Chapter 10 Chemical Quantities Yes you will need











- Slides: 35

Chapter 10 “Chemical Quantities” Yes, you will need a calculator for this chapter!

Mole Activity #1

How do we measure items? § You can measure mass, volume, or you can count pieces. § We measure mass in grams. liters. § We count pieces in MOLES. § We measure volume in

The Mole Song http: //www. youtube. com/watch? v=Pv. T 51 M 0 ek 5 c Yes, you must sing along with the chorus!

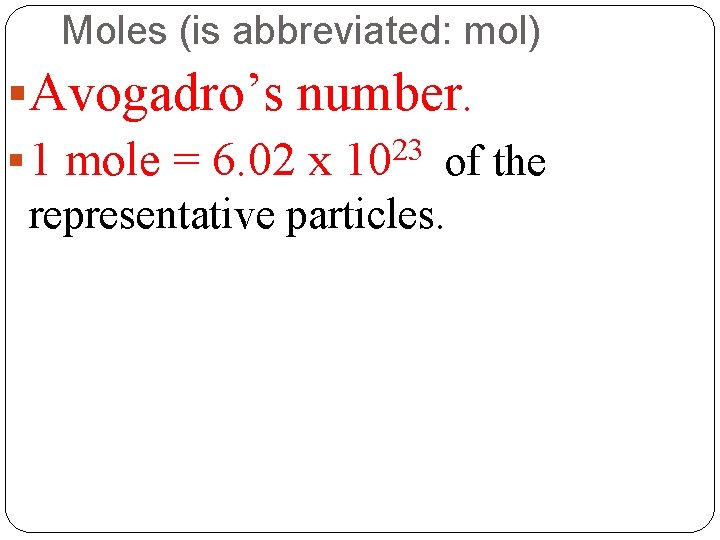
Moles (is abbreviated: mol) §Avogadro’s number. § 1 mole = 6. 02 x 1023 of the representative particles.

Representative Particles § The smallest pieces of a substance § Most elements = atoms § Br. INCl. HOF = molecules because they always come in two’s § Br 2 I 2 O 2 etc § Covalent compounds = molecules § H 2 O, SO 2 § Ionic compounds = formula units or ions § Na. Cl = 1 formula unit or § Na. Cl = 2 ions Na+ and Cl§ Something with a charge= Ion

Representative Particles CO 2 Fe Ca. O Li+1 Nitrogen gas Sulfur

8

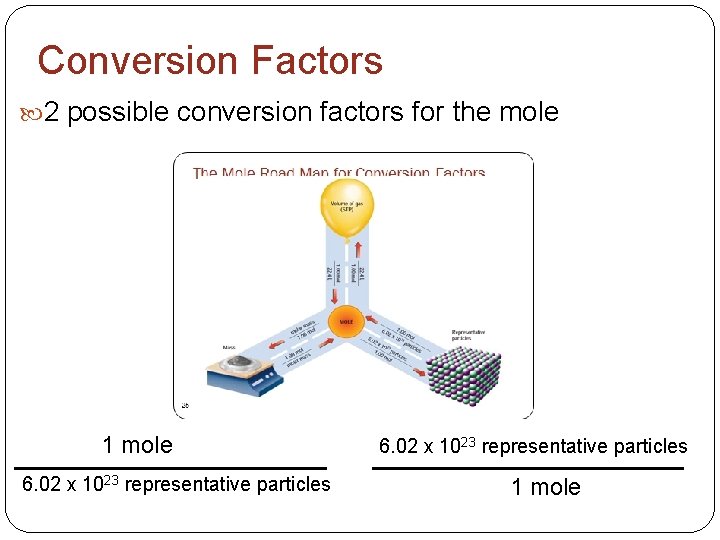
Conversion Factors 2 possible conversion factors for the mole OR 1 mole 6. 02 x 1023 representative particles 1 mole

Conversion Factors Using the conversion factors we can determine the number of atoms that are in a mole of a compound or how many moles are in a sample of a compound How many molecules are in 2. 12 mole of carbon dioxide? 2. 12 mole CO 2 1 6. 02 x 10 23 molecules of CO 2 1 mole CO 2 = _____ 1. 28 x 1024 molecules of CO 2

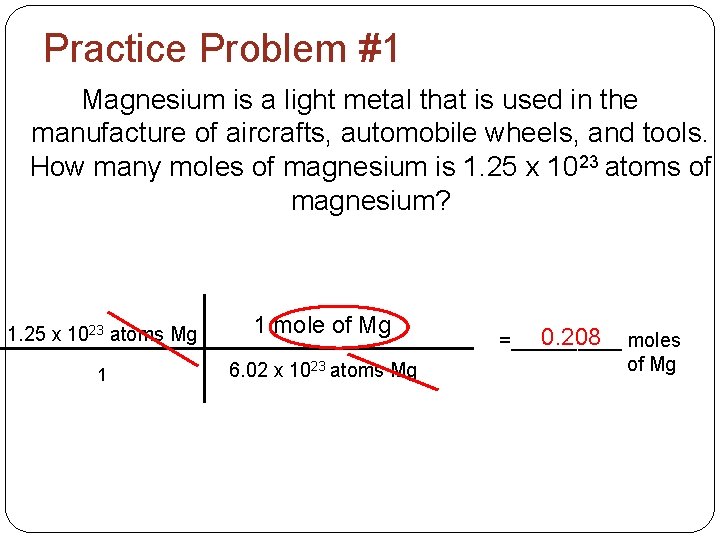
Practice Problem #1 Magnesium is a light metal that is used in the manufacture of aircrafts, automobile wheels, and tools. How many moles of magnesium is 1. 25 x 1023 atoms of magnesium? 1. 25 x 1023 atoms Mg 1 mole of Mg 1 6. 02 x 1023 atoms Mg 0. 208 moles =_____ of Mg

Try This How many moles are in 2. 4 x 1023 formula units Li. O How many formula units are in 1. 2 mol Li. O

V. Chemical Measurements Atomic Mass measured in amu Atomic mass units Bottom number on the periodic table Find the atomic mass of each atom Mass of O 15. 999 amu Mass of Fe 55. 847 amu Mass of C 12. 011 amu

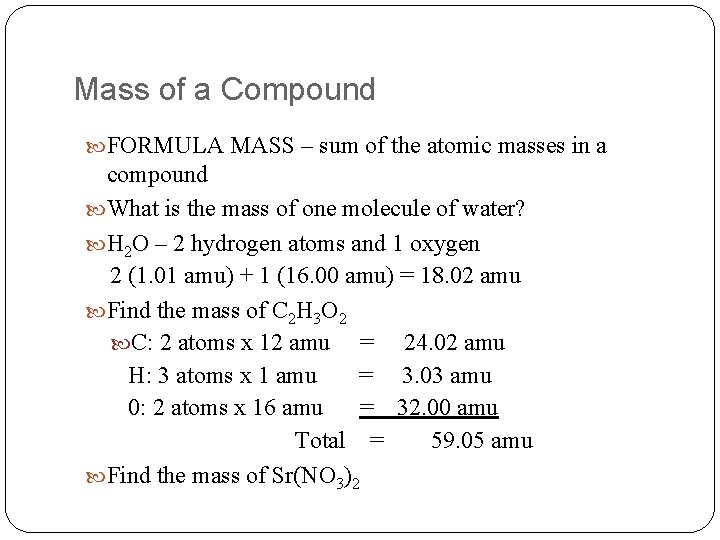
Mass of a Compound FORMULA MASS – sum of the atomic masses in a compound What is the mass of one molecule of water? H 2 O – 2 hydrogen atoms and 1 oxygen 2 (1. 01 amu) + 1 (16. 00 amu) = 18. 02 amu Find the mass of C 2 H 3 O 2 C: 2 atoms x 12 amu = 24. 02 amu H: 3 atoms x 1 amu = 3. 03 amu 0: 2 atoms x 16 amu = 32. 00 amu Total = 59. 05 amu Find the mass of Sr(NO 3)2

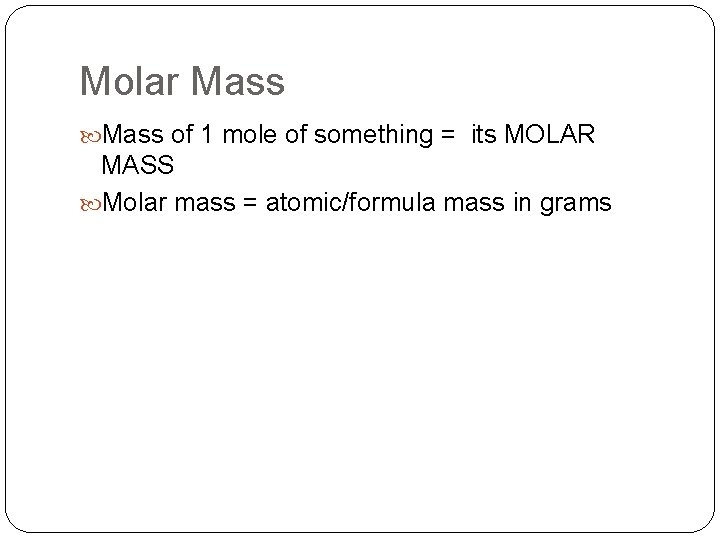
Molar Mass of 1 mole of something = its MOLAR MASS Molar mass = atomic/formula mass in grams

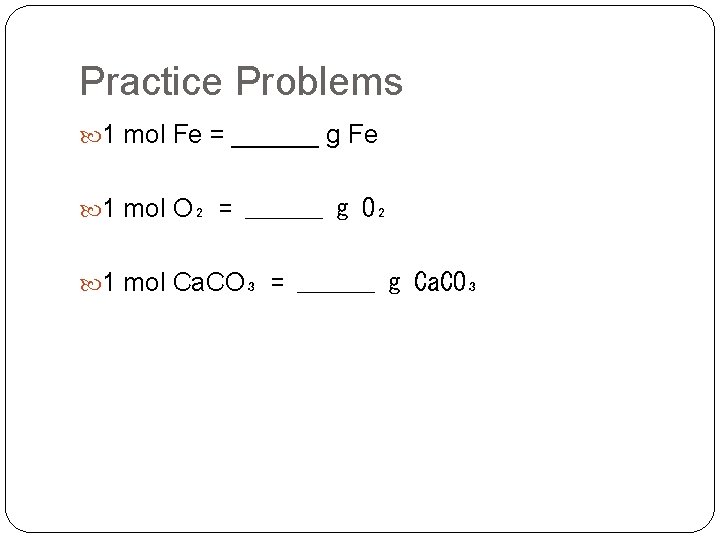
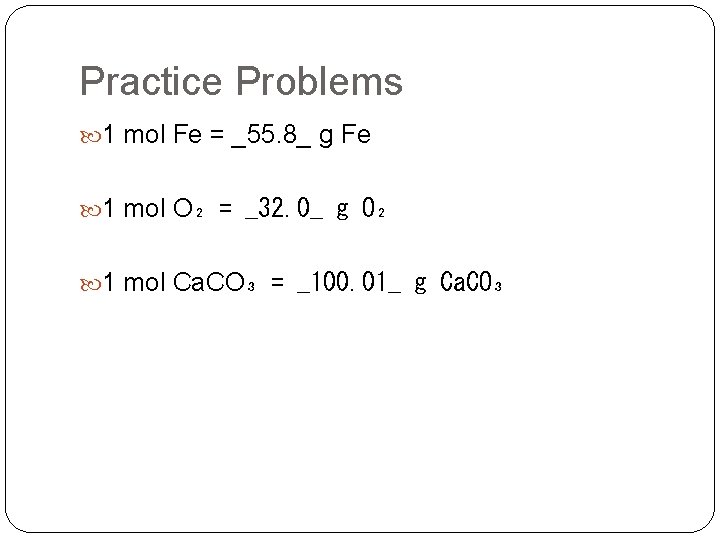
Practice Problems 1 mol Fe = ______ g Fe 1 mol O₂ = ______ g O₂ 1 mol Ca. CO₃ = ______ g Ca. CO₃

Practice Problems 1 mol Fe = _55. 8_ g Fe 1 mol O₂ = _32. 0_ g O₂ 1 mol Ca. CO₃ = _100. 01_ g Ca. CO₃

10. 2 18

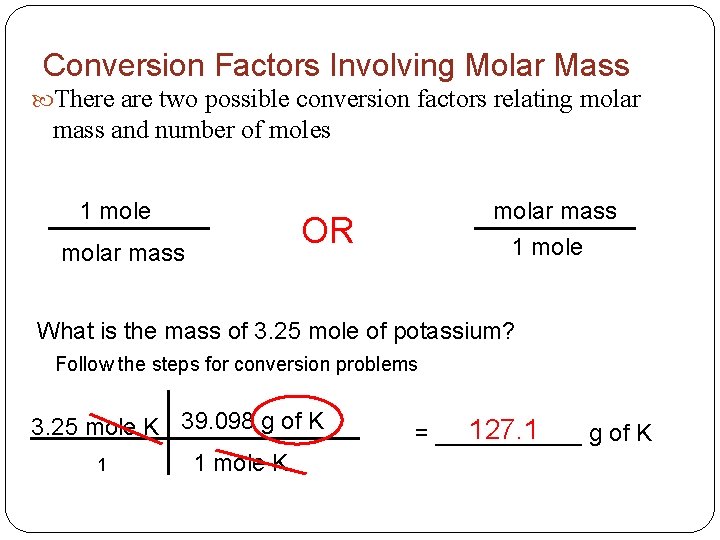
Conversion Factors Involving Molar Mass There are two possible conversion factors relating molar mass and number of moles 1 mole molar mass 1 mole OR What is the mass of 3. 25 mole of potassium? Follow the steps for conversion problems 3. 25 mole K 39. 098 g of K 1 mole K 1 127. 1 = ______ g of K

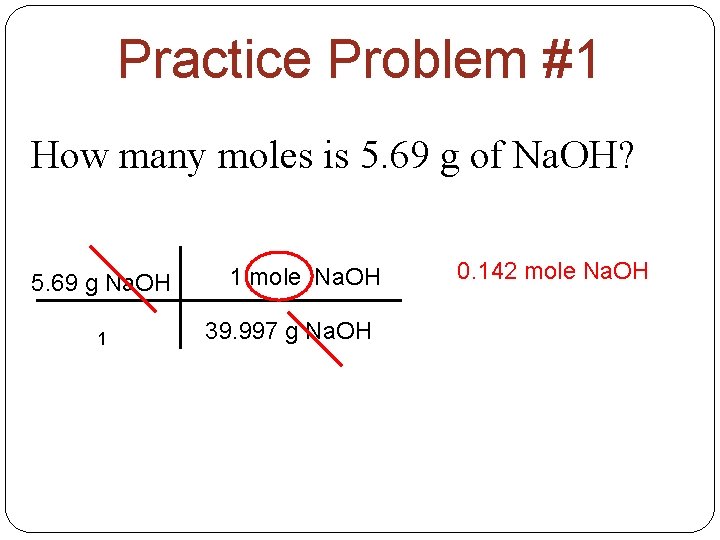
Practice Problem #1 How many moles is 5. 69 g of Na. OH? 5. 69 g Na. OH 1 1 mole Na. OH 39. 997 g Na. OH 0. 142 mole Na. OH

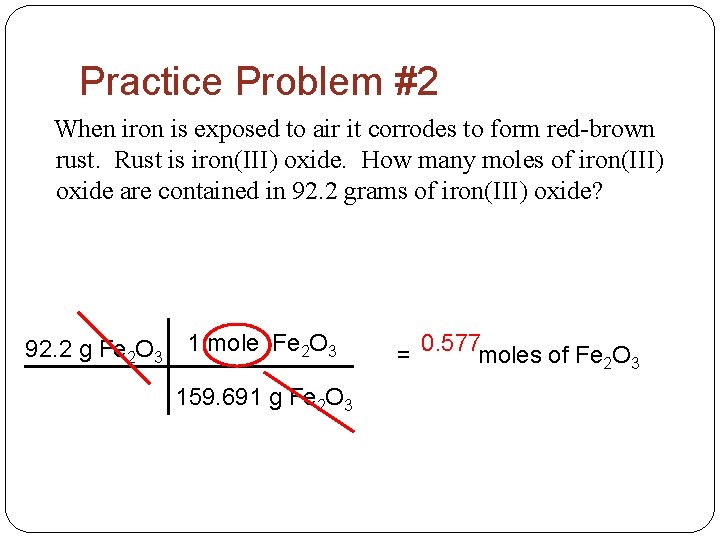
Practice Problem #2 When iron is exposed to air it corrodes to form red-brown rust. Rust is iron(III) oxide. How many moles of iron(III) oxide are contained in 92. 2 grams of iron(III) oxide? 92. 2 g Fe 2 O 3 1 mole Fe 2 O 3 159. 691 g Fe 2 O 3 = 0. 577 moles of Fe 2 O 3

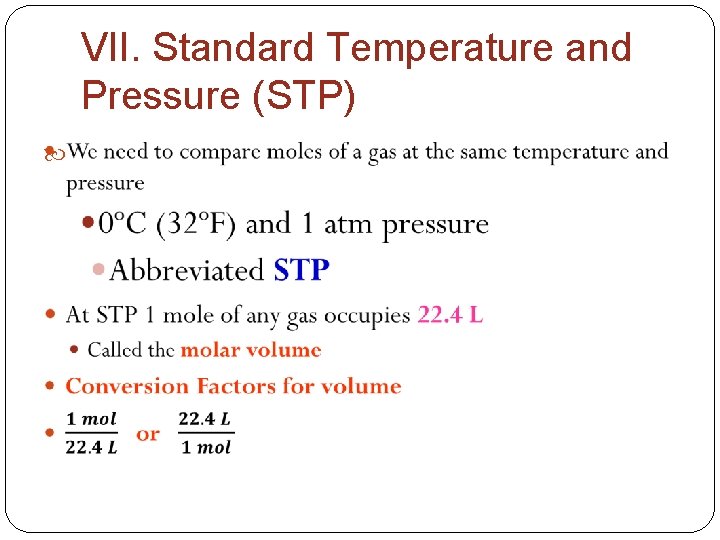
VII. Standard Temperature and Pressure (STP)

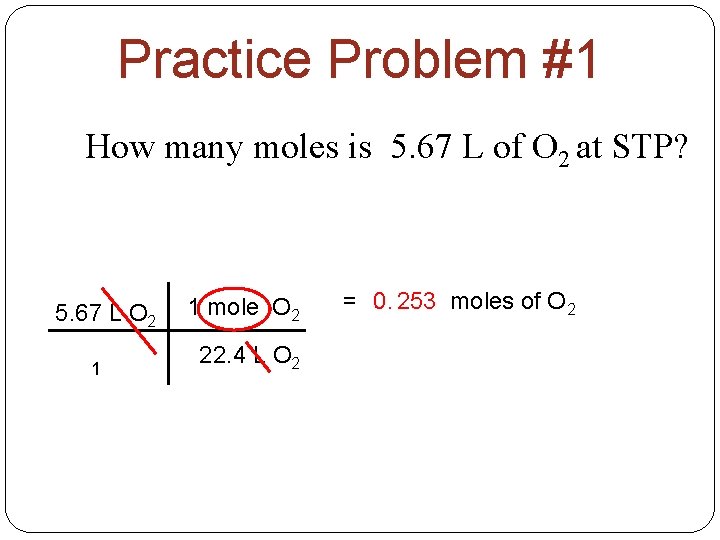
Practice Problem #1 How many moles is 5. 67 L of O 2 at STP? 5. 67 L O 2 1 1 mole O 2 22. 4 L O 2 = 0. 253 moles of O 2

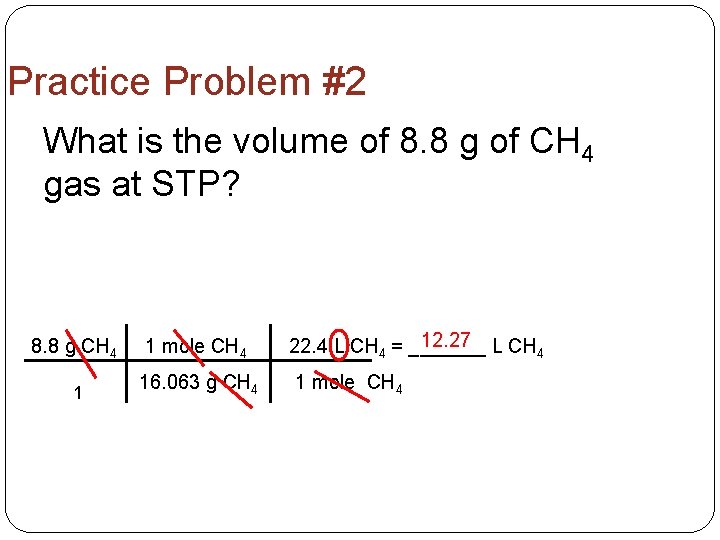
Practice Problem #2 What is the volume of 8. 8 g of CH 4 gas at STP? 8. 8 g CH 4 1 mole CH 4 12. 27 L CH 22. 4 L CH 4 = _______ 4 1 16. 063 g CH 4 1 mole CH 4

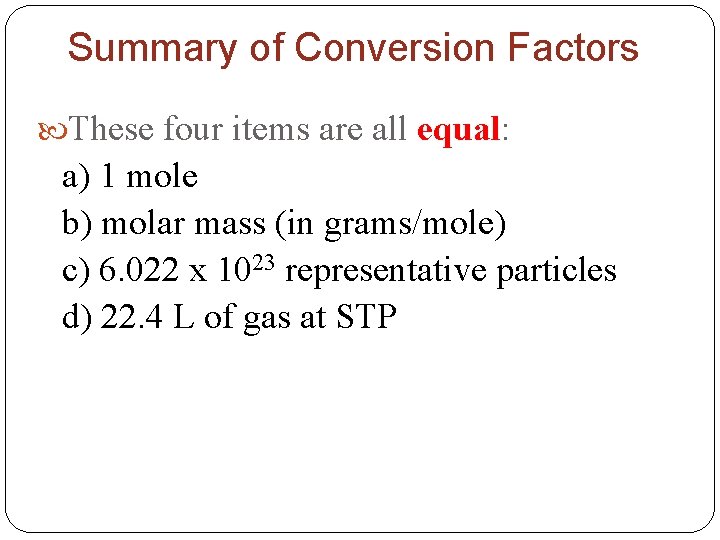
Summary of Conversion Factors These four items are all equal: a) 1 mole b) molar mass (in grams/mole) c) 6. 022 x 1023 representative particles d) 22. 4 L of gas at STP

The Mole Road Map for Conversion Factors

Chapter 10 Section 3 Chemistry

Essential Questions How do you calculate percent composition? What is an empirical formula? How can you tell the difference between an empirical formula and molecular formulas?

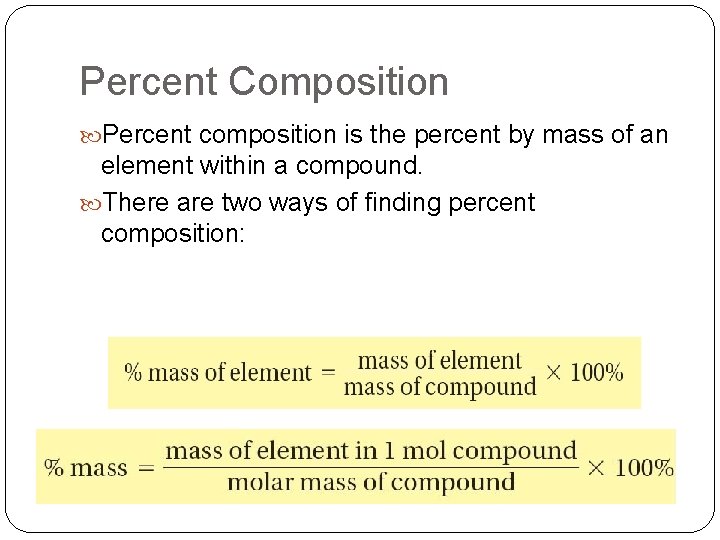
Percent Composition Percent composition is the percent by mass of an element within a compound. There are two ways of finding percent composition:

Percent Composition (cont) Propane (C 3 H 8) is a fuel used commonly in gas grills, find the percent composition of propane. When a 13. 60 g sample of a compound containing only magnesium and oxygen is decomposed, 5. 40 g of oxygen is obtained. What is the percent composition of this compound?

Percent Composition (cont) Find the percent comp for a 2. 50 g sample containing 0. 0625 g Cu and 2. 44 g Zn. Find the percent comp of CO 2 Find percent comp (NH 4)2 CO 3 Find percent comp of NH 3 Find percent comp of Fe 2(SO 4)3

Formulas The empirical formula of a compound shows the smallest whole-number ratio of the atoms in the compound. The molecular formula of a compound is either the same as its empirical formula, or it is a simple whole -number multiple of its empirical formula.

Formulas (cont) Identify if the following formulas are empirical, molecular or both. N 2 O 4 H 2 N CH C 3 H 6

Essential Questions How do you calculate percent composition? What is an empirical formula? How can you tell the difference between an empirical formula and molecular formulas?

Chapter 10 Section 3 P 312 #41 -44, 46 P 315 -317 #63, 67