Chapter 11 Chemical Reactions Writing Chemical Equations Word



























- Slides: 41

Chapter 11 Chemical Reactions

Writing Chemical Equations • Word Equations – Names of reactants on the left of an arrow separated by plus signs – Names of products to the right of the arrow separated by plus signs – Ex: flour + water + yeast + salt bread - Ex: carbon + oxygen carbon dioxide

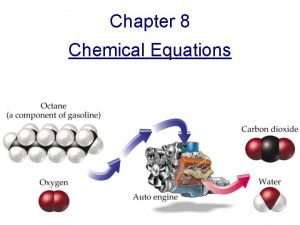
Word equation • Methane + Oxygen Carbon dioxide + Water

Chemical Reaction • When one or more substances (reactants) are changed into one or more new substances (products), a CHEMICAL REACTION has occurred • represented as a chemical equation Reactants products

If you mix two things together how do you know a chemical reaction has occurred?

Evidence a reaction has occurred: • • • Color change Gas released Precipitate formed Temperature change (endo or exothermic) Odor produced Smoke Light Flames p. H change Flammable to nonflammable or vice versa

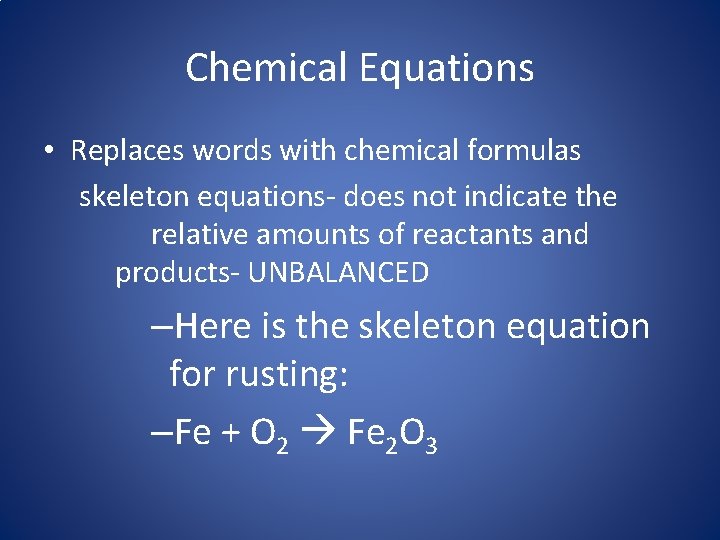
Chemical Equations • Replaces words with chemical formulas skeleton equations- does not indicate the relative amounts of reactants and products- UNBALANCED –Here is the skeleton equation for rusting: –Fe + O 2 Fe 2 O 3


Symbols for reactions • • • + separates 2 reactants or 2 products (s) solid (l) liquid (g) gas (aq) aqueous – solid dissolved in a liquid usually water (c) crystal precipitate produced gas produced Δ energy is needed yields or produces- separates reactants from products

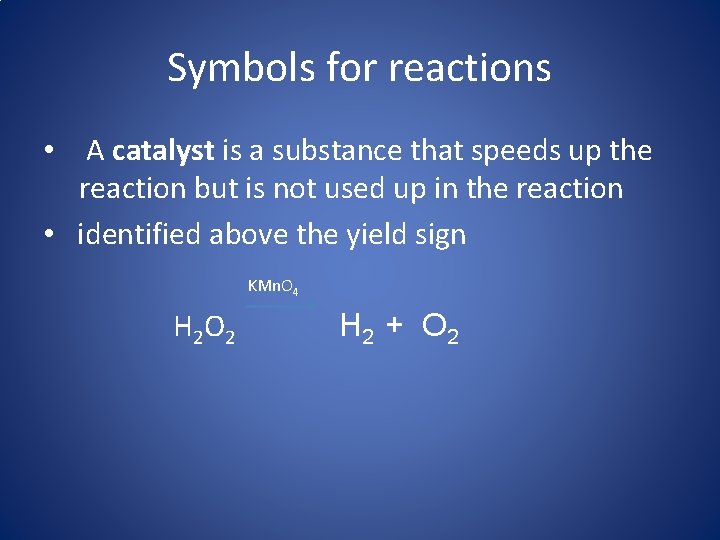
Symbols for reactions • A catalyst is a substance that speeds up the reaction but is not used up in the reaction • identified above the yield sign KMn. O 4 H 2 O 2 H 2 + O 2

Law of Conservation of Mass • Demo match • Silver nitrate

Law of Conservation of Mass • Mass is never created or destroyed-ALL must be conserved and accounted for during a chemical reaction • The same number of atoms of reactant elements must equal the atoms of product elements • By balancing equations, we satisfy the Law of Conservation of Mass

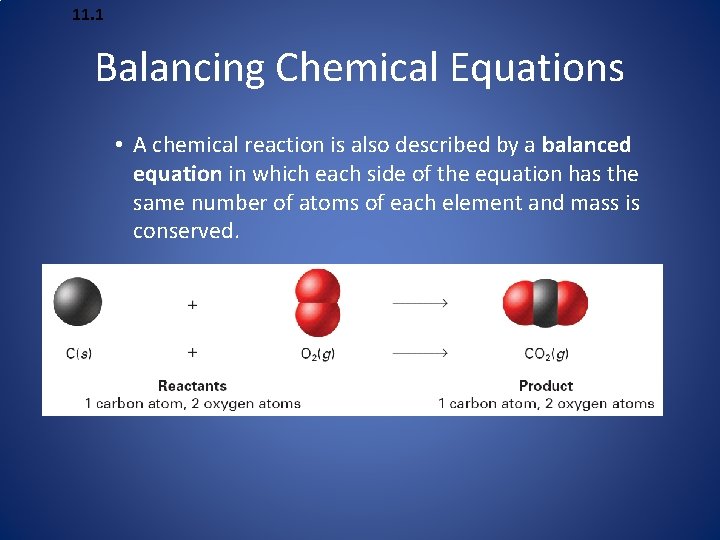
11. 1 Balancing Chemical Equations • A chemical reaction is also described by a balanced equation in which each side of the equation has the same number of atoms of each element and mass is conserved.

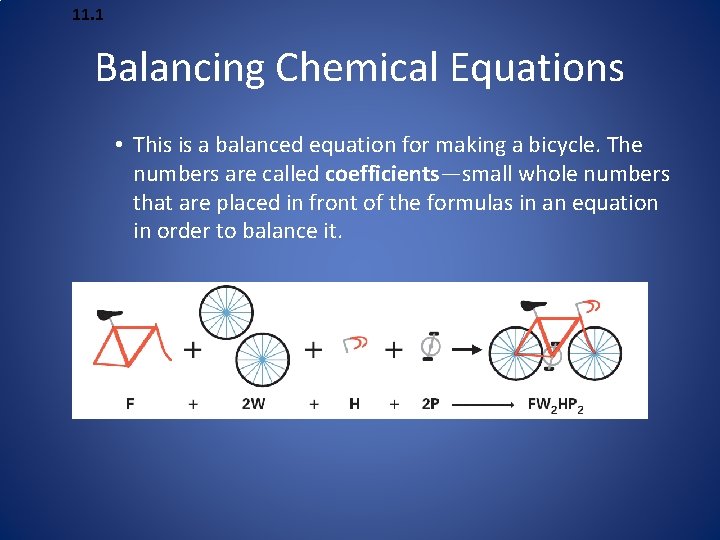
11. 1 Balancing Chemical Equations • This is a balanced equation for making a bicycle. The numbers are called coefficients—small whole numbers that are placed in front of the formulas in an equation in order to balance it.

11. 1 Balancing Chemical Equations – To write a balanced chemical equation, first write the skeleton equation. Then use coefficients to balance the equation so that it obeys the law of conservation of mass.

Rules for balancing equations: • Write correct skeleton formula • Determine number of atoms of each element of reactants and products. COUNT POLYATOMIC ION AS A SINGLE UNIT if it appears unchanged on both sides of the equation • Balance elements one at a time by using coefficients. NEVER change subscripts • Begin with the easiest elements first • Check both sides to see if they match • Make sure coefficients are in the lowest possible ratio

Remember diatomics!! • When you write the skeleton equation, remember these elements must be written as 2 atoms when they are not involved in a compound…. • Br 2 I 2 N 2 Cl 2 H 2 O 2 F 2



Types of Reactions

Types of Chemical Reactions • • • Synthesis (combination) Decomposition Single Replacement Double Replacement Combustion

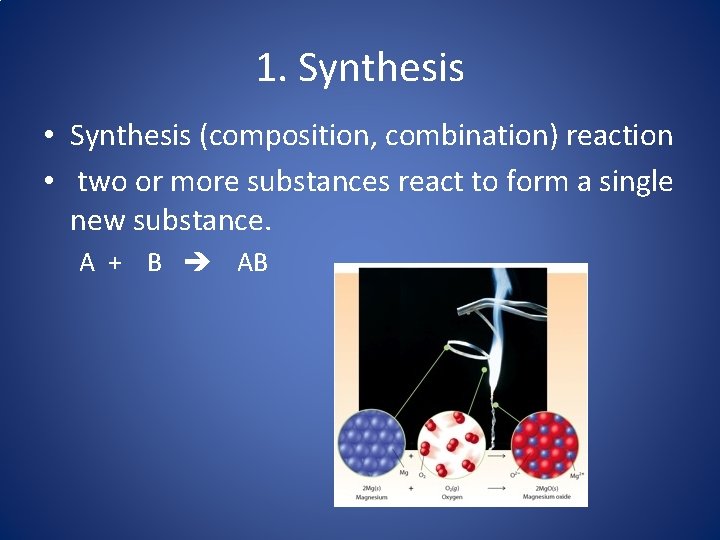
1. Synthesis • Synthesis (composition, combination) reaction • two or more substances react to form a single new substance. A + B AB

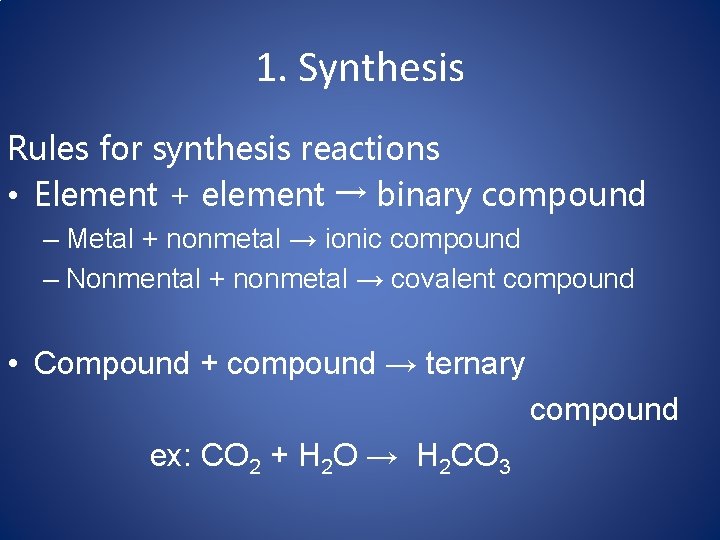
1. Synthesis Rules for synthesis reactions • Element + element → binary compound – Metal + nonmetal → ionic compound – Nonmental + nonmetal → covalent compound • Compound + compound → ternary compound ex: CO 2 + H 2 O → H 2 CO 3

Practice • Predict the products. Write and balance the following synthesis reaction equations. • Sodium metal reacts with chlorine gas Na(s) + Cl 2(g) • Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g) • Aluminum metal reacts with fluorine gas Al(s) + F 2(g)

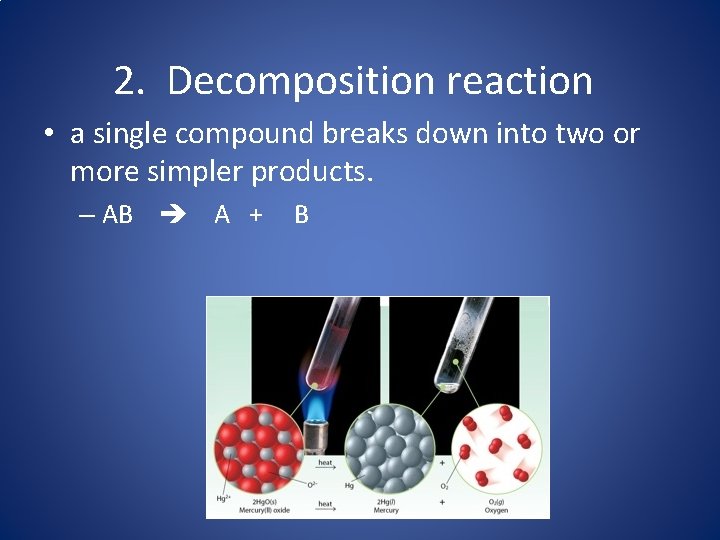
2. Decomposition reaction • a single compound breaks down into two or more simpler products. – AB A + B

2. Decomposition Reactions Rules for Decomposition Reactions: • All binary compounds (ex: Mg. Cl 2) will break down into their elements • All carbonates (CO 32 -) break down into oxide and carbon dioxide • Chlorates (Cl. O 3 -) break down into binary salt and oxygen • Bases (OH group) and compounds with an H and an O will break down into water and an oxide

Practice • Predict the products. Then, write and balance the following decomposition reaction equations: • Solid Lead (IV) oxide decomposes Pb. O 2(s) • Aluminum nitride decomposes Al. N(s)

• http: //educationportal. com/academy/lesson/decompositionand-synthesis-reactions. html#lesson

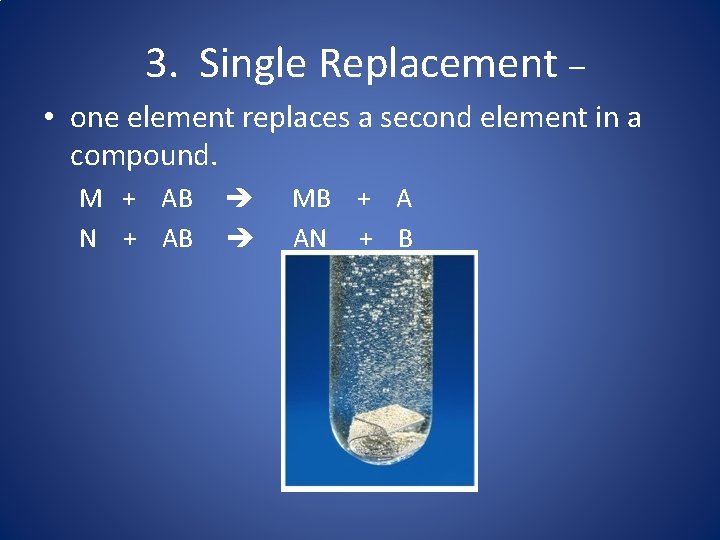
3. Single Replacement – • one element replaces a second element in a compound. M + AB N + AB MB + A AN + B

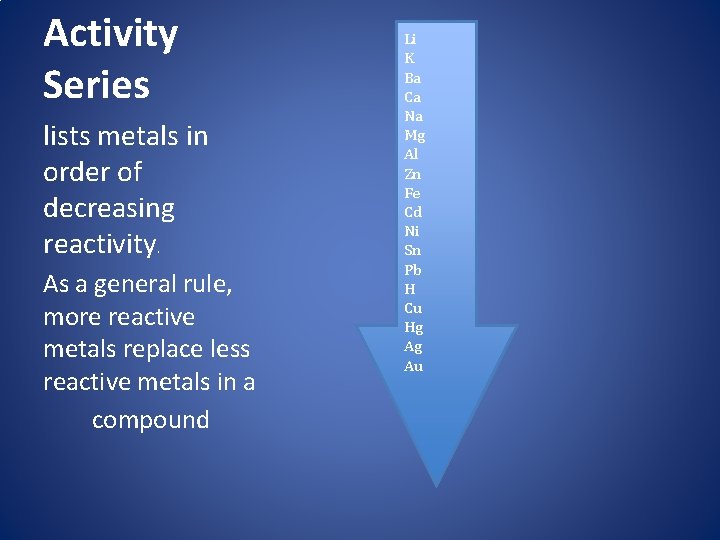
Activity Series lists metals in order of decreasing reactivity. As a general rule, more reactive metals replace less reactive metals in a compound Li K Ba Ca Na Mg Al Zn Fe Cd Ni Sn Pb H Cu Hg Ag Au

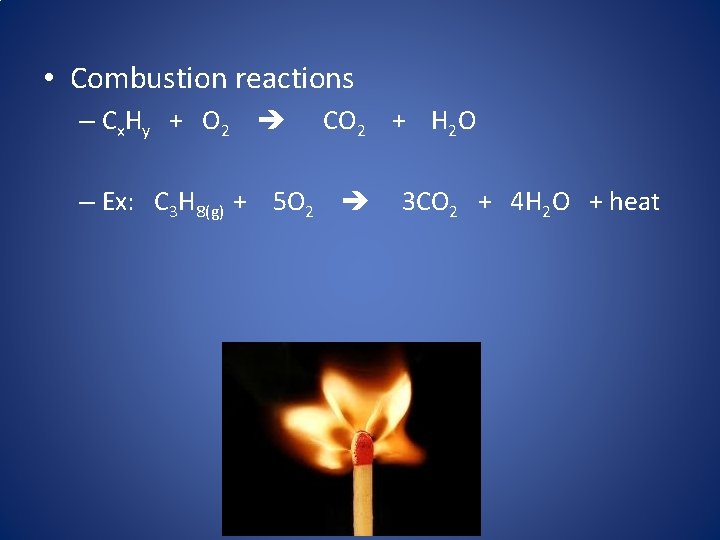
4. Combustion Reactions • Combustion reactions occur when a fuel reacts with oxygen gas, which produces heat! Fuel + O 2 (+ Heat) Product

• Combustion reactions – C x Hy + O 2 CO 2 + H 2 O – Ex: C 3 H 8(g) + 5 O 2 3 CO 2 + 4 H 2 O + heat

Hydrocarbon Combustion Reactions • Products in combustion are ALWAYS carbon dioxide and water. (although incomplete burning does cause some by-products like carbon monoxide) • Combustion is used to heat homes (CH 4)and run automobiles (octane: C 8 H 18)

Carbon Monoxide Effects Edgar Allen Poe’s drooping eyes and mouth are potential signs of CO poisoning.

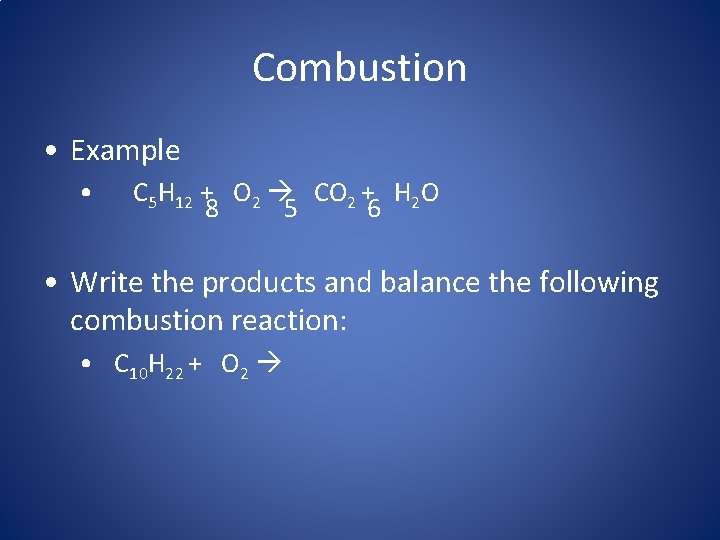
Combustion • Example • C 5 H 12 + O 2 CO 2 + H 2 O 6 8 5 • Write the products and balance the following combustion reaction: • C 10 H 22 + O 2

5. Double Replacement (precipitation) • Double replacement- also known as precipitation reaction, (and sometimes neutralization reaction) AB + CD AD + CB • The attractive forces between t oppositely charged ions is greater than the forces of attraction between the water molecules and the ions

1. Double Replacement cont Colors of precipitants Precipitant formed • Pb. I 2 • Cd. S • Pb. S • Ag 2 S • Ni(OH)2 • Al(OH)3 • Pb. SO 4 • Ba. SO 4 • Ca 3(PO 4)2 • Ag. Cl Color Bright yellow Dark yellow Black Green White white

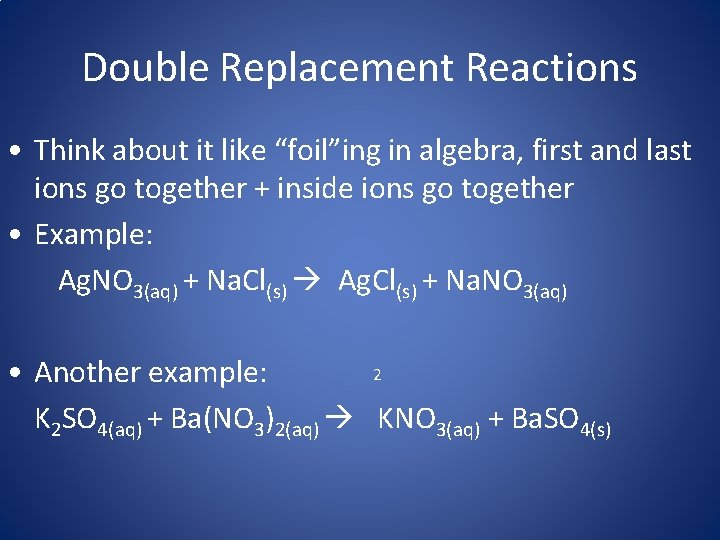
Double Replacement Reactions • Think about it like “foil”ing in algebra, first and last ions go together + inside ions go together • Example: Ag. NO 3(aq) + Na. Cl(s) Ag. Cl(s) + Na. NO 3(aq) 2 • Another example: K 2 SO 4(aq) + Ba(NO 3)2(aq) KNO 3(aq) + Ba. SO 4(s)

Solubility rules

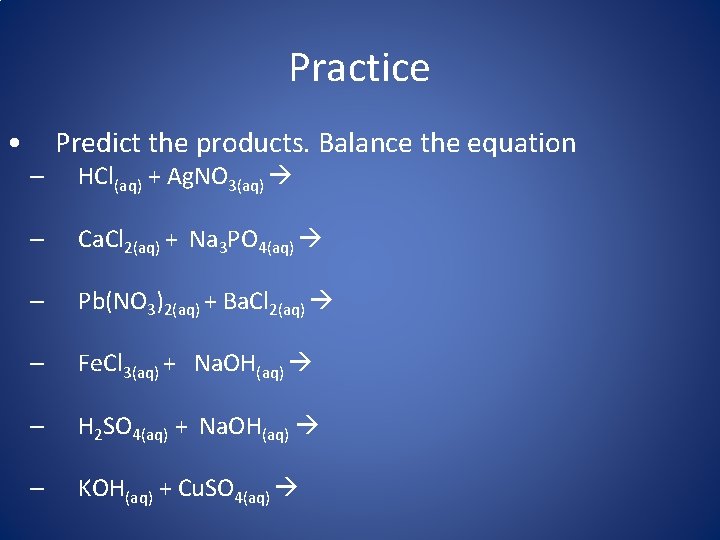
Practice • Predict the products. Balance the equation – HCl(aq) + Ag. NO 3(aq) – Ca. Cl 2(aq) + Na 3 PO 4(aq) – Pb(NO 3)2(aq) + Ba. Cl 2(aq) – Fe. Cl 3(aq) + Na. OH(aq) – H 2 SO 4(aq) + Na. OH(aq) – KOH(aq) + Cu. SO 4(aq)

Mixed Practice • State the type, predict the products, and balance the following reactions: 1. Ba. Cl 2 + H 2 SO 4 2. C 6 H 12 + O 2 3. Zn + Cu. SO 4 4. Cs + Br 2 5. Fe. CO 3
 Are kc and kp equal
Are kc and kp equal Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Synthesis reaction
Synthesis reaction Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Translate word equations to chemical equations
Translate word equations to chemical equations Toxic reactions chemical equations
Toxic reactions chemical equations Examples of chemical change
Examples of chemical change Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Toxic reactions chemical equations
Toxic reactions chemical equations Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Chapter 9 chapter assessment chemical reactions
Chapter 9 chapter assessment chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Examples of redox reaction
Examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems 5 types of chemical reactions
5 types of chemical reactions Chapter 9 chemical reactions
Chapter 9 chemical reactions Co2+h2o- c6h12o6+o2
Co2+h2o- c6h12o6+o2 Describing chemical reactions worksheet answers
Describing chemical reactions worksheet answers Water vapour chemical formula
Water vapour chemical formula How to write equations from word problems
How to write equations from word problems Writing linear equations from word problems worksheet
Writing linear equations from word problems worksheet Word equation examples
Word equation examples Stoichiometry island diagram
Stoichiometry island diagram Redox rules
Redox rules Identify types of reactions
Identify types of reactions Types of reactions chemistry
Types of reactions chemistry Types of reactions
Types of reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions More predicting products of chemical reactions
More predicting products of chemical reactions