Chapter 18 The Second Law of Thermodynamics Directions









- Slides: 25

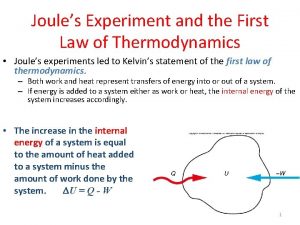
Chapter 18: The Second Law of Thermodynamics Directions of a thermodynamic process Reversible processes: Thermodynamic processes which can be reversed with a small change in conditions. Requires near equilibrium conditions (quasi-equilibrium) that take place slowly (quasi-statically). Irreversible processes generally non-equilibrium processes have a preferred direction (towards increasing “disorder, increasing entropy). 203/4 c 18: 1

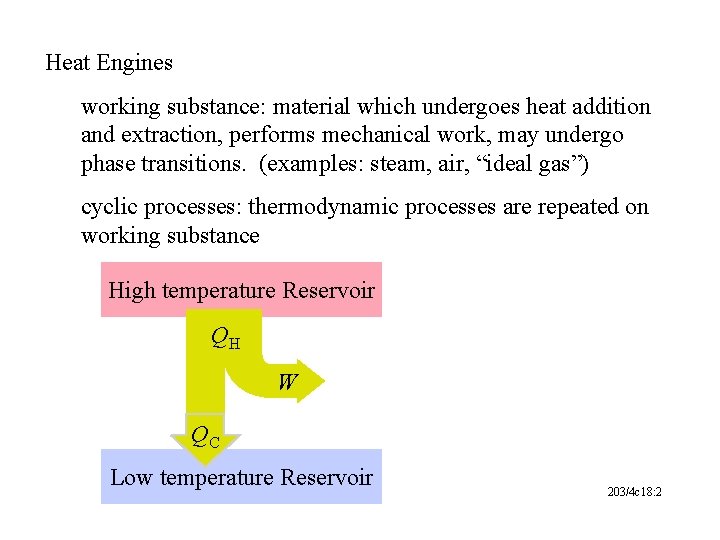
Heat Engines working substance: material which undergoes heat addition and extraction, performs mechanical work, may undergo phase transitions. (examples: steam, air, “ideal gas”) cyclic processes: thermodynamic processes are repeated on working substance High temperature Reservoir QH W QC Low temperature Reservoir 203/4 c 18: 2

High temperature Reservoir QH W QC Low temperature Reservoir Goal: Analyze several cyclic processes, and by example study the process of analyzing the application of thermodynamics. 203/4 c 18: 3

Otto Cycle Approximation of gasoline engine. c QH a-b adiabatic compression *V -> r. V p b-c constant volume heat addition *QH c-d adiabatic expansion d-a constant volume heat release a-e, e-a exhaust, intake b e W d a V QC r. V 203/4 c 18: 4

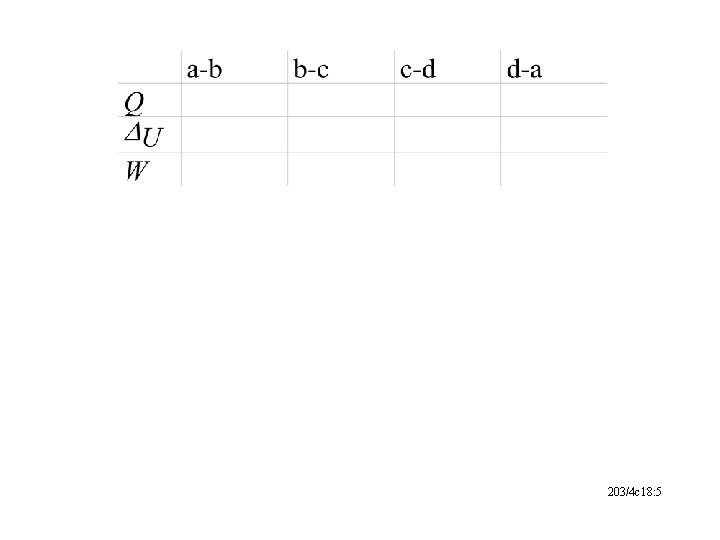
203/4 c 18: 5

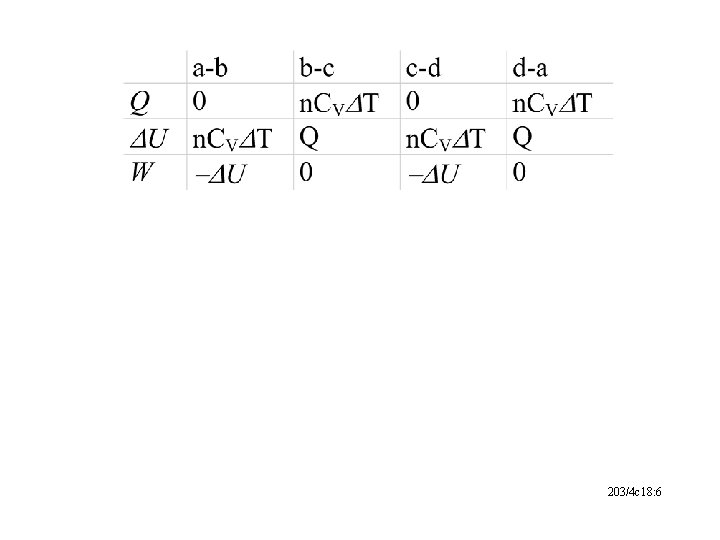
203/4 c 18: 6

203/4 c 18: 7

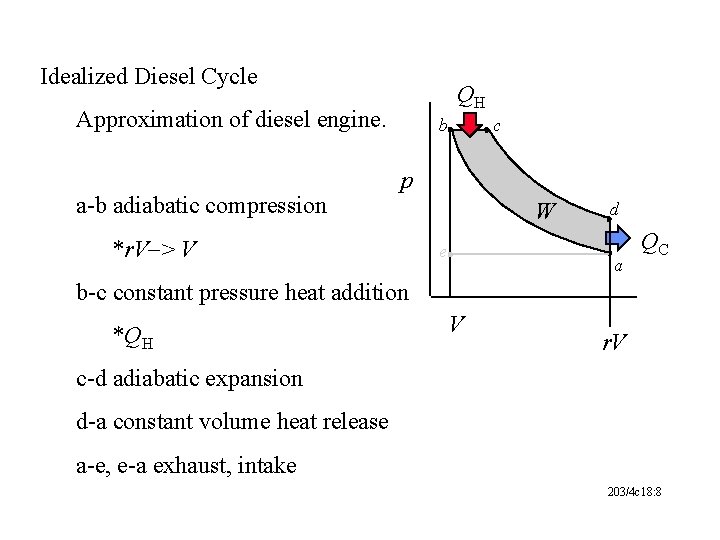
Idealized Diesel Cycle QH Approximation of diesel engine. a-b adiabatic compression b c p *r. V-> V W e d a QC b-c constant pressure heat addition *QH V r. V c-d adiabatic expansion d-a constant volume heat release a-e, e-a exhaust, intake 203/4 c 18: 8

Problem 38: Consider a Diesel cycle that starts with 1. 20 L of air at 300 K and a pressure of 1. 00 E 5 Pa. If Tc is 1200 K, derive an expression for the efficiency in terms of the compression ratio r. 203/4 c 18: 9

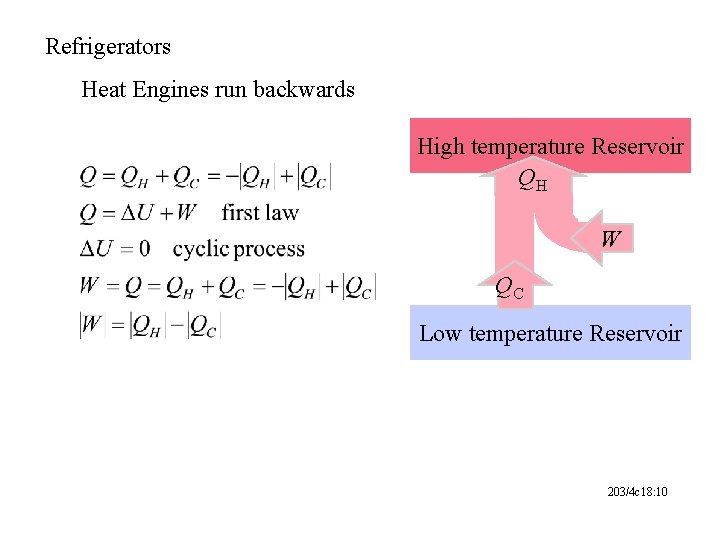
Refrigerators Heat Engines run backwards High temperature Reservoir QH W QC Low temperature Reservoir 203/4 c 18: 10

High temperature Reservoir QH W QC Low temperature Reservoir 203/4 c 18: 11

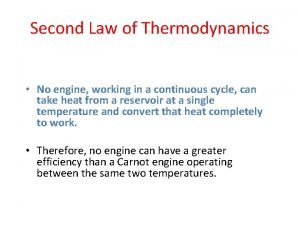
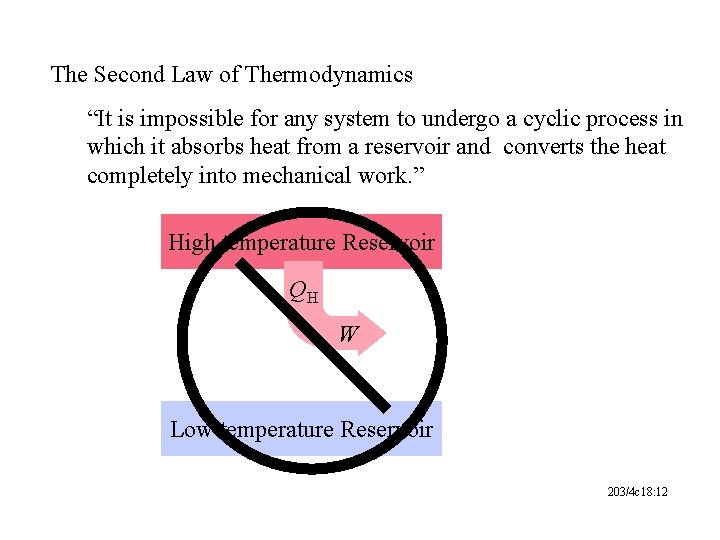
The Second Law of Thermodynamics “It is impossible for any system to undergo a cyclic process in which it absorbs heat from a reservoir and converts the heat completely into mechanical work. ” High temperature Reservoir QH W Low temperature Reservoir 203/4 c 18: 12

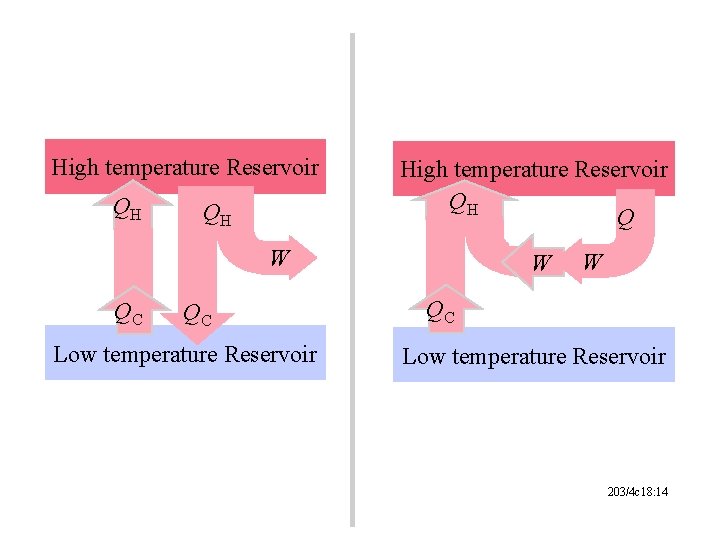
The Second Law of Thermodynamics, equivalent form for refrigeration: “It is impossible for any process to have as its sole result the transfer of heat from cooler body to a warmer one. ” High temperature Reservoir QC QC Low temperature Reservoir 203/4 c 18: 13

High temperature Reservoir QH QH High temperature Reservoir QH Q W QC QC Low temperature Reservoir W W QC Low temperature Reservoir 203/4 c 18: 14

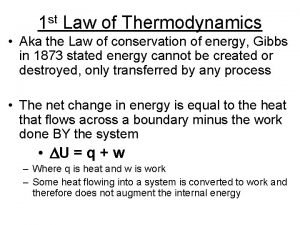
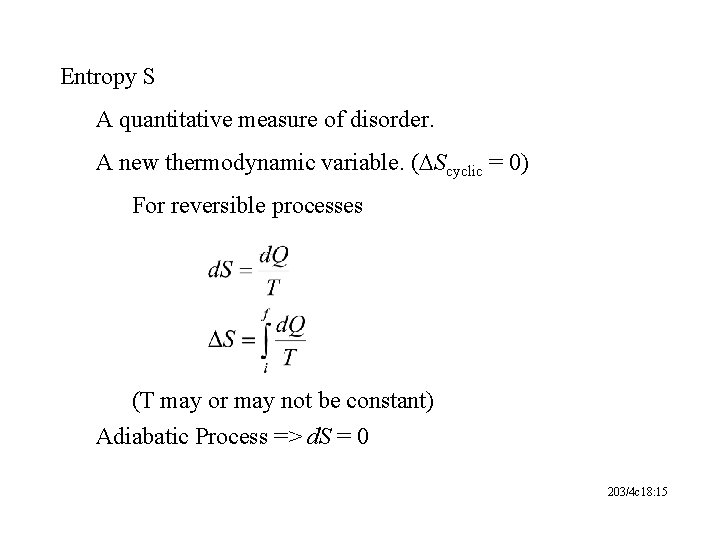
Entropy S A quantitative measure of disorder. A new thermodynamic variable. (DScyclic = 0) For reversible processes (T may or may not be constant) Adiabatic Process => d. S = 0 203/4 c 18: 15

Melt ice at 0°C: DS = ? (LF = 3. 34 x 105 J/Kg) Heat water from 0°C to 100°C : DS = ? (c = 4190 J/Kg-°C) Boil water at 100°C: DS = ? (LV = 2. 256 x 106 J/Kg) 203/4 c 18: 16

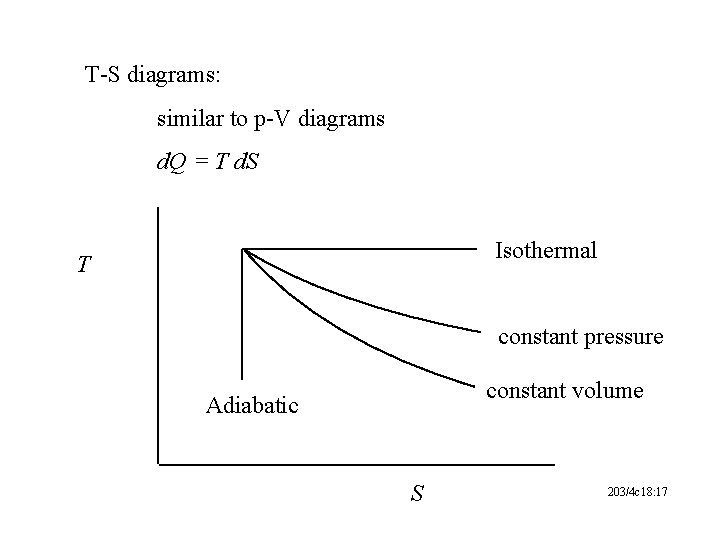
T-S diagrams: similar to p-V diagrams d. Q = T d. S Isothermal T constant pressure constant volume Adiabatic S 203/4 c 18: 17

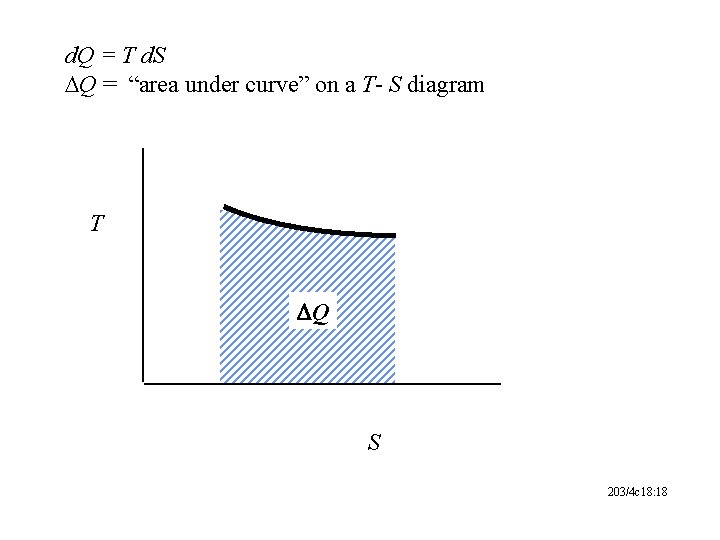
d. Q = T d. S DQ = “area under curve” on a T- S diagram T DQ S 203/4 c 18: 18

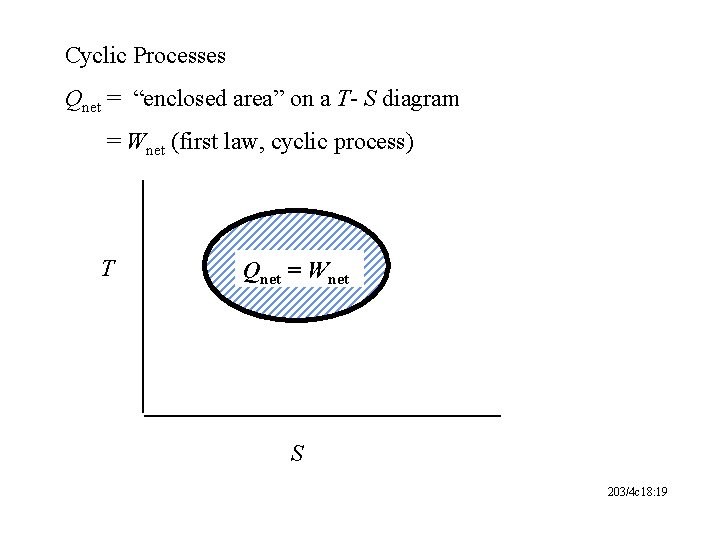
Cyclic Processes Qnet = “enclosed area” on a T- S diagram = Wnet (first law, cyclic process) T Qnet = Wnet S 203/4 c 18: 19

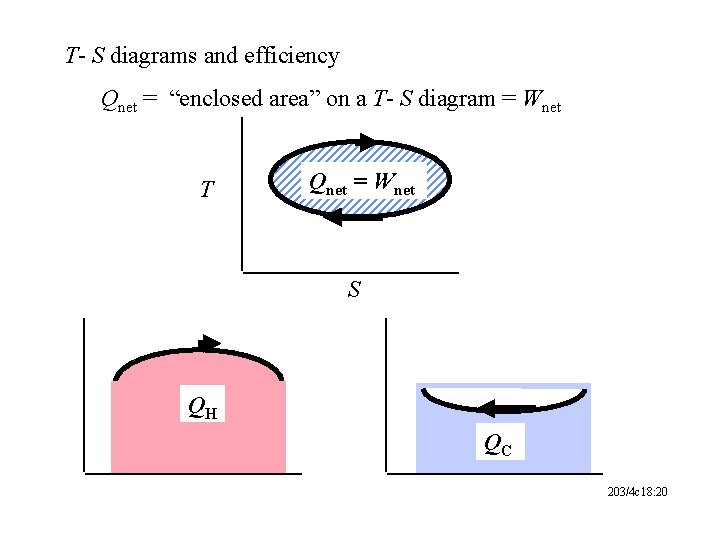
T- S diagrams and efficiency Qnet = “enclosed area” on a T- S diagram = Wnet T Qnet = Wnet S QH QC 203/4 c 18: 20

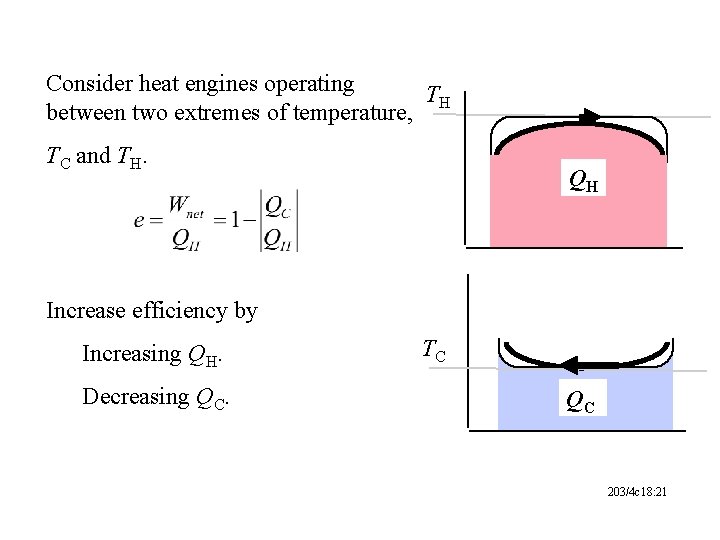
Consider heat engines operating TH between two extremes of temperature, TC and TH. QH Increase efficiency by Increasing QH. Decreasing QC. TC QC 203/4 c 18: 21

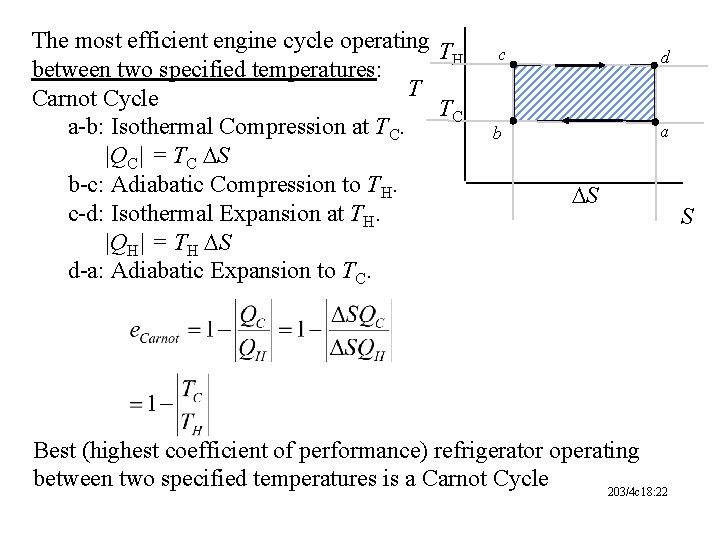
The most efficient engine cycle operating T H between two specified temperatures: T Carnot Cycle TC a-b: Isothermal Compression at TC. |QC| = TC DS b-c: Adiabatic Compression to TH. c-d: Isothermal Expansion at TH. |QH| = TH DS d-a: Adiabatic Expansion to TC. c d b a DS Best (highest coefficient of performance) refrigerator operating between two specified temperatures is a Carnot Cycle 203/4 c 18: 22 S

Example 18 -2: A Carnot engine takes 2000 J of heat from a reservoir at 500 K. does some work, and discards the remainder to a heat reservoir at 300 K. How much work does it do, how much heat is discarded, and what is thermal efficiency? 203/4 c 18: 23

Example 18 -3: . 200 moles of an ideal gas (g = 1. 40) undergoes a Carnot cycle operating between 400 K and 300 K. The initial pressure (just before the isothermal expansion) is 10. 0 E 5 Pa, and during the isothermal expansion, the volume doubles. 203/4 c 18: 24

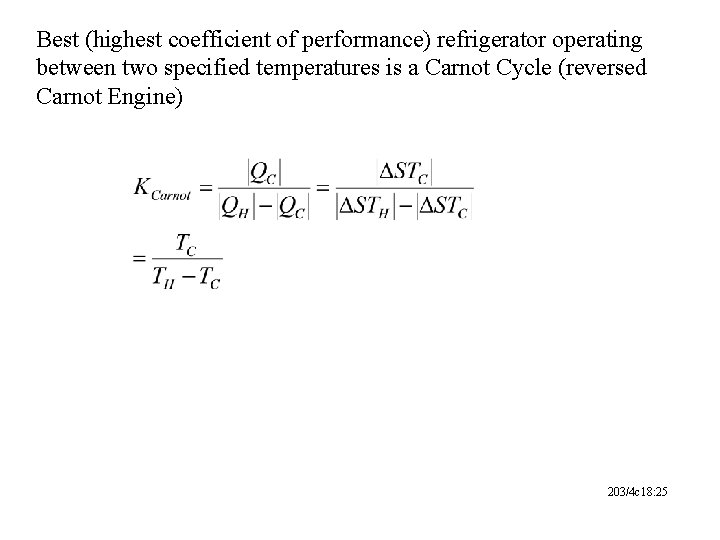
Best (highest coefficient of performance) refrigerator operating between two specified temperatures is a Carnot Cycle (reversed Carnot Engine) 203/4 c 18: 25
 Second law of thermodynamics
Second law of thermodynamics State second law of thermodynamics
State second law of thermodynamics What is second law of thermodynamics
What is second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics definition
Second law of thermodynamics definition Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law Chapter 2 lesson 3 newton's second law
Chapter 2 lesson 3 newton's second law 186 282 miles per second into meters per second
186 282 miles per second into meters per second Energy balance equation thermodynamics open system
Energy balance equation thermodynamics open system Zeroth law of thermodynamics
Zeroth law of thermodynamics Law of thermodynamics in chemistry
Law of thermodynamics in chemistry Zeroth law of thermodynamics examples
Zeroth law of thermodynamics examples First law of thermodynamics
First law of thermodynamics First law of thermodynamics cyclic process
First law of thermodynamics cyclic process The joule experiment
The joule experiment Quasi static process
Quasi static process Steady flow process in thermodynamics
Steady flow process in thermodynamics Law of thermodynamics
Law of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt First law of thermodynamics sign convention
First law of thermodynamics sign convention 1st law of thermodynamics
1st law of thermodynamics