Word Equations Lesson 10 n A word equation












- Slides: 16

Word Equations Lesson 10

n A word equation is a way of representing a chemical reaction: it tells you what reacts and what is produced. Word equations are an efficient way to describe chemical changes, to help chemists recognise patterns, and to predict the products of a chemical reaction.

Writing word equations n They are written in a particular order. Reactants (what you start with) are always on the left side of the arrow and products (what you make) are always on the right side of the arrow. Multiple reactants or products are separated by a + sign.

Word Equations examples. n Silver nitrate + copper silver + copper (II) nitrate Reactants Products n Hydrogen + Oxygen water vapour Reactants Products

The Conservation of Mass In a chemical reaction, the total mass of the reactants is always equal to the total mass of the products. n This tells us a few things. n Atoms do not change in a reaction. The molecules that they form can be changed but the atoms themselves are not. n Mass cannot be destroyed. If it could we could use E = MC 2 to create energy n

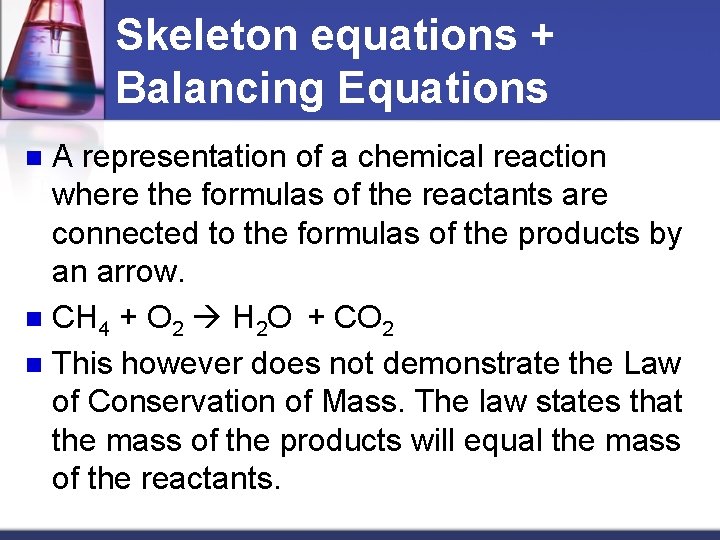
Example n Methane + oxygen water + carbon dioxide n

Skeleton equations + Balancing Equations A representation of a chemical reaction where the formulas of the reactants are connected to the formulas of the products by an arrow. n CH 4 + O 2 H 2 O + CO 2 n This however does not demonstrate the Law of Conservation of Mass. The law states that the mass of the products will equal the mass of the reactants. n

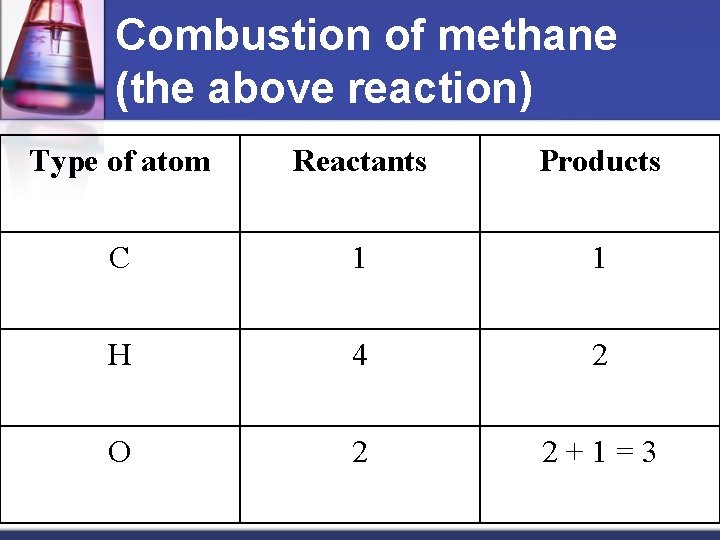
Combustion of methane (the above reaction) Type of atom Reactants Products C 1 1 H 4 2 O 2 2+1=3

n We can’t change the formulas of the products or reactants so the only thing we can do is change the number of molecules instead of their formulas.

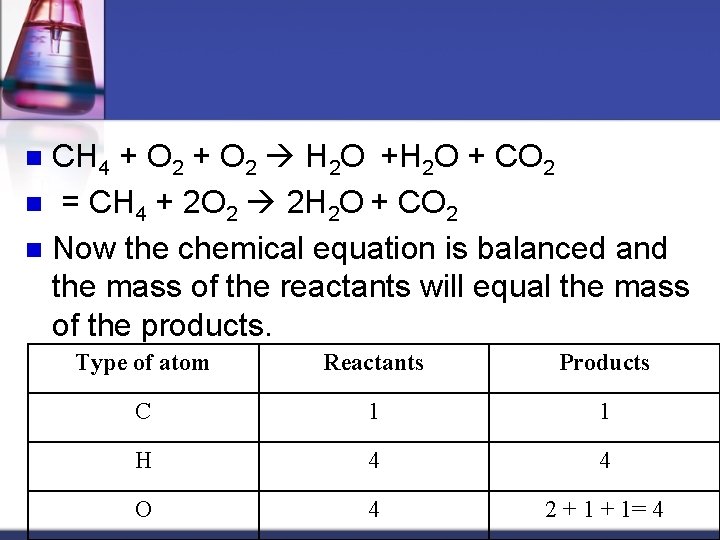
CH 4 + O 2 H 2 O + CO 2 n = CH 4 + 2 O 2 2 H 2 O + CO 2 n Now the chemical equation is balanced and the mass of the reactants will equal the mass of the products. n Type of atom Reactants Products C 1 1 H 4 4 O 4 2 + 1= 4

Steps to balancing an equation Step 1 n Write the word equation of the reaction n Aluminum + bromine aluminum bromide Step 2 n Write the skeleton equation by replacing each name with a correct formula. n Al +Br 2 Al. Br 3

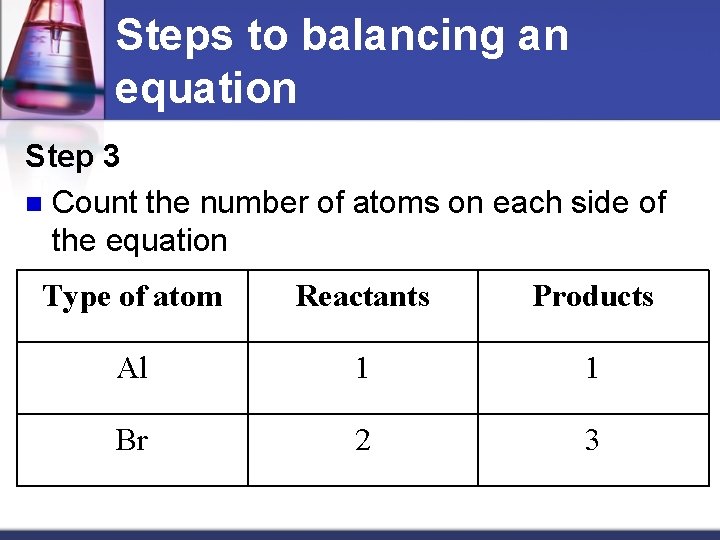
Steps to balancing an equation Step 3 n Count the number of atoms on each side of the equation Type of atom Reactants Products Al 1 1 Br 2 3

Steps to balancing an equation Step 4 n Multiply each of the formulas by the appropriate coefficients to balance the number of atoms.

Start out by picking the element with the most number of atoms and try to balance it first. We will start with Bromine. The 2 and 3 will be balanced if we multiply the reactant side by 3 which would give it 6 Br, and multiply the product side by 2 to give us 6 Br. Now we have 2 Al products which need to be balanced so we add a 2 to the Al on the reactant side. n 2 Al + 3 Br 2 2 Al. Br 3 n

More examples on the board Hydrogen gas + Chlorine gas hydrogen chloride n n Sodium + chlorine sodium chloride n n Nitrogen + hydrogen ammonia (hydrogen nitride) n N 2 + H 2 NH 3 = N 2 + H 2 NH 3 n

n Complete the worksheet and hand in.