Chapter 18 Gene Expression Prokaryotes and eukaryotes alter





- Slides: 52

Chapter 18 -Gene Expression • Prokaryotes and eukaryotes alter gene expression in response to their changing environment • In multicellular eukaryotes, gene expression regulates development and is responsible for differences in cell types • RNA molecules play many roles in regulating gene expression in eukaryotes © 2011 Pearson Education, Inc.

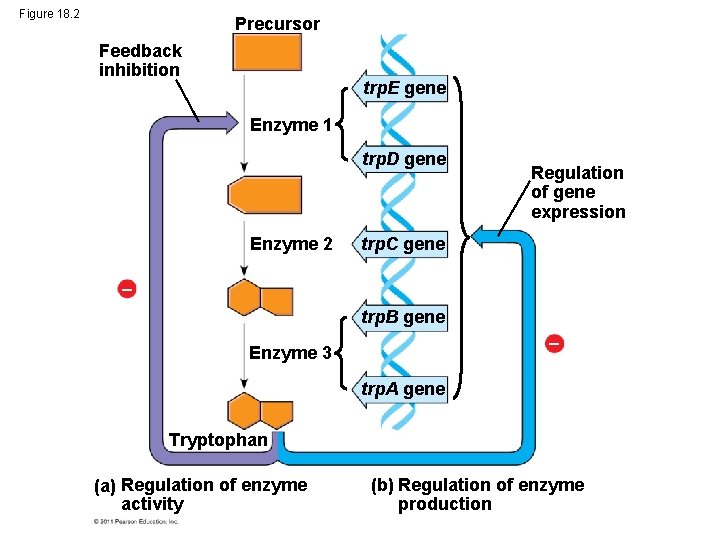
Bacteria often respond to environmental change by regulating transcription • Natural selection has favored bacteria that produce only the products needed by that cell • A cell can regulate the production of enzymes by feedback inhibition or by gene regulation • Gene expression in bacteria is controlled by the operon model © 2011 Pearson Education, Inc.

Figure 18. 2 Precursor Feedback inhibition trp. E gene Enzyme 1 trp. D gene Enzyme 2 Regulation of gene expression trp. C gene trp. B gene Enzyme 3 trp. A gene Tryptophan (a) Regulation of enzyme activity (b) Regulation of enzyme production

Operons: The Basic Concept • A cluster of functionally related genes can be under coordinated control by a single “on-off switch” • The regulatory “switch” is a segment of DNA called an operator usually positioned within the promoter • An operon is the entire stretch of DNA that includes the operator, the promoter, and the genes that they control © 2011 Pearson Education, Inc.

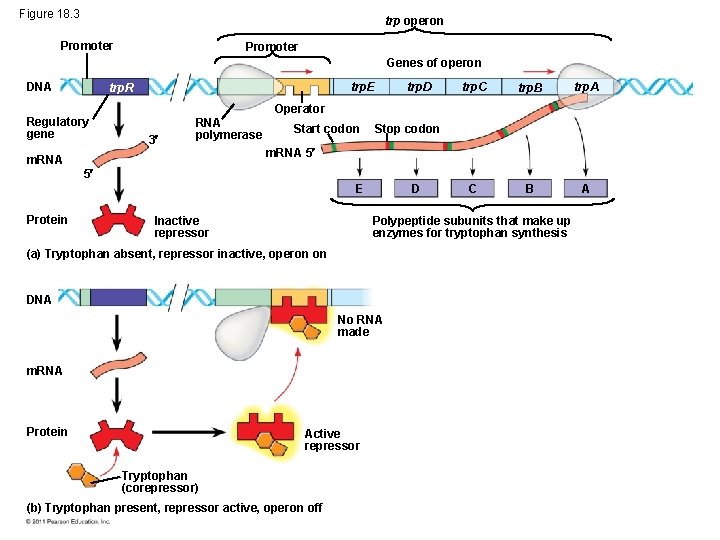
• The operon can be switched off by a repressor • The repressor binds to the operator and blocks RNA polymerase=no transcription • The repressor is the product of a separate regulatory gene • The repressor can be in an active or inactive form • A corepressor cooperates with a repressor protein to switch an operon off • For example, E. coli can synthesize the amino acid tryptophan © 2011 Pearson Education, Inc.

• By default the trp operon is on and the genes for tryptophan synthesis are transcribed • When tryptophan is present, it binds to the trp repressor protein, which turns the operon off • The repressor is active only in the presence of its corepressor tryptophan; thus the trp operon is turned off (repressed) if tryptophan levels are high © 2011 Pearson Education, Inc.

Figure 18. 3 trp operon Promoter Genes of operon DNA trp. E trp. R Regulatory gene trp. D trp. C trp. B trp. A C B A Operator 3 RNA polymerase m. RNA Start codon Stop codon m. RNA 5 5 E Protein Inactive repressor D Polypeptide subunits that make up enzymes for tryptophan synthesis (a) Tryptophan absent, repressor inactive, operon on DNA No RNA made m. RNA Protein Active repressor Tryptophan (corepressor) (b) Tryptophan present, repressor active, operon off

Repressible and Inducible Operons: Two Types of Negative Gene Regulation • A repressible operon is one that is usually on; binding of a repressor to the operator shuts off transcription • The trp operon is a repressible operon • An inducible operon is one that is usually off; a molecule called an inducer inactivates the repressor and turns on transcription © 2011 Pearson Education, Inc.

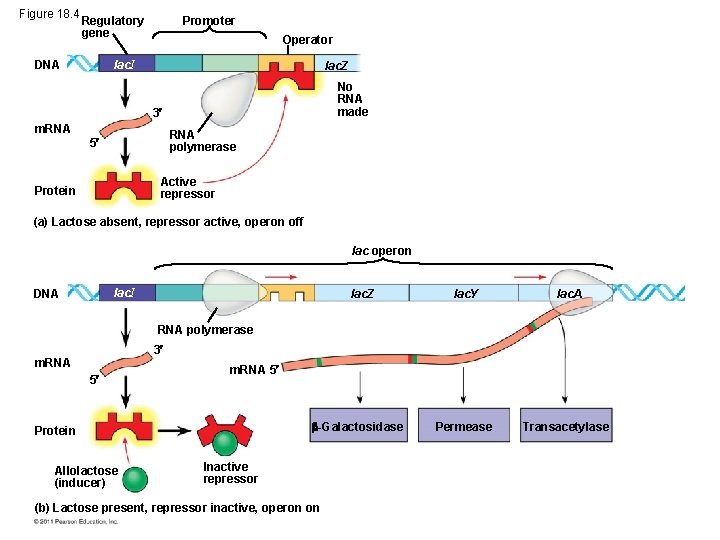
• The lac operon is an inducible operon and contains genes that code for enzymes used in the hydrolysis and metabolism of lactose • By itself, the lac repressor is active and switches the lac operon off • A molecule called an inducer inactivates the repressor to turn the lac operon on © 2011 Pearson Education, Inc.

Figure 18. 4 Regulatory gene DNA Promoter Operator lac. I lac. Z No RNA made 3 m. RNA polymerase 5 Active repressor Protein (a) Lactose absent, repressor active, operon off lac operon lac. I DNA lac. Z lac. Y lac. A Permease Transacetylase RNA polymerase 3 m. RNA 5 -Galactosidase Protein Allolactose (inducer) Inactive repressor (b) Lactose present, repressor inactive, operon on

• Inducible enzymes usually function in catabolic pathways; their synthesis is induced by a chemical signal • Repressible enzymes usually function in anabolic pathways; their synthesis is repressed by high levels of the end product • Regulation of the trp and lac operons involves negative control of genes because operons are switched off by the active form of the repressor © 2011 Pearson Education, Inc.

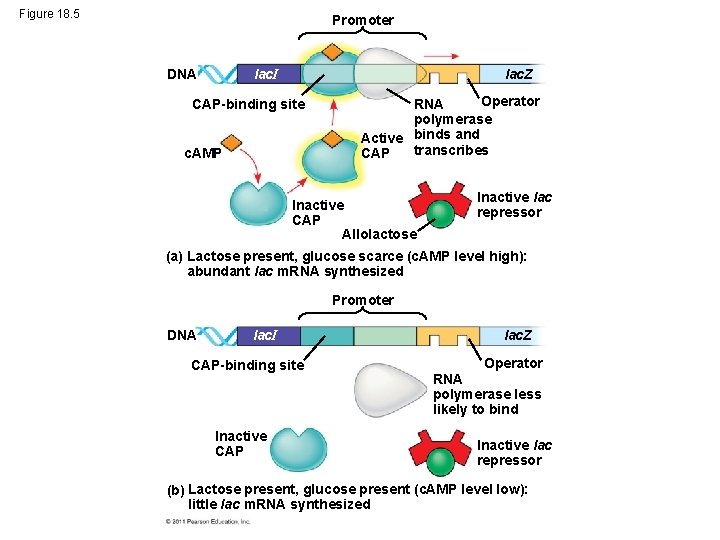
Positive Gene Regulation • Some operons are also subject to positive control through a stimulatory protein, such as catabolite activator protein (CAP), an activator of transcription • When glucose (a preferred food source of E. coli) is scarce, CAP is activated by binding with cyclic AMP (c. AMP) • Activated CAP attaches to the promoter of the lac operon and increases the affinity of RNA polymerase, thus accelerating transcription © 2011 Pearson Education, Inc.

• When glucose levels increase, CAP detaches from the lac operon, and transcription returns to a normal rate • CAP helps regulate other operons that encode enzymes used in catabolic pathways © 2011 Pearson Education, Inc.

Figure 18. 5 Promoter lac. I DNA lac. Z CAP-binding site c. AMP Operator RNA polymerase Active binds and transcribes CAP Inactive CAP Allolactose Inactive lac repressor (a) Lactose present, glucose scarce (c. AMP level high): abundant lac m. RNA synthesized Promoter DNA lac. I CAP-binding site Inactive CAP lac. Z Operator RNA polymerase less likely to bind Inactive lac repressor (b) Lactose present, glucose present (c. AMP level low): little lac m. RNA synthesized

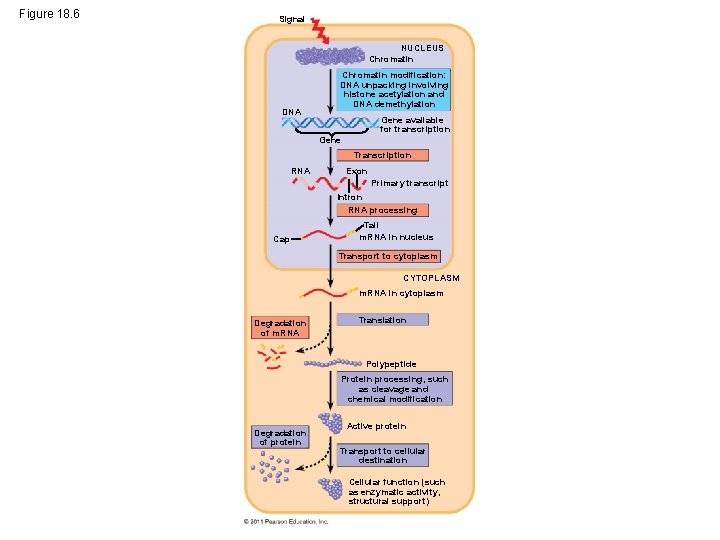
Eukaryotic gene expression • All organisms must regulate which genes are expressed at any given time • Almost all the cells in an organism are genetically identical. • Differences between cell types result from differential gene expression, the expression of different genes by cells with the same genome. • Errors in gene expression can lead to diseases including cancer. • Gene expression is regulated at many stages. © 2011 Pearson Education, Inc.

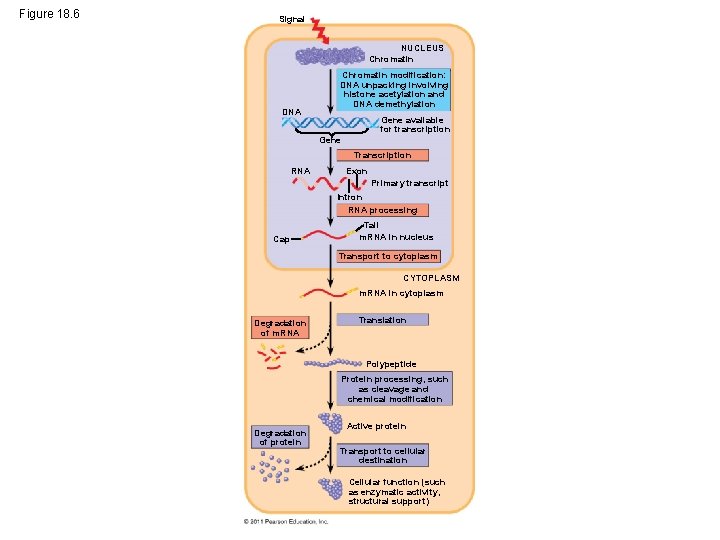
Figure 18. 6 Signal NUCLEUS Chromatin DNA Chromatin modification: DNA unpacking involving histone acetylation and DNA demethylation Gene available for transcription Gene Transcription RNA Exon Primary transcript Intron RNA processing Cap Tail m. RNA in nucleus Transport to cytoplasm CYTOPLASM m. RNA in cytoplasm Degradation of m. RNA Translation Polypeptide Protein processing, such as cleavage and chemical modification Degradation of protein Active protein Transport to cellular destination Cellular function (such as enzymatic activity, structural support)

Regulation of Chromatin Structure • Genes within highly packed heterochromatin are usually not expressed • Chemical modifications to histones and DNA of chromatin influence both chromatin structure and gene expression • The histone code hypothesis proposes that specific combinations of modifications, as well as the order in which they occur, help determine chromatin configuration and influence transcription © 2011 Pearson Education, Inc.

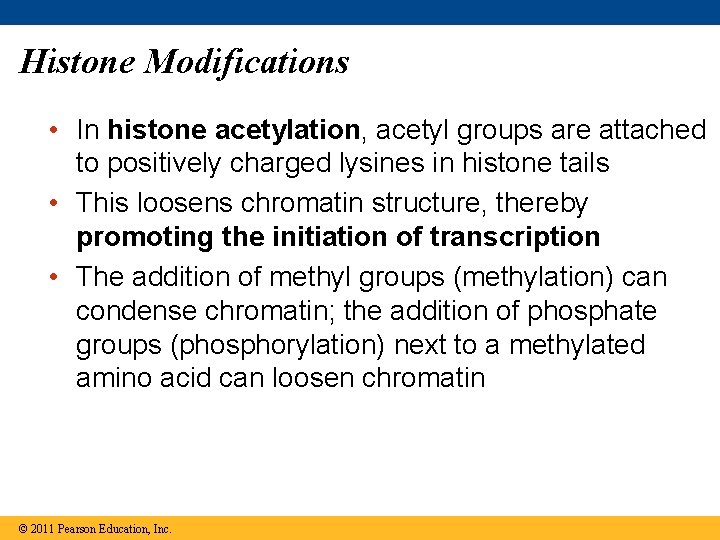
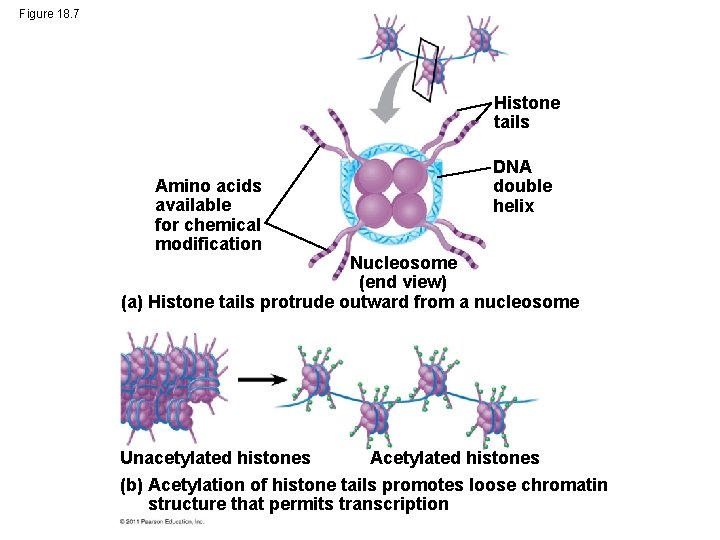
Histone Modifications • In histone acetylation, acetyl groups are attached to positively charged lysines in histone tails • This loosens chromatin structure, thereby promoting the initiation of transcription • The addition of methyl groups (methylation) can condense chromatin; the addition of phosphate groups (phosphorylation) next to a methylated amino acid can loosen chromatin © 2011 Pearson Education, Inc.

Figure 18. 7 Histone tails Amino acids available for chemical modification DNA double helix Nucleosome (end view) (a) Histone tails protrude outward from a nucleosome Acetylated histones Unacetylated histones (b) Acetylation of histone tails promotes loose chromatin structure that permits transcription

DNA Methylation • DNA methylation, the addition of methyl groups to certain bases in DNA, is associated with reduced transcription in some species • DNA methylation cause long-term inactivation of genes in cellular differentiation • In genomic imprinting, methylation regulates expression of either the maternal or paternal alleles of certain genes at the start of development © 2011 Pearson Education, Inc.

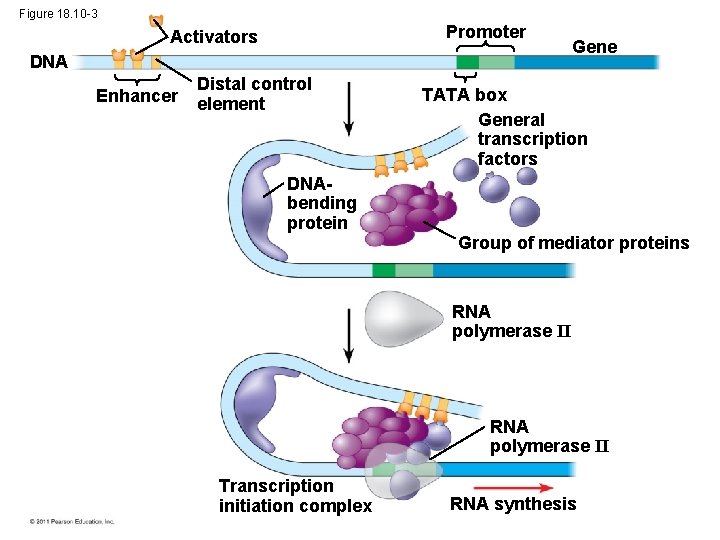
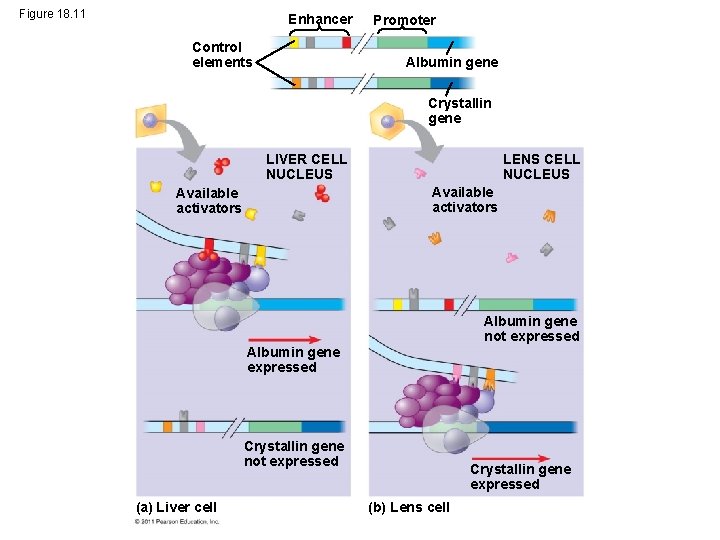
Organization of a Typical Eukaryotic Gene • Associated with most eukaryotic genes are multiple control elements, segments of noncoding DNA that serve as binding sites for transcription factors that help regulate transcription • Control elements and the transcription factors they bind are critical to the precise regulation of gene expression in different cell types © 2011 Pearson Education, Inc.

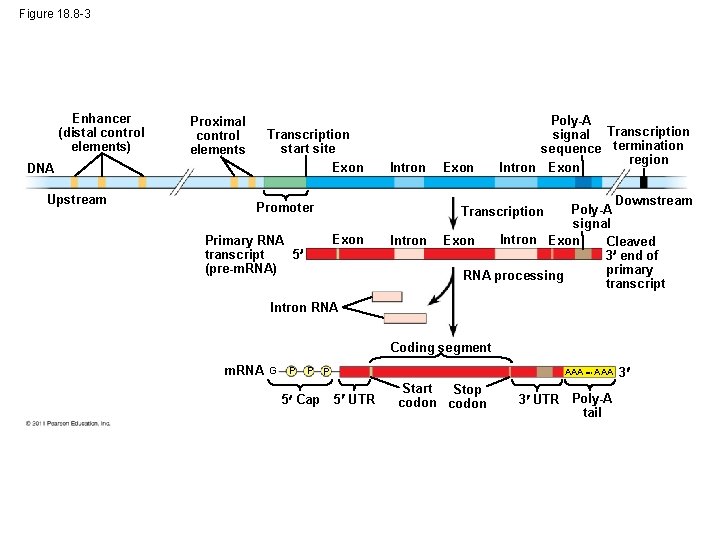
Figure 18. 8 -3 Enhancer (distal control elements) Proximal control elements Transcription start site Exon DNA Upstream Intron Exon Intron Downstream Poly-A signal Intron Exon Cleaved 3 end of primary RNA processing transcript Promoter Transcription Exon Primary RNA 5 transcript (pre-m. RNA) Poly-A signal Transcription sequence termination region Intron Exon Intron RNA Coding segment m. RNA G P P P 5 Cap AAA 5 UTR Start Stop codon 3 UTR Poly-A tail 3

The Roles of Transcription Factors • To initiate transcription, eukaryotic RNA polymerase requires the assistance of proteins called transcription factors • General transcription factors are essential for the transcription of all protein-coding genes • In eukaryotes, high levels of transcription of particular genes depend on control elements interacting with specific transcription factors © 2011 Pearson Education, Inc.

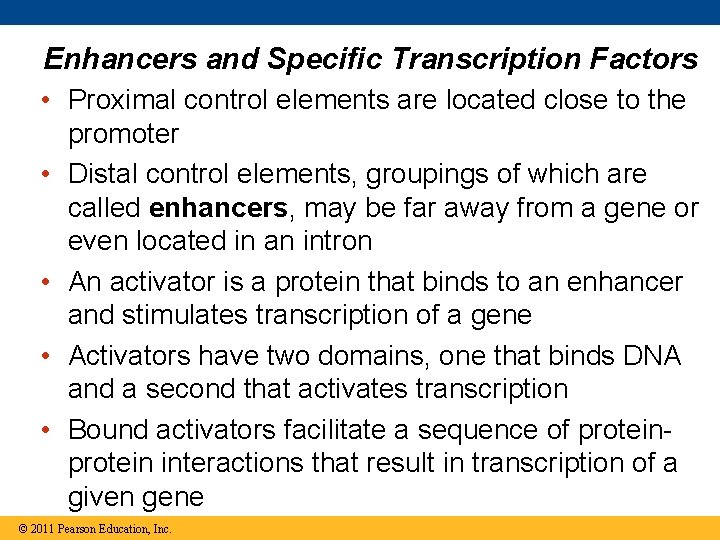
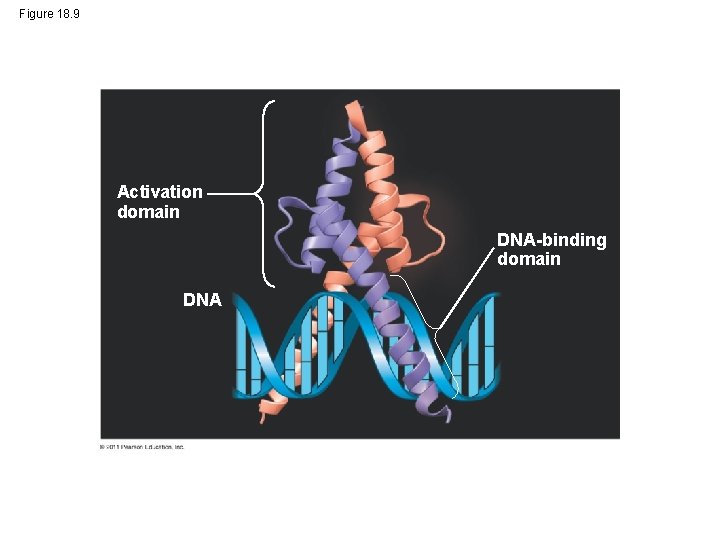
Enhancers and Specific Transcription Factors • Proximal control elements are located close to the promoter • Distal control elements, groupings of which are called enhancers, may be far away from a gene or even located in an intron • An activator is a protein that binds to an enhancer and stimulates transcription of a gene • Activators have two domains, one that binds DNA and a second that activates transcription • Bound activators facilitate a sequence of protein interactions that result in transcription of a given gene © 2011 Pearson Education, Inc.

Figure 18. 9 Activation domain DNA-binding domain DNA

Figure 18. 10 -3 Promoter Activators DNA Enhancer Distal control element Gene TATA box General transcription factors DNAbending protein Group of mediator proteins RNA polymerase II Transcription initiation complex RNA synthesis

Figure 18. 11 Enhancer Control elements Promoter Albumin gene Crystallin gene LENS CELL NUCLEUS LIVER CELL NUCLEUS Available activators Albumin gene not expressed Albumin gene expressed Crystallin gene not expressed (a) Liver cell Crystallin gene expressed (b) Lens cell

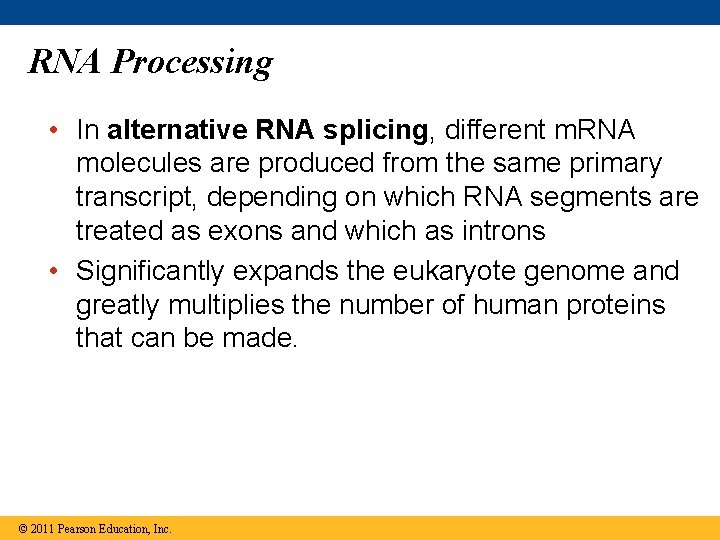
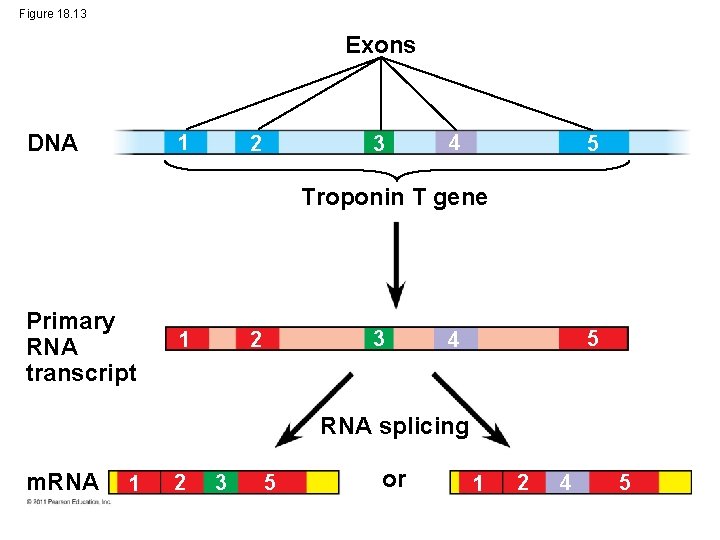
RNA Processing • In alternative RNA splicing, different m. RNA molecules are produced from the same primary transcript, depending on which RNA segments are treated as exons and which as introns • Significantly expands the eukaryote genome and greatly multiplies the number of human proteins that can be made. © 2011 Pearson Education, Inc.

Figure 18. 13 Exons DNA 1 3 2 4 5 Troponin T gene Primary RNA transcript 3 2 1 5 4 RNA splicing m. RNA 1 2 3 5 or 1 2 4 5

Mechanisms of Post-Transcriptional Regulation • Transcription alone does not account for gene expression • Regulatory mechanisms can operate at various stages after transcription • Such mechanisms allow a cell to fine-tune gene expression rapidly in response to environmental changes © 2011 Pearson Education, Inc.

Figure 18. 6 Signal NUCLEUS Chromatin DNA Chromatin modification: DNA unpacking involving histone acetylation and DNA demethylation Gene available for transcription Gene Transcription RNA Exon Primary transcript Intron RNA processing Cap Tail m. RNA in nucleus Transport to cytoplasm CYTOPLASM m. RNA in cytoplasm Degradation of m. RNA Translation Polypeptide Protein processing, such as cleavage and chemical modification Degradation of protein Active protein Transport to cellular destination Cellular function (such as enzymatic activity, structural support)

1. m. RNA Degradation • The life span of m. RNA molecules in the cytoplasm is a key to determining protein synthesis • Eukaryotic m. RNA is more long lived than prokaryotic m. RNA • Nucleotide sequences that influence the lifespan of m. RNA in eukaryotes reside in the untranslated region (UTR) at the 3 end of the molecule © 2011 Pearson Education, Inc.

2. Initiation of Translation • The initiation of translation of selected m. RNAs can be blocked by regulatory proteins that bind to sequences or structures of the m. RNA • Alternatively, translation of all m. RNAs in a cell may be regulated simultaneously • For example, translation initiation factors are simultaneously activated in an egg following fertilization © 2011 Pearson Education, Inc.

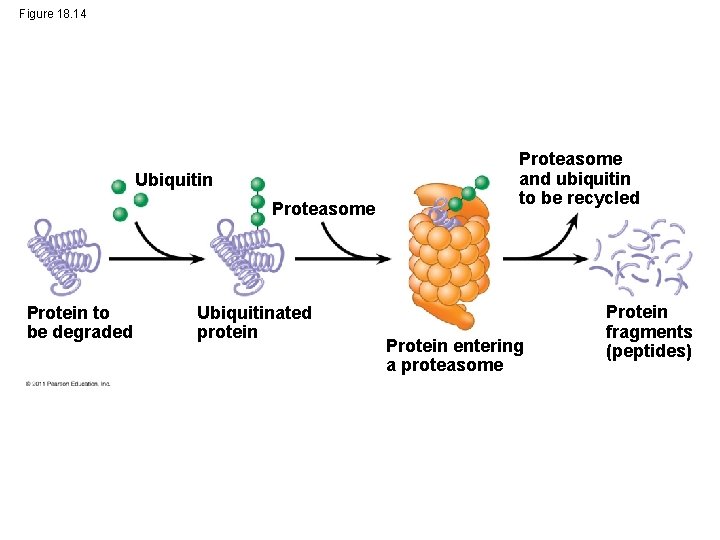
3 -4. Protein Processing and Degradation • After translation, various types of protein processing, including cleavage and the addition of chemical groups, are subject to control • Proteasomes are giant protein complexes that bind protein molecules and degrade them © 2011 Pearson Education, Inc.

Figure 18. 14 Ubiquitin Proteasome Protein to be degraded Ubiquitinated protein Proteasome and ubiquitin to be recycled Protein entering a proteasome Protein fragments (peptides)

Concept 18. 3: Noncoding RNAs play multiple roles in controlling gene expression • Only a small fraction of DNA codes for proteins, and a very small fraction of the non-protein-coding DNA consists of genes for RNA such as r. RNA and t. RNA • A significant amount of the genome may be transcribed into noncoding RNAs (nc. RNAs) • Noncoding RNAs regulate gene expression at two points: m. RNA translation and chromatin configuration © 2011 Pearson Education, Inc.

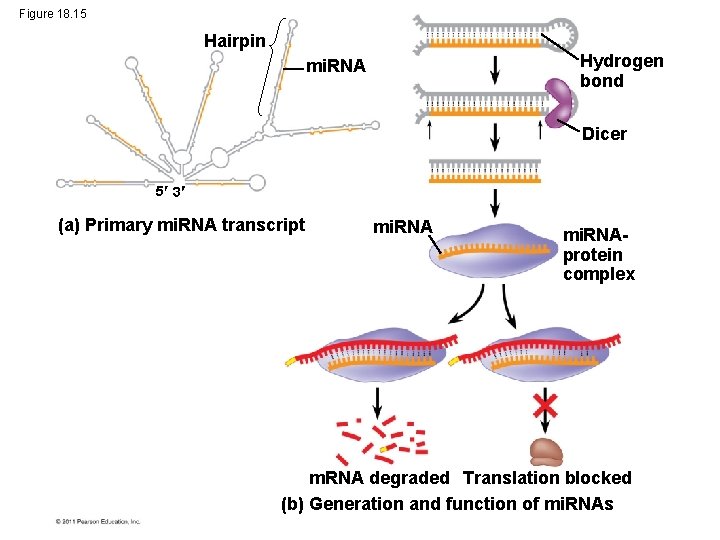
Effects on m. RNAs by Micro. RNAs and Small Interfering RNAs • Micro. RNAs (mi. RNAs) are small single-stranded RNA molecules that can bind to m. RNA • These can degrade m. RNA or block its translation © 2011 Pearson Education, Inc.

Figure 18. 15 Hairpin Hydrogen bond mi. RNA Dicer 5 3 (a) Primary mi. RNA transcript mi. RNAprotein complex m. RNA degraded Translation blocked (b) Generation and function of mi. RNAs

• The phenomenon of inhibition of gene expression by RNA molecules is called RNA interference (RNAi) • RNAi is caused by small interfering RNAs (si. RNAs) • si. RNAs and mi. RNAs are similar but form from different RNA precursors © 2011 Pearson Education, Inc.

Chromatin Remodeling and Effects on Transcription by nc. RNAs • In some yeasts si. RNAs play a role in heterochromatin formation and can block large regions of the chromosome • Small nc. RNAs called piwi-associated RNAs (pi. RNAs) induce heterochromatin, blocking the expression of parasitic DNA elements in the genome, known as transposons • RNA-based mechanisms may also block transcription of single genes © 2011 Pearson Education, Inc.

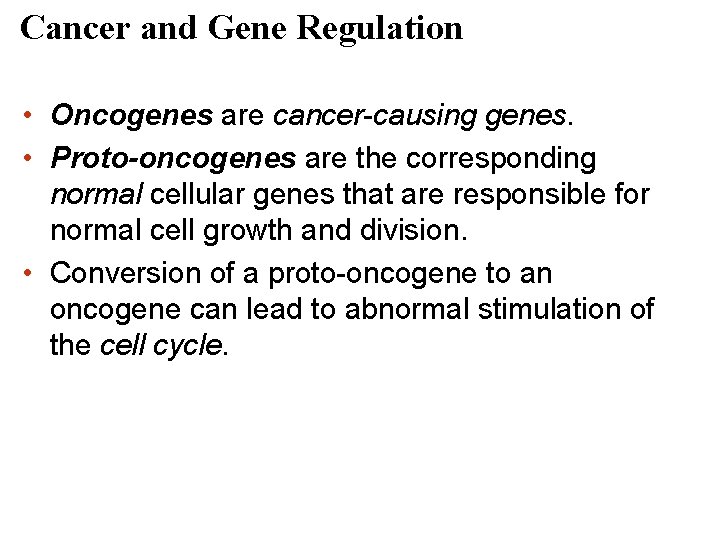
Cancer and Gene Regulation • Oncogenes are cancer-causing genes. • Proto-oncogenes are the corresponding normal cellular genes that are responsible for normal cell growth and division. • Conversion of a proto-oncogene to an oncogene can lead to abnormal stimulation of the cell cycle.

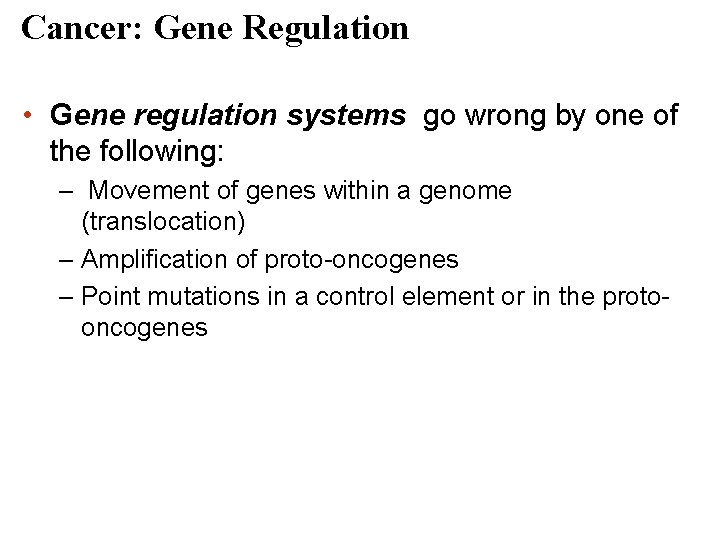
Cancer: Gene Regulation • Gene regulation systems go wrong by one of the following: – Movement of genes within a genome (translocation) – Amplification of proto-oncogenes – Point mutations in a control element or in the protooncogenes

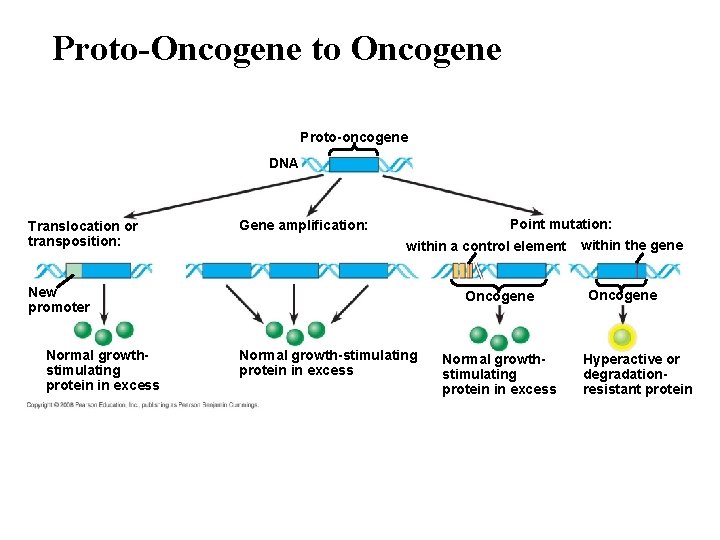
Proto-Oncogene to Oncogene Proto-oncogene DNA Translocation or transposition: Point mutation: Gene amplification: within a control element New promoter Normal growthstimulating protein in excess Oncogene Normal growth-stimulating protein in excess Normal growthstimulating protein in excess within the gene Oncogene Hyperactive or degradationresistant protein

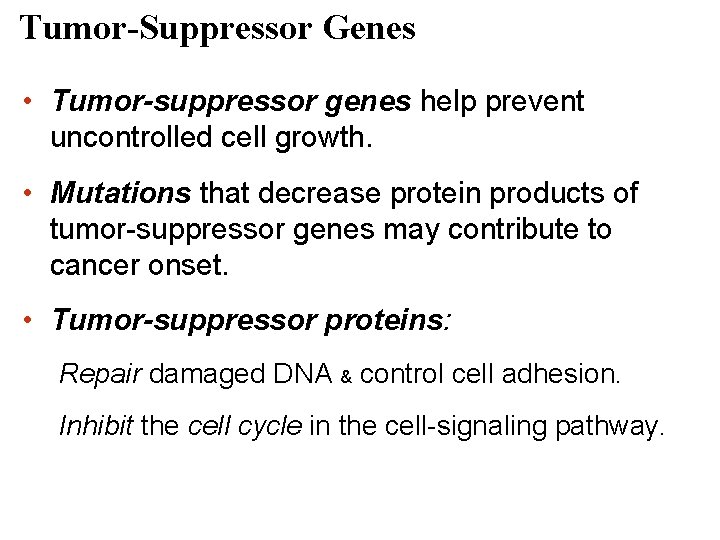
Tumor-Suppressor Genes • Tumor-suppressor genes help prevent uncontrolled cell growth. • Mutations that decrease protein products of tumor-suppressor genes may contribute to cancer onset. • Tumor-suppressor proteins: Repair damaged DNA & control cell adhesion. Inhibit the cell cycle in the cell-signaling pathway.

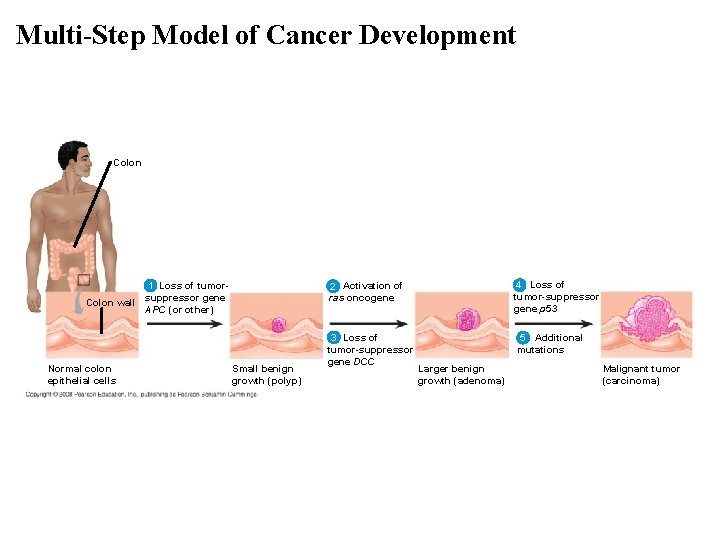
The Multistep Model of Cancer Development • Multiple mutations are generally needed for fullfledged cancer; thus the incidence increases with age. • At the DNA level, a cancerous cell is usually characterized by at least one active oncogene and the mutation of several tumor-suppressor genes.

Multi-Step Model of Cancer Development Colon EFFECTS OF MUTATIONS 1 Loss of tumorsuppressor gene Colon wall APC (or other) Normal colon epithelial cells Small benign growth (polyp) 2 Activation of ras oncogene 4 Loss of tumor-suppressor gene p 53 3 Loss of tumor-suppressor gene DCC 5 Additional mutations Larger benign growth (adenoma) Malignant tumor (carcinoma)

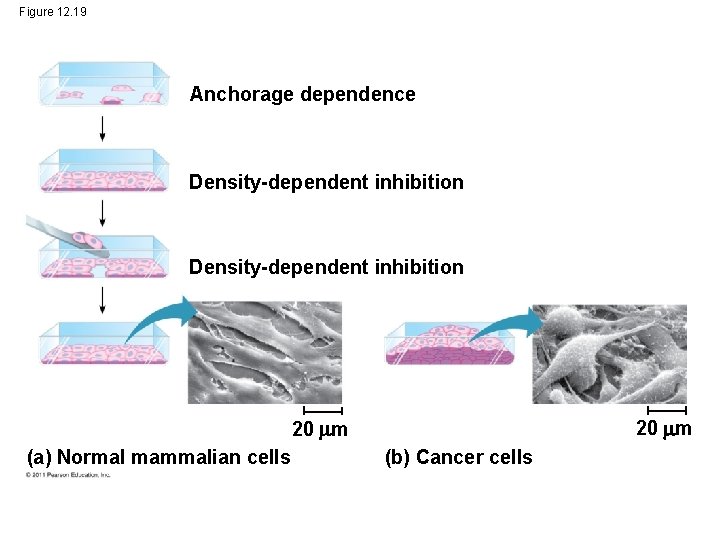
Cancer: Signaling • A clear example of external signals is densitydependent inhibition, in which crowded cells stop dividing • Most animal cells also exhibit anchorage dependence, in which they must be attached to a substratum in order to divide • Cancer cells exhibit neither density-dependent inhibition nor anchorage dependence © 2011 Pearson Education, Inc.

Figure 12. 19 Anchorage dependence Density-dependent inhibition 20 m (a) Normal mammalian cells (b) Cancer cells

Cancer Cells: Cell cycle control • Cancer cells do not respond normally to the body’s control mechanisms • Cancer cells may not need growth factors to grow and divide – They make their own growth factor – They may convey a growth factor’s signal without the presence of the growth factor – They may have an abnormal cell cycle control system © 2011 Pearson Education, Inc.

• A normal cell is converted to a cancerous cell by a process called transformation • Cancer cells that are not eliminated by the immune system, form tumors, masses of abnormal cells within otherwise normal tissue • If abnormal cells remain at the original site, the lump is called a benign tumor • Malignant tumors invade surrounding tissues and can metastasize, exporting cancer cells to other parts of the body, where they may form additional tumors © 2011 Pearson Education, Inc.

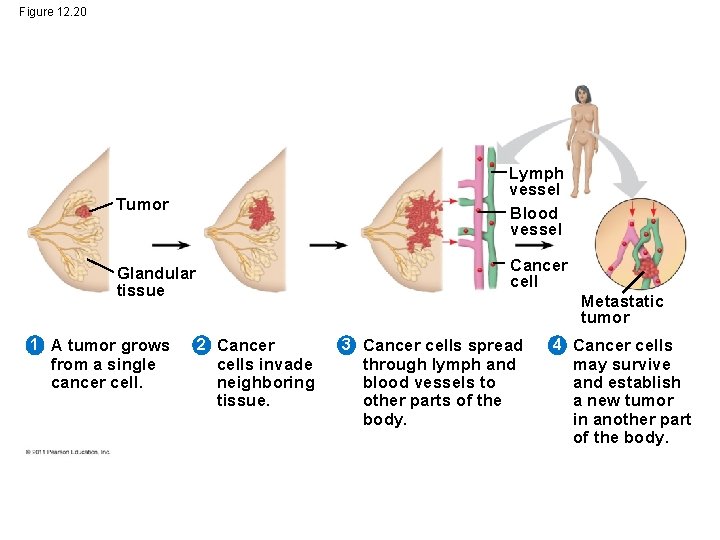
Figure 12. 20 Tumor Lymph vessel Blood vessel Glandular tissue Cancer cell 1 A tumor grows from a single cancer cell. Metastatic tumor 2 Cancer cells invade neighboring tissue. 3 Cancer cells spread through lymph and blood vessels to other parts of the body. 4 Cancer cells may survive and establish a new tumor in another part of the body.

• Recent advances in understanding the cell cycle and cell cycle signaling have led to advances in cancer treatment © 2011 Pearson Education, Inc.