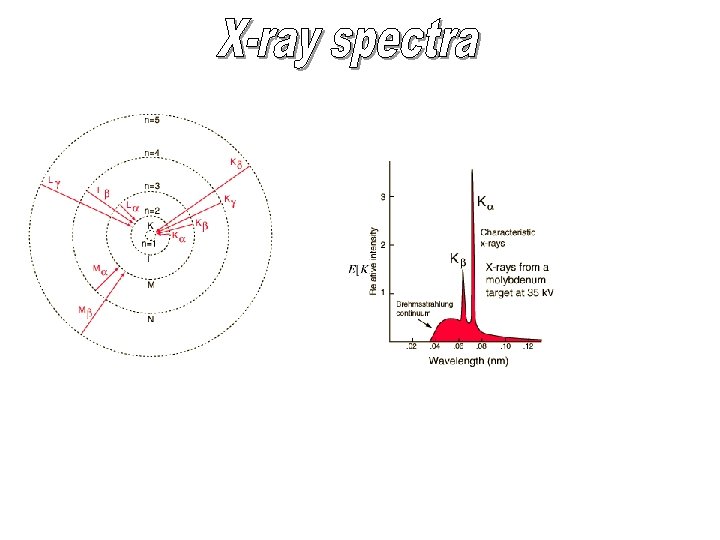
orbiting electron “sees” net charge of +e…like hydrogen nucleus: charge +3 e closed s “shell”: total charge -2 e Note that for higher orbital angular momentum, the energy more nearly equals to the corresponding levels in hydrogen. The more nuclear charge an electron “sees”,

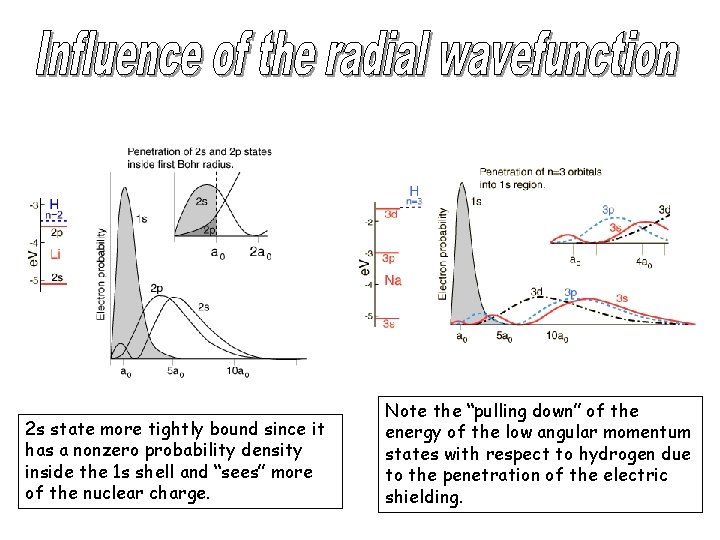
2 s state more tightly bound since it has a nonzero probability density inside the 1 s shell and “sees” more of the nuclear charge. Note the “pulling down” of the energy of the low angular momentum states with respect to hydrogen due to the penetration of the electric shielding.

Order of filling, then, is not what we naively expect. The degree of charge screening plays a BIG role.


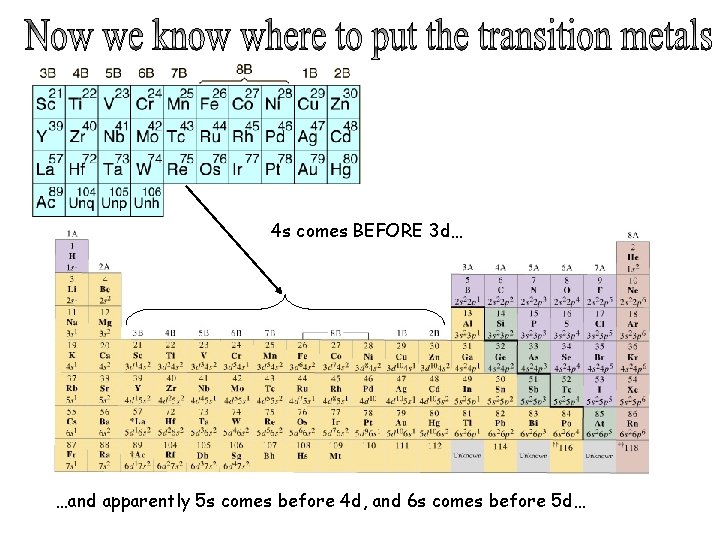
4 s comes BEFORE 3 d… …and apparently 5 s comes before 4 d, and 6 s comes before 5 d…

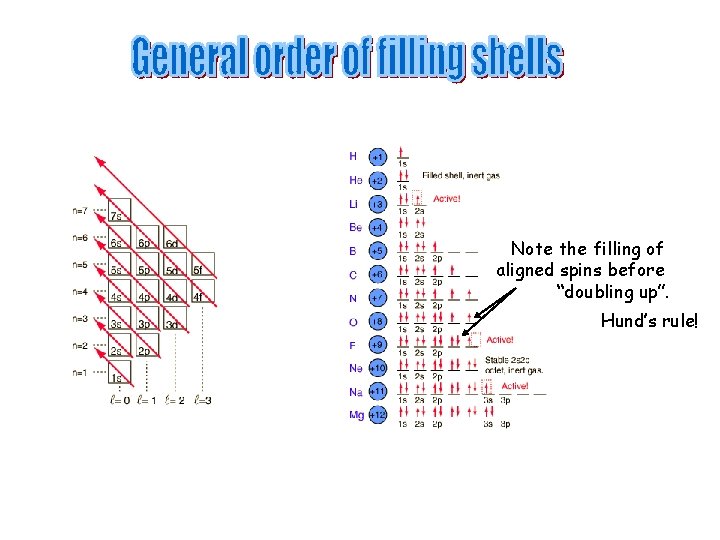
Note the filling of aligned spins before “doubling up”. Hund’s rule!

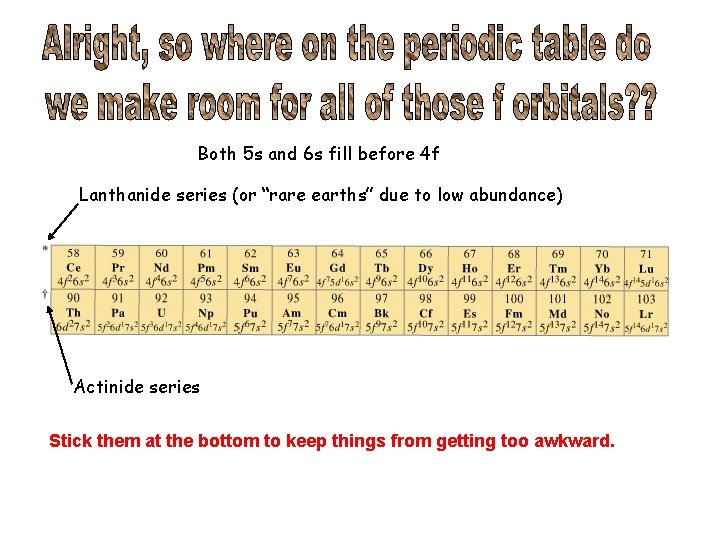
Both 5 s and 6 s fill before 4 f Lanthanide series (or “rare earths” due to low abundance) Actinide series Stick them at the bottom to keep things from getting too awkward.



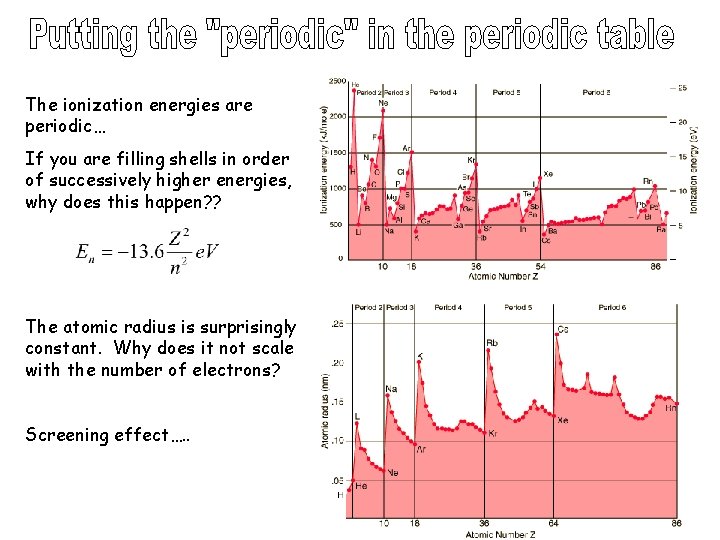
The ionization energies are periodic… If you are filling shells in order of successively higher energies, why does this happen? ? The atomic radius is surprisingly constant. Why does it not scale with the number of electrons? Screening effect…. .


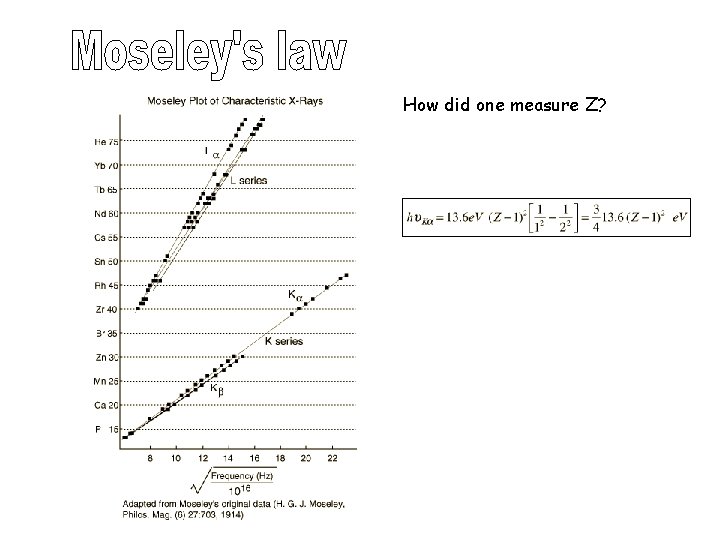
How did one measure Z?


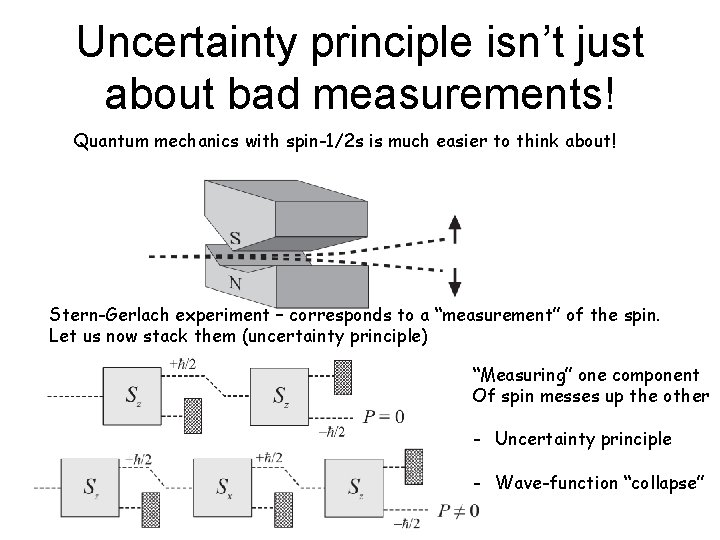
Uncertainty principle isn’t just about bad measurements! Quantum mechanics with spin-1/2 s is much easier to think about! Stern-Gerlach experiment – corresponds to a “measurement” of the spin. Let us now stack them (uncertainty principle) “Measuring” one component Of spin messes up the other - Uncertainty principle - Wave-function “collapse”

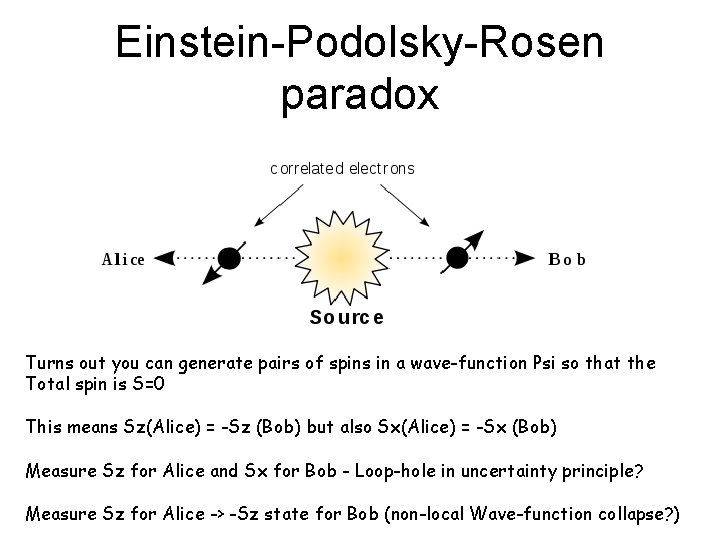
Einstein-Podolsky-Rosen paradox Turns out you can generate pairs of spins in a wave-function Psi so that the Total spin is S=0 This means Sz(Alice) = -Sz (Bob) but also Sx(Alice) = -Sx (Bob) Measure Sz for Alice and Sx for Bob - Loop-hole in uncertainty principle? Measure Sz for Alice -> -Sz state for Bob (non-local Wave-function collapse? )

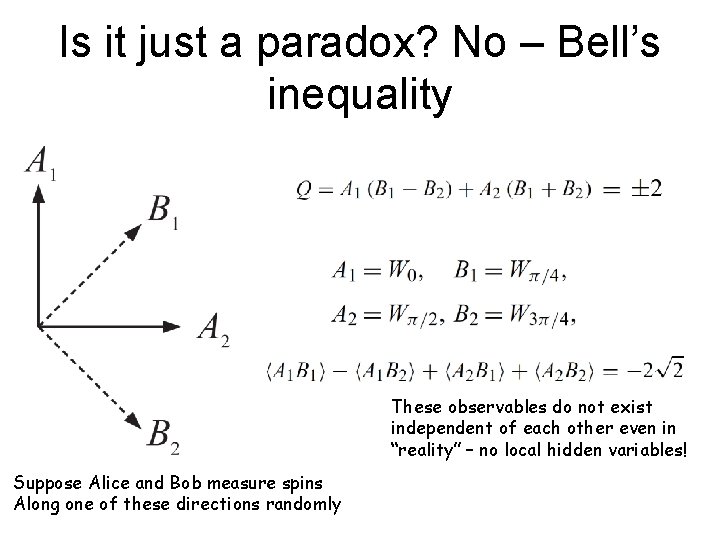
Is it just a paradox? No – Bell’s inequality These observables do not exist independent of each other even in “reality” – no local hidden variables! Suppose Alice and Bob measure spins Along one of these directions randomly

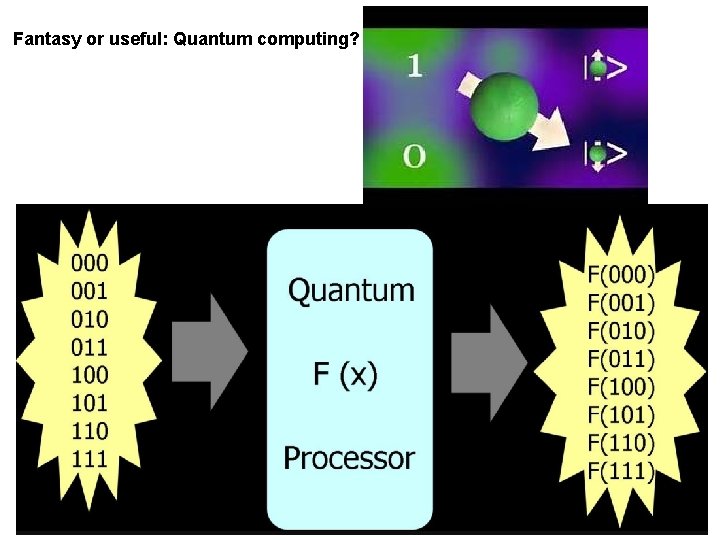
Fantasy or useful: Quantum computing?