Chapter 10 Chemical Quantities You will need a

















- Slides: 41

Chapter 10 “Chemical Quantities” You will need a calculator for this chapter!

The Mole: A Measurement of Matter
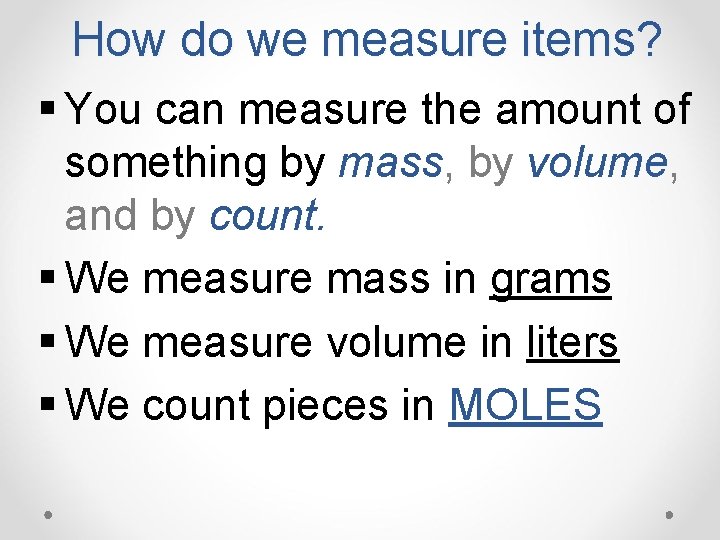
How do we measure items? § You can measure the amount of something by mass, by volume, and by count. § We measure mass in grams § We measure volume in liters § We count pieces in MOLES

Other Ways to Measure Amount § § Pair: 1 pair of socks = 2 socks Dozen: 1 dozen donuts = 12 donuts Gross: 1 gross of pencils = 144 pencils (12 dozen) Ream: 1 ream of paper = 500 sheets of paper

Practice Problem #2 • Assume 2. 0 kg of apples is 1 dozen and that each apple has 8 seeds. How many apple seeds are in 14 kg of apples? (work INDEPENDENTLY to solve)

What is the mole? Not this kind of mole!

Moles (abbreviated mol) § Derived from German word molekül (molecule) § SI measurement of an amount § 1 mole = 6. 02 x 1023 of representative particles, or…. . § # of carbon atoms in exactly 12 g of Carbon-12 isotope § Called Avogadro’s number

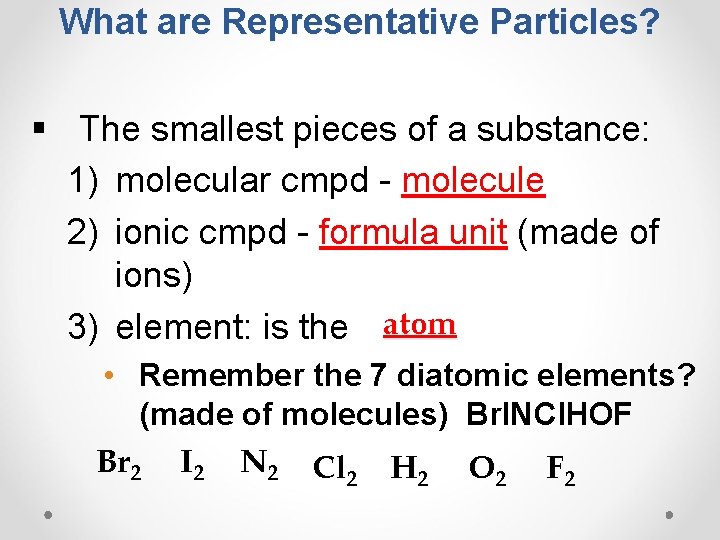
What are Representative Particles? § The smallest pieces of a substance: 1) molecular cmpd - molecule 2) ionic cmpd - formula unit (made of ions) 3) element: is the atom • Remember the 7 diatomic elements? (made of molecules) Br. INCl. HOF Br 2 I 2 N 2 Cl 2 H 2 O 2 F 2

Consider these questions: • How many oxygen atoms in the following? Ca. CO 3 3 atoms of oxygen Al 2(SO 4)3 12 (3 x 4) atoms of oxygen • How many ions in the following? Ca. Cl 2 3 total ions (1 Ca ion and 2 Cl ions) Na. OH 2 total ions (1 Na ion and 1 OH ion) Al 2(SO 4)3 2+ 1+ 1 - 1 - 5 total ions (2 Al 3+ + 3 SO 42 - ions)

The Mass of a Mole of an Element § Atomic mass of element (mass of 1 atom) expressed in amu - atomic masses - relative masses based on mass of C-12 (12. 0 amu) - 1 amu is 1/12 mass of C-12 atom

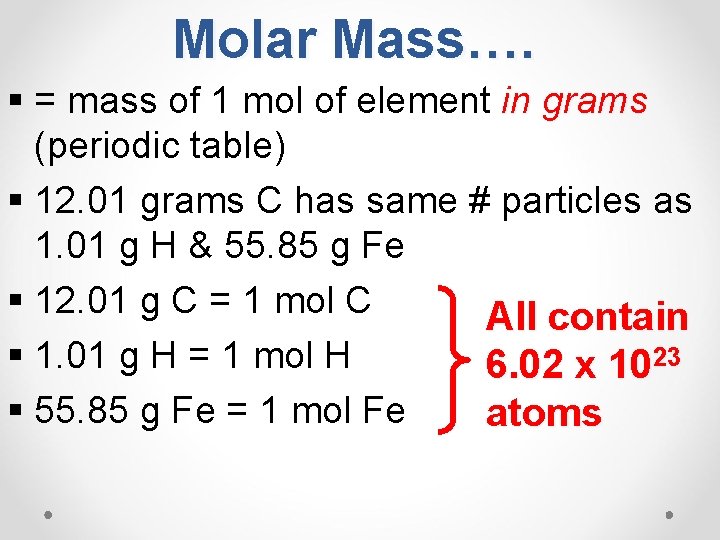
Molar Mass…. § = mass of 1 mol of element in grams (periodic table) § 12. 01 grams C has same # particles as 1. 01 g H & 55. 85 g Fe § 12. 01 g C = 1 mol C All contain § 1. 01 g H = 1 mol H 6. 02 x 1023 § 55. 85 g Fe = 1 mol Fe atoms

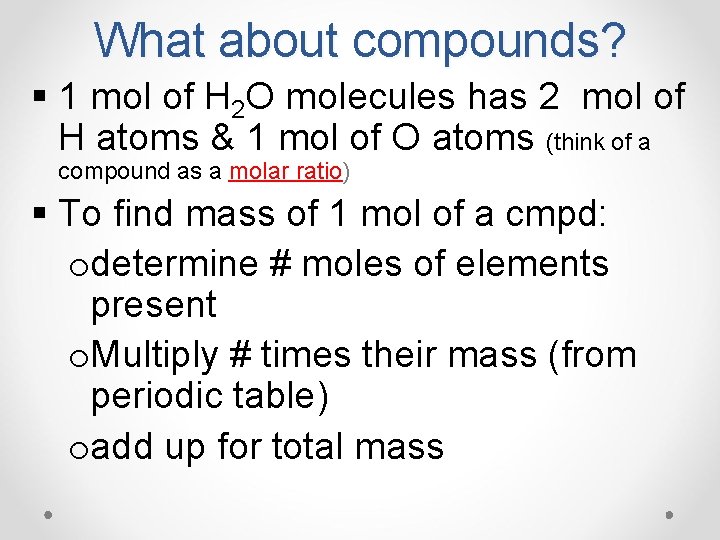
What about compounds? § 1 mol of H 2 O molecules has 2 mol of H atoms & 1 mol of O atoms (think of a compound as a molar ratio) § To find mass of 1 mol of a cmpd: odetermine # moles of elements present o. Multiply # times their mass (from periodic table) oadd up for total mass

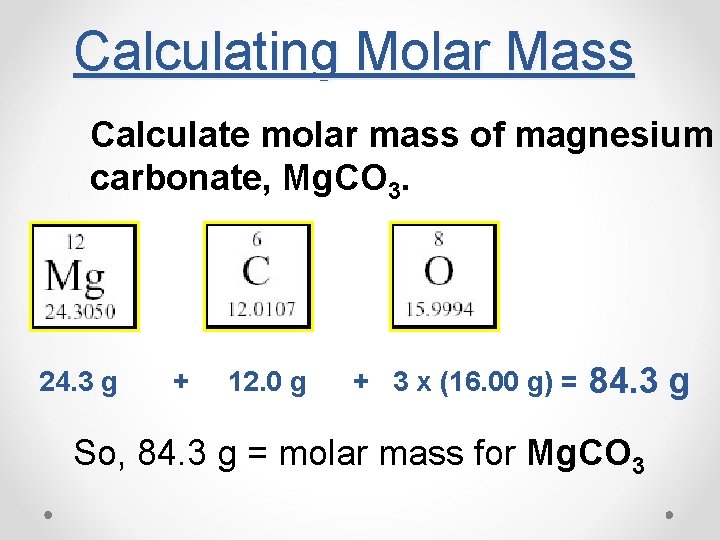
Calculating Molar Mass Calculate molar mass of magnesium carbonate, Mg. CO 3. 24. 3 g + 12. 0 g + 3 x (16. 00 g) = 84. 3 g So, 84. 3 g = molar mass for Mg. CO 3

Mole-Mass and Mole. Volume Relationships

Molar Mass § Molar mass - generic term for mass of 1 mol of any substance (expressed in grams/mol) § Same as: 1) Gram Molecular Mass (for molecules) 2) Gram Formula Mass (ionic compounds) 3) Gram Atomic Mass (for elements) o molar mass is more broad term than these other specific masses

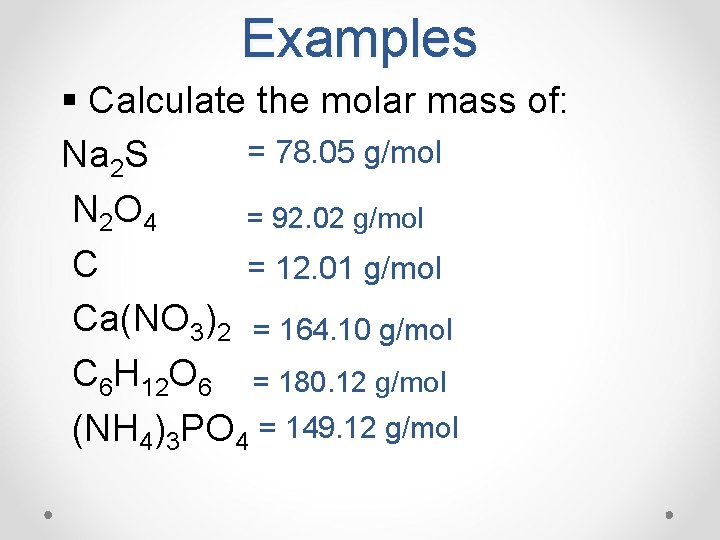
Examples § Calculate the molar mass of: = 78. 05 g/mol Na 2 S N 2 O 4 = 92. 02 g/mol C = 12. 01 g/mol Ca(NO 3)2 = 164. 10 g/mol C 6 H 12 O 6 = 180. 12 g/mol (NH 4)3 PO 4 = 149. 12 g/mol

Molar Mass is… § # of g in 1 mol of atoms, formula units, or molecules § Make conversion factors from these - To change btwn g of cmpd and mol of cmpd

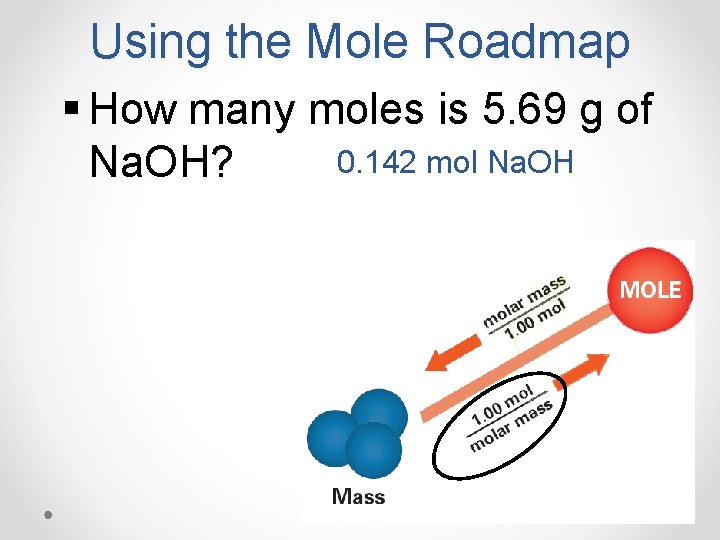
Using the Mole Roadmap § How many moles is 5. 69 g of 0. 142 mol Na. OH?

The Mole-Volume Relationship §gases - hard to determine mass § how many moles of gas? § 2 things affect gas V: § a) Temp & b) Pressure § compare all gases at = temp & pressure

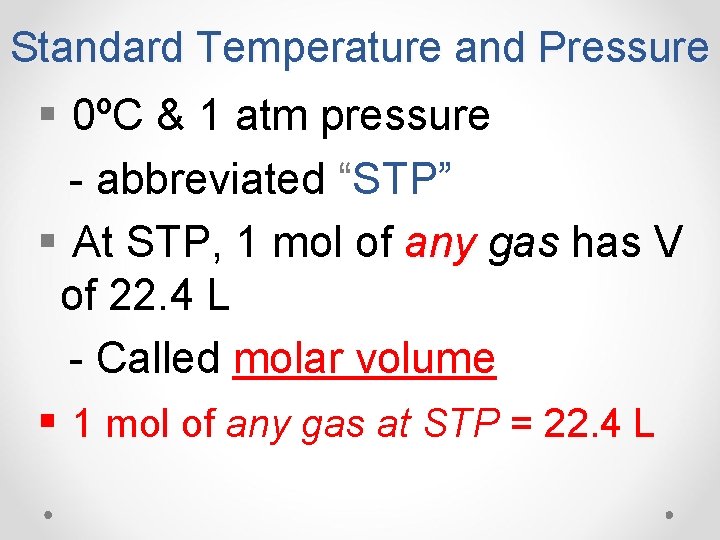
Standard Temperature and Pressure § 0ºC & 1 atm pressure - abbreviated “STP” § At STP, 1 mol of any gas has V of 22. 4 L - Called molar volume § 1 mol of any gas at STP = 22. 4 L

Mole Day Celebrated on October 23 rd from 6: 02 am until 6: 02 pm (6: 02 on 10 -23)

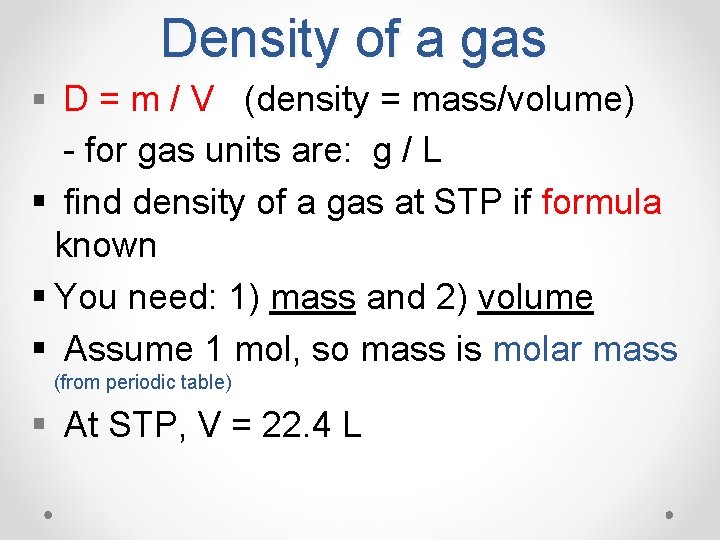
Density of a gas § D = m / V (density = mass/volume) - for gas units are: g / L § find density of a gas at STP if formula known § You need: 1) mass and 2) volume § Assume 1 mol, so mass is molar mass (from periodic table) § At STP, V = 22. 4 L

Practice Examples (D=m/V)

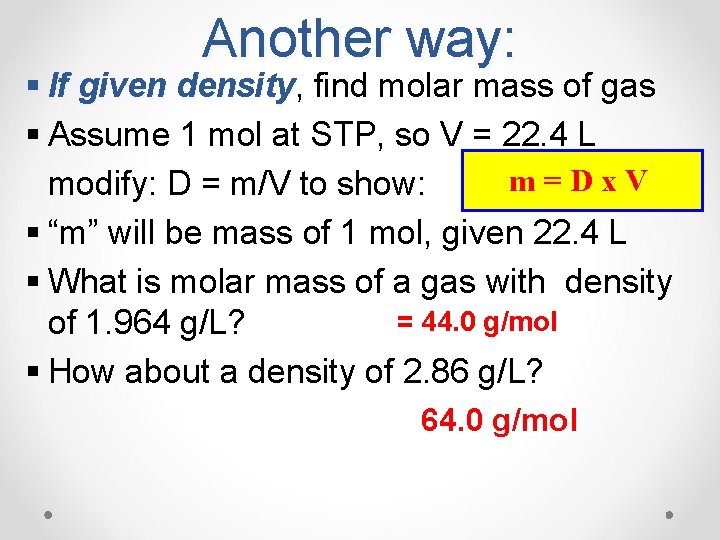
Another way: § If given density, density find molar mass of gas § Assume 1 mol at STP, so V = 22. 4 L m=Dx. V modify: D = m/V to show: § “m” will be mass of 1 mol, given 22. 4 L § What is molar mass of a gas with density = 44. 0 g/mol of 1. 964 g/L? § How about a density of 2. 86 g/L? 64. 0 g/mol

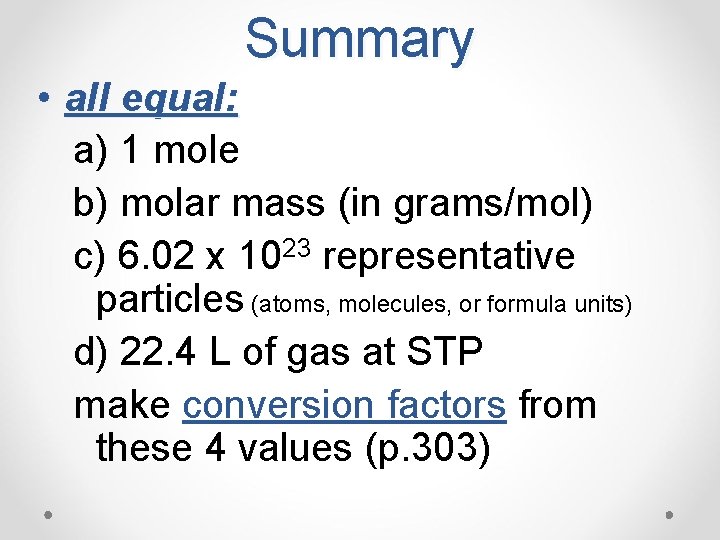
Summary • all equal: a) 1 mole b) molar mass (in grams/mol) c) 6. 02 x 1023 representative particles (atoms, molecules, or formula units) d) 22. 4 L of gas at STP make conversion factors from these 4 values (p. 303)

Notice all conversions must go through the MOLE!

Percent Composition and Chemical Formulas

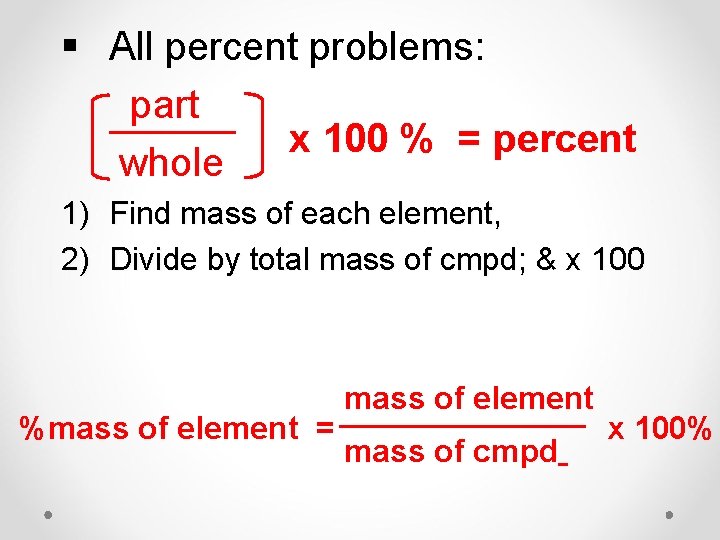
§ All percent problems: part x 100 % = percent whole 1) Find mass of each element, 2) Divide by total mass of cmpd; & x 100 %mass of element = mass of element mass of cmpd x 100%

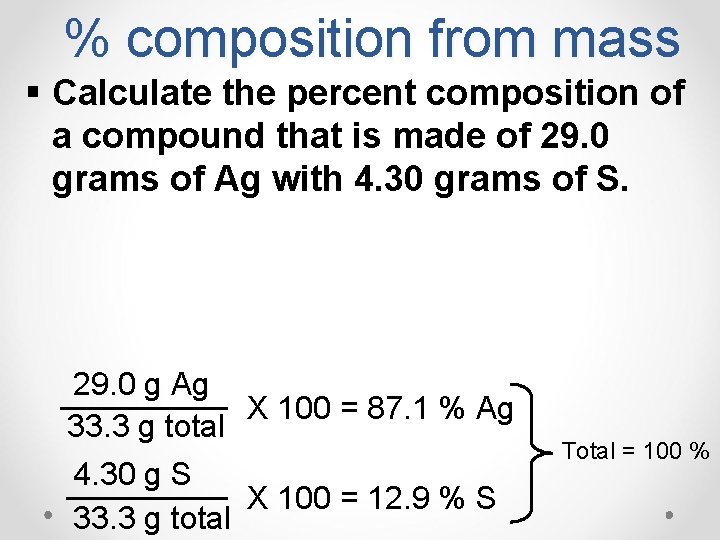
% composition from mass § Calculate the percent composition of a compound that is made of 29. 0 grams of Ag with 4. 30 grams of S. 29. 0 g Ag X 100 = 87. 1 % Ag 33. 3 g total 4. 30 g S X 100 = 12. 9 % S 33. 3 g total Total = 100 %

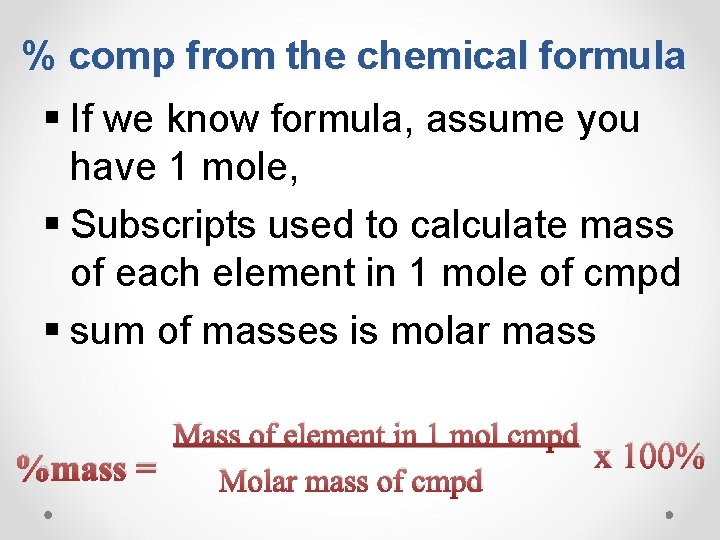
% comp from the chemical formula § If we know formula, assume you have 1 mole, § Subscripts used to calculate mass of each element in 1 mole of cmpd § sum of masses is molar mass Mass of element in 1 mol cmpd x 100% %mass = Molar mass of cmpd

% Composition Examples

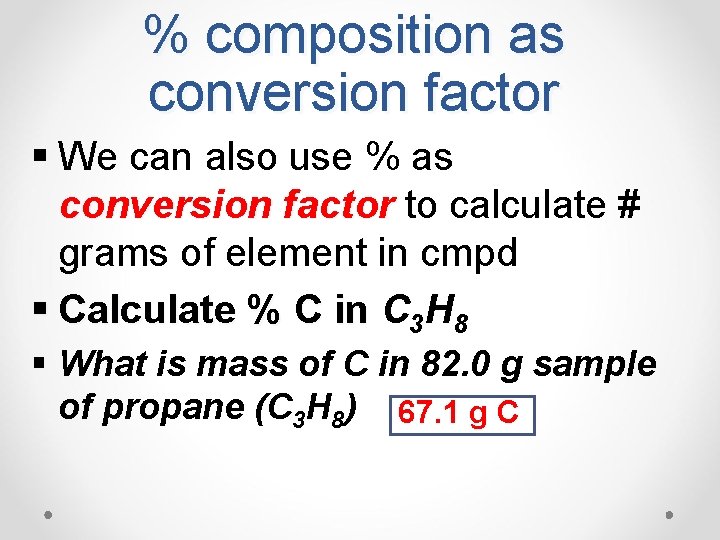
% composition as conversion factor § We can also use % as conversion factor to calculate # grams of element in cmpd § Calculate % C in C 3 H 8 § What is mass of C in 82. 0 g sample of propane (C 3 H 8) 67. 1 g C

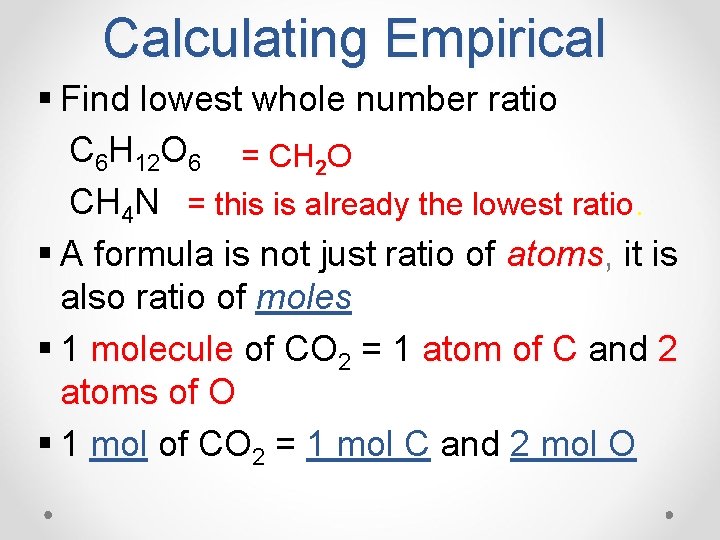
Calculating Empirical § Find lowest whole number ratio C 6 H 12 O 6 = CH 2 O CH 4 N = this is already the lowest ratio. § A formula is not just ratio of atoms, atoms it is also ratio of moles § 1 molecule of CO 2 = 1 atom of C and 2 atoms of O § 1 mol of CO 2 = 1 mol C and 2 mol O

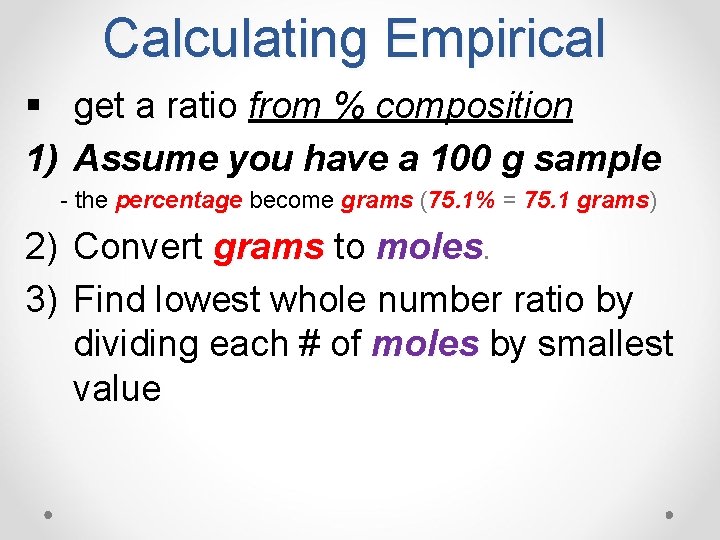
Calculating Empirical § get a ratio from % composition 1) Assume you have a 100 g sample - the percentage become grams (75. 1% = 75. 1 grams) grams 2) Convert grams to moles 3) Find lowest whole number ratio by dividing each # of moles by smallest value

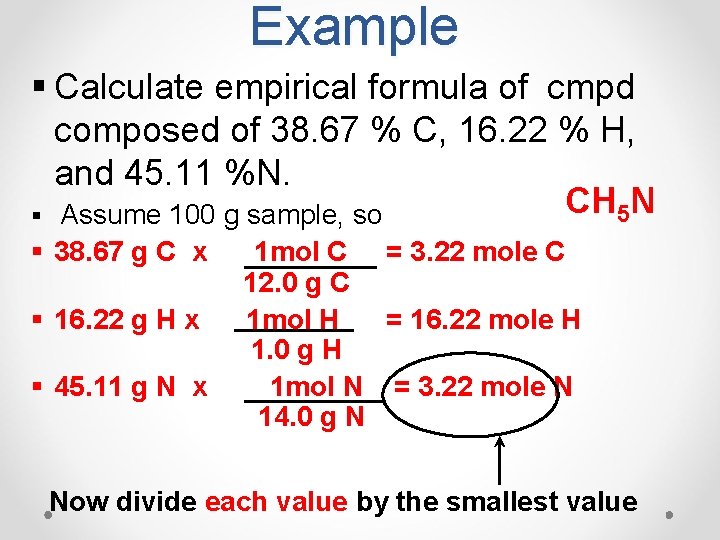
Example § Calculate empirical formula of cmpd composed of 38. 67 % C, 16. 22 % H, and 45. 11 %N. CH 5 N § Assume 100 g sample, so § 38. 67 g C x § 16. 22 g H x § 45. 11 g N x 1 mol C = 3. 22 mole C 12. 0 g C 1 mol H = 16. 22 mole H 1. 0 g H 1 mol N = 3. 22 mole N 14. 0 g N Now divide each value by the smallest value

Example § The ratio is 3. 22 mol C = 1 mol C 3. 22 mol N 1 mol N § The ratio is 16. 22 mol H = 5 mol H 3. 22 mol N 1 mol N = C 1 H 5 N 1 which is = CH 5 N

Practice Problem Calculate the empirical formula of: 94. 1% O, 5. 9% H

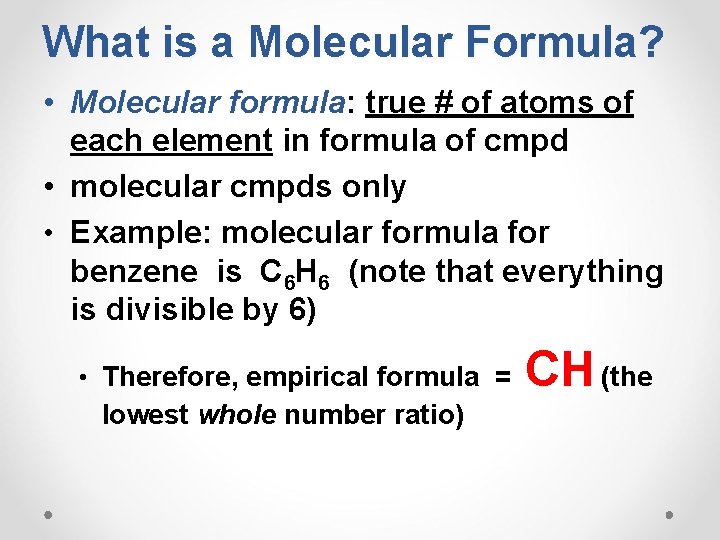
What is a Molecular Formula? • Molecular formula: true # of atoms of each element in formula of cmpd • molecular cmpds only • Example: molecular formula for benzene is C 6 H 6 (note that everything is divisible by 6) • Therefore, empirical formula = lowest whole number ratio) CH (the

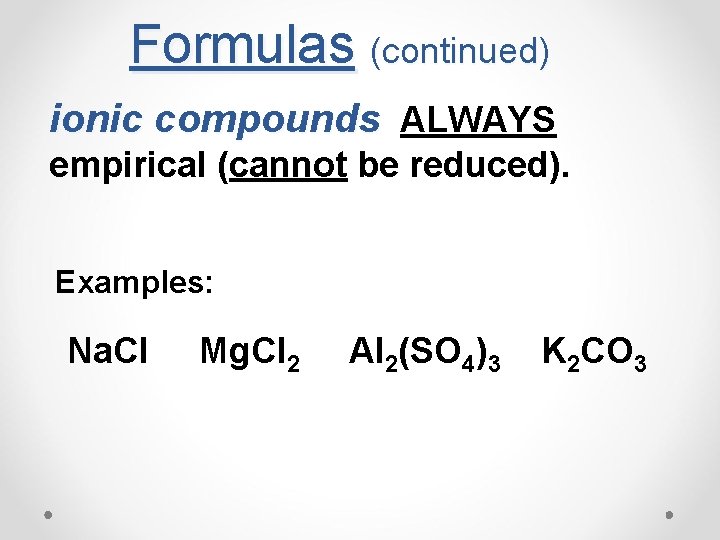
Formulas (continued) ionic compounds ALWAYS empirical (cannot be reduced). Examples: Na. Cl Mg. Cl 2 Al 2(SO 4)3 K 2 CO 3

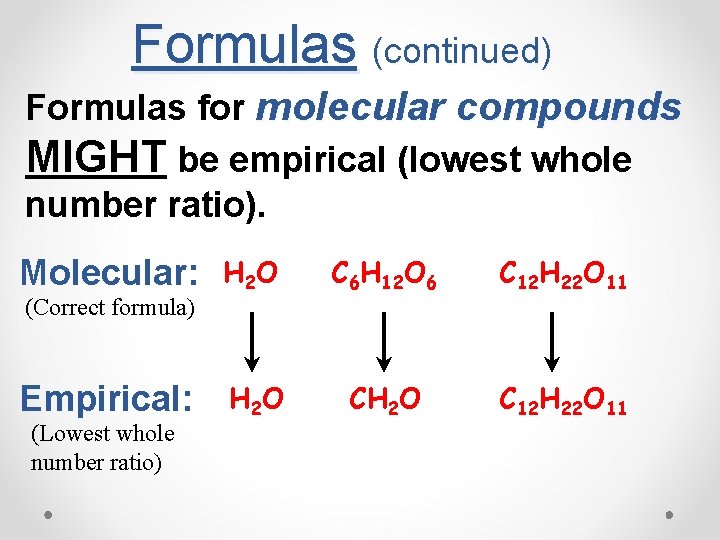
Formulas (continued) Formulas for molecular compounds MIGHT be empirical (lowest whole number ratio). Molecular: (Correct formula) Empirical: (Lowest whole number ratio) H 2 O C 6 H 12 O 6 C 12 H 22 O 11 H 2 O CH 2 O C 12 H 22 O 11

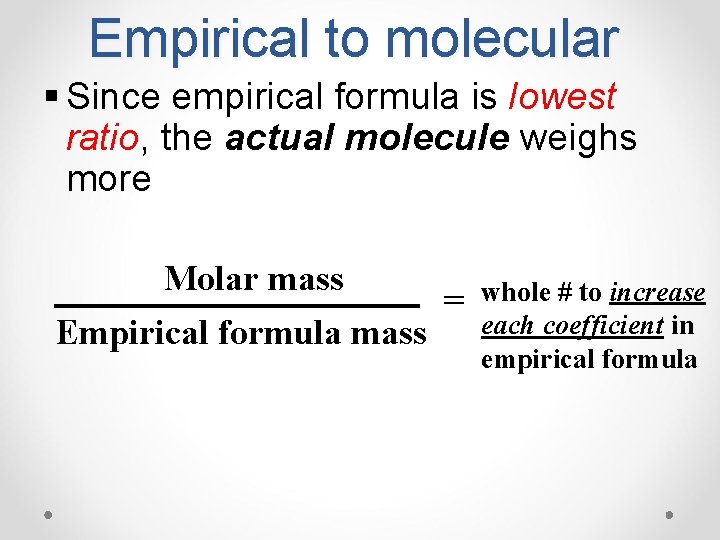
Empirical to molecular § Since empirical formula is lowest ratio, the actual molecule weighs more Molar mass = Empirical formula mass whole # to increase each coefficient in empirical formula