Calorimetry and Enthalpy SCH 4 U Specific heat





- Slides: 26

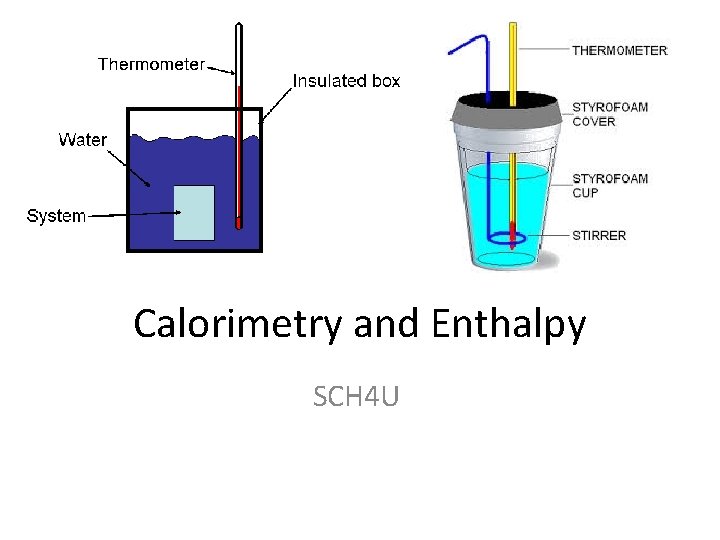
Calorimetry and Enthalpy SCH 4 U

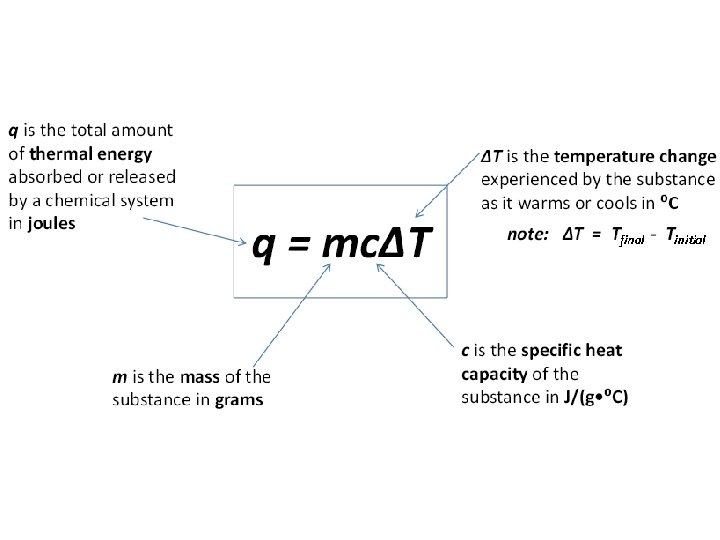
Specific heat capacity (c): • quantity of thermal energy required to raise the temperature of 1 g of a substance by 1⁰C • units for specific heat capacity are J/(g • ⁰C)

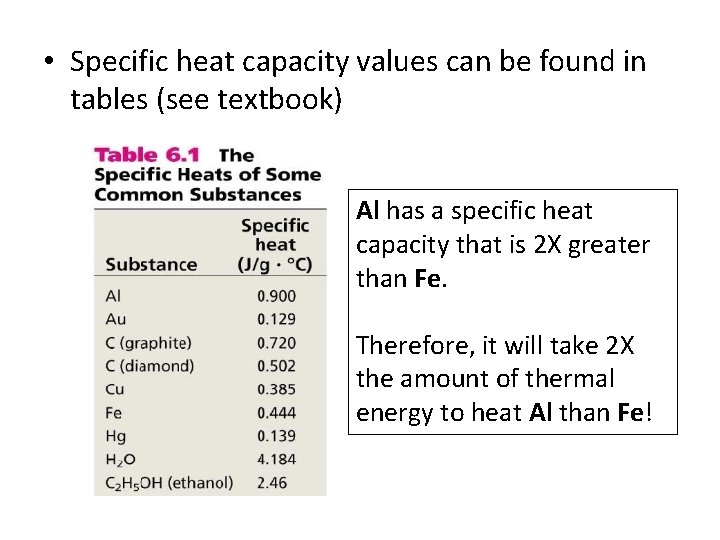
• Specific heat capacity values can be found in tables (see textbook) Al has a specific heat capacity that is 2 X greater than Fe. Therefore, it will take 2 X the amount of thermal energy to heat Al than Fe!

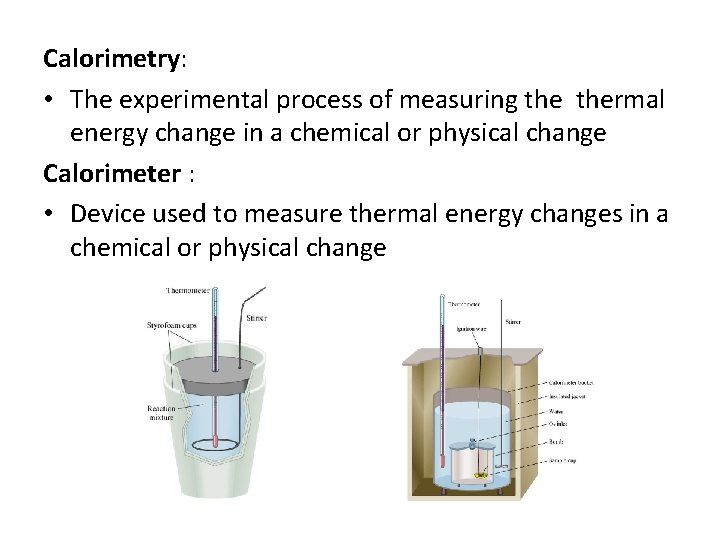
Calorimetry: • The experimental process of measuring thermal energy change in a chemical or physical change Calorimeter : • Device used to measure thermal energy changes in a chemical or physical change


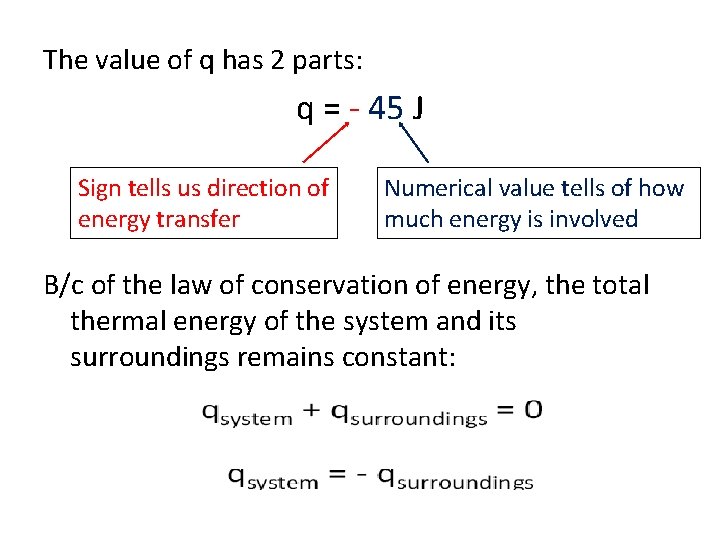
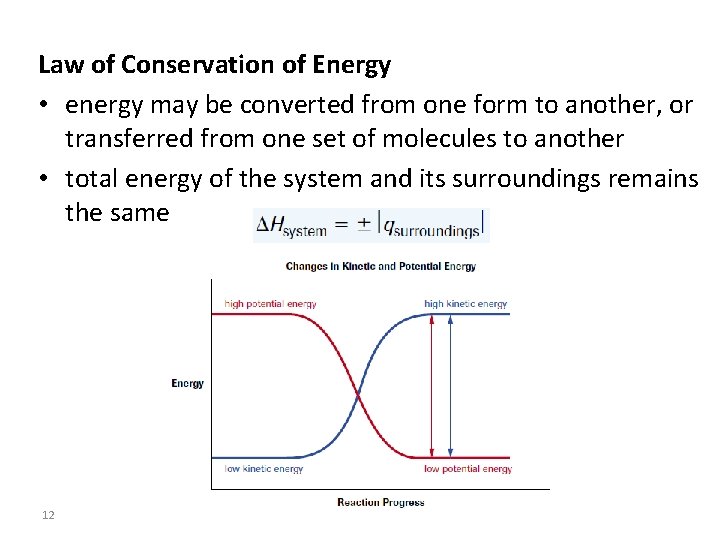
The value of q has 2 parts: q = - 45 J Sign tells us direction of energy transfer Numerical value tells of how much energy is involved B/c of the law of conservation of energy, the total thermal energy of the system and its surroundings remains constant:

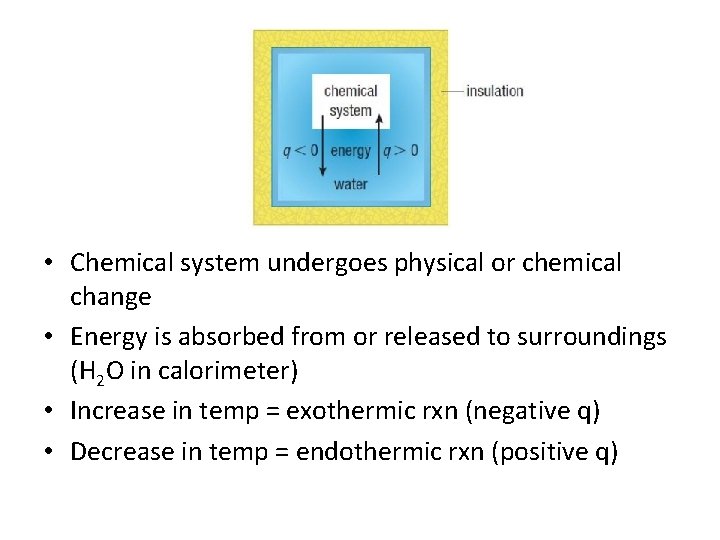
• Chemical system undergoes physical or chemical change • Energy is absorbed from or released to surroundings (H 2 O in calorimeter) • Increase in temp = exothermic rxn (negative q) • Decrease in temp = endothermic rxn (positive q)

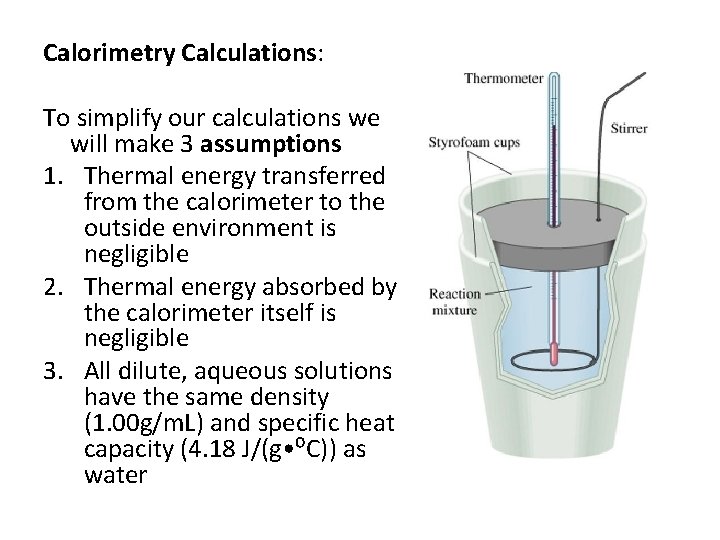
Calorimetry Calculations: To simplify our calculations we will make 3 assumptions 1. Thermal energy transferred from the calorimeter to the outside environment is negligible 2. Thermal energy absorbed by the calorimeter itself is negligible 3. All dilute, aqueous solutions have the same density (1. 00 g/m. L) and specific heat capacity (4. 18 J/(g • ⁰C)) as water

Example 1: 600 m. L of water in an electric kettle is heated from 20⁰C to 85⁰C to make a cup of tea. How much thermal energy is absorbed by the water?

Example 2: The temperature of an aluminum fence post at 5 pm is 20⁰C. The same fence post has a temperature of 6⁰C by 11 pm. If the fence post releases 315 k. J of thermal energy to its surroundings, what is the mass of the fence post?

Enthalpy (H) • total amount of thermal energy in a substance Enthalpy Change (ΔH) • energy released to or absorbed from the surroundings during a chemical or physical change

Law of Conservation of Energy • energy may be converted from one form to another, or transferred from one set of molecules to another • total energy of the system and its surroundings remains the same 12

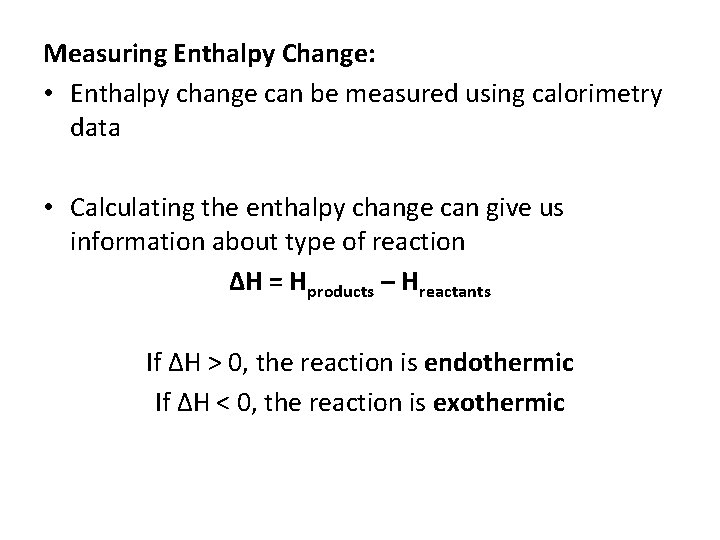
Measuring Enthalpy Change: • Enthalpy change can be measured using calorimetry data • Calculating the enthalpy change can give us information about type of reaction ΔH = Hproducts – Hreactants If ΔH > 0, the reaction is endothermic If ΔH < 0, the reaction is exothermic

Types of Energy Changes: • Physical changes Kinetic Energy changes – moving electrons within atoms – the vibration of atoms connected by chemical bonds – the rotation and translation of molecules that are made up of these atoms • Chemical/Nuclear changes Potential Energy changes – Nuclear: potential energy of protons and neutrons in atomic nuclei – Chemical: electronic potential energy of atoms connected by chemical bonds 14

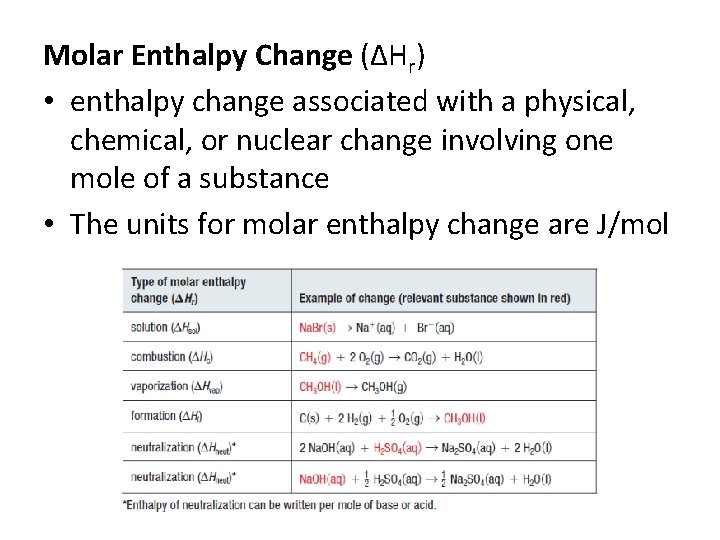
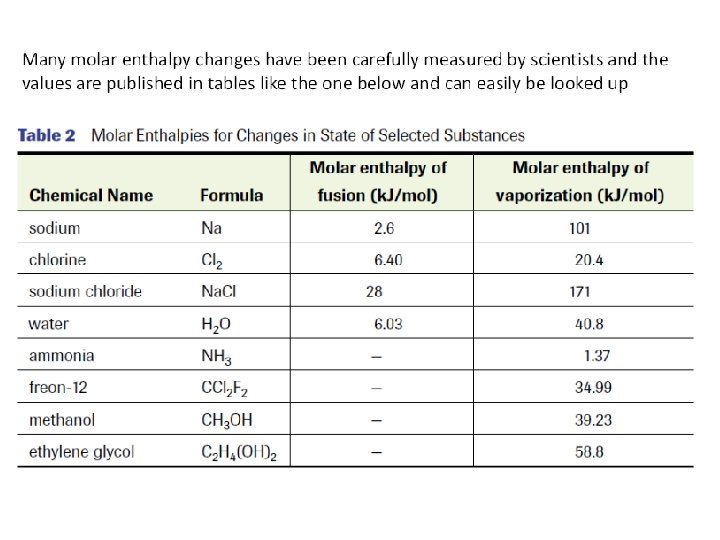
Molar Enthalpy Change (ΔHr) • enthalpy change associated with a physical, chemical, or nuclear change involving one mole of a substance • The units for molar enthalpy change are J/mol


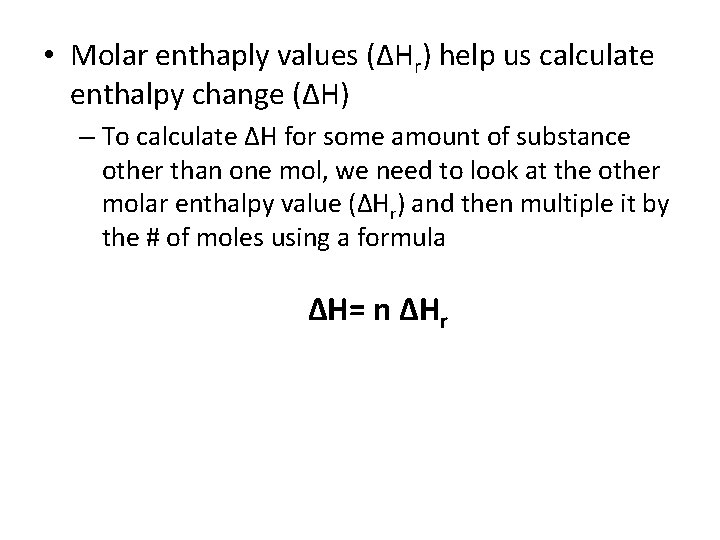
• Molar enthaply values (ΔHr) help us calculate enthalpy change (ΔH) – To calculate ΔH for some amount of substance other than one mol, we need to look at the other molar enthalpy value (ΔHr) and then multiple it by the # of moles using a formula ΔH= n ΔHr

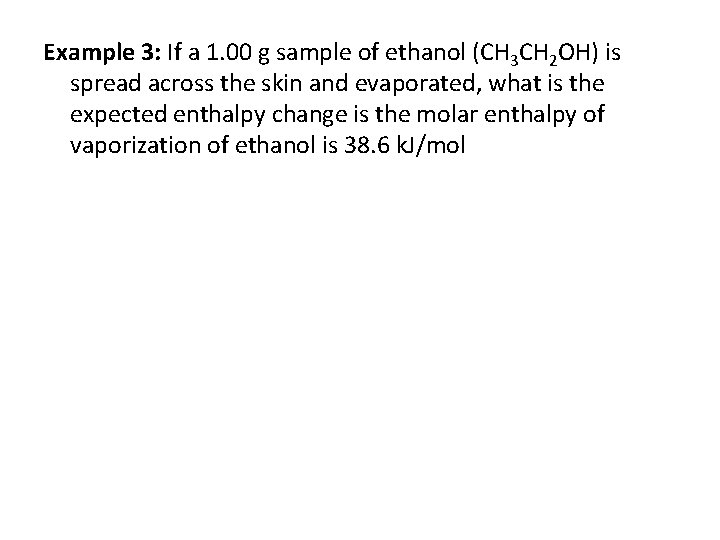
Example 3: If a 1. 00 g sample of ethanol (CH 3 CH 2 OH) is spread across the skin and evaporated, what is the expected enthalpy change is the molar enthalpy of vaporization of ethanol is 38. 6 k. J/mol

Example 4: A student places 125 g of liquid water at 24. 2 ⁰C into a coffee-cup calorimeter and then adds 10. 5 g of KBr(s), also at 24. 2 ⁰C. He stirs the liquid until the KBr dissolves, then determines the temperature is 21. 1 ⁰C. Calculate the molar enthalpy for this dissolution reaction ΔHsol. Assume c is 4. 18 J/(g ⁰C ).

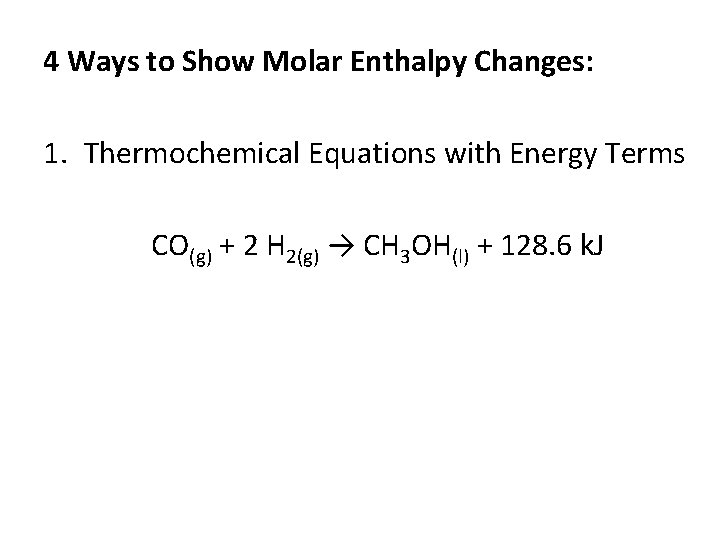
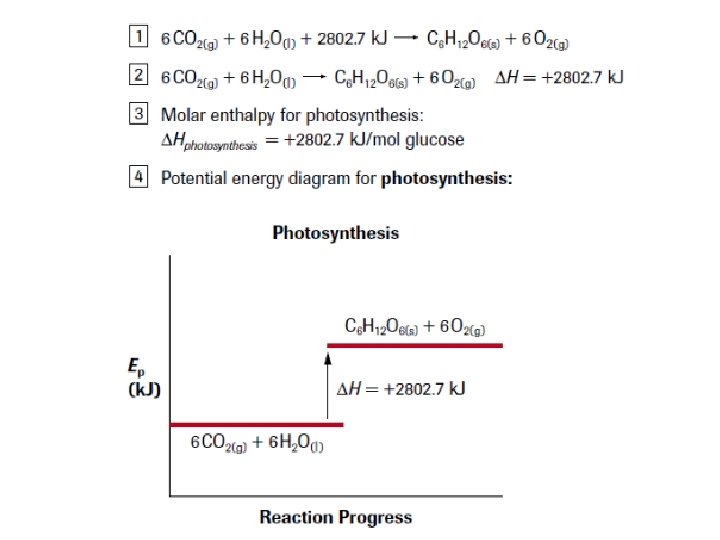
4 Ways to Show Molar Enthalpy Changes: 1. Thermochemical Equations with Energy Terms CO(g) + 2 H 2(g) → CH 3 OH(l) + 128. 6 k. J

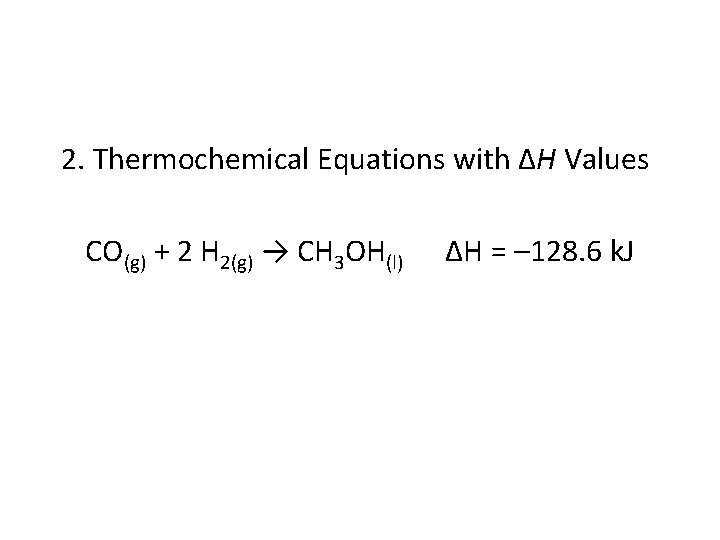
2. Thermochemical Equations with ∆H Values CO(g) + 2 H 2(g) → CH 3 OH(l) ∆H = – 128. 6 k. J

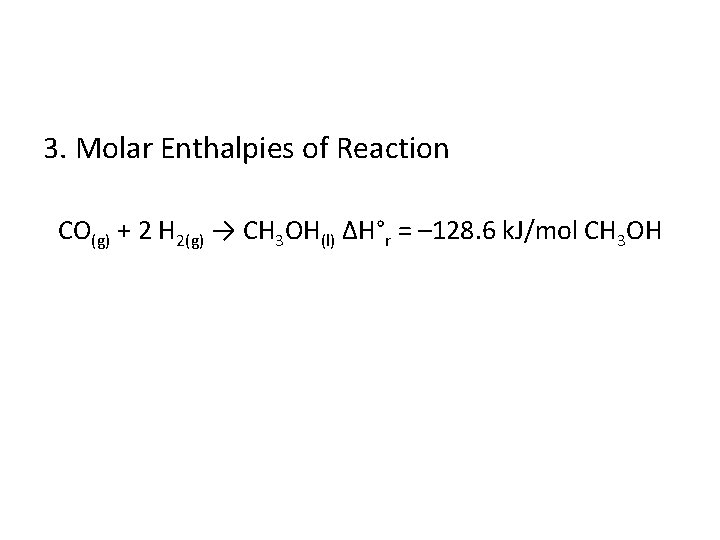
3. Molar Enthalpies of Reaction CO(g) + 2 H 2(g) → CH 3 OH(l) ∆H°r = – 128. 6 k. J/mol CH 3 OH

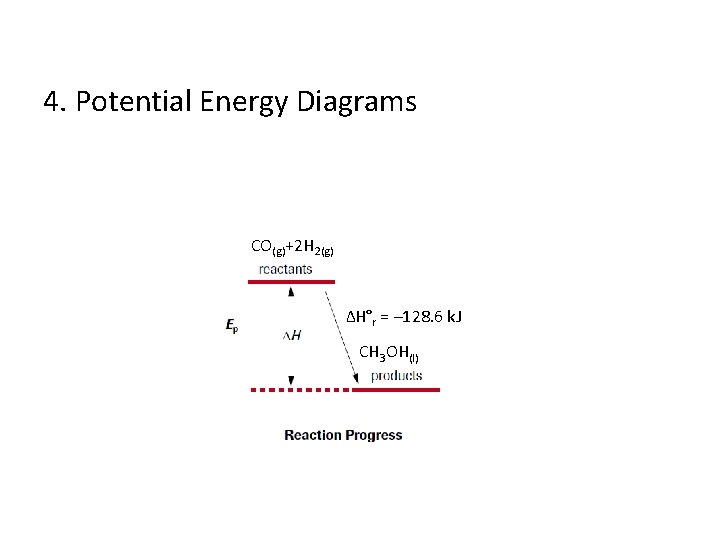
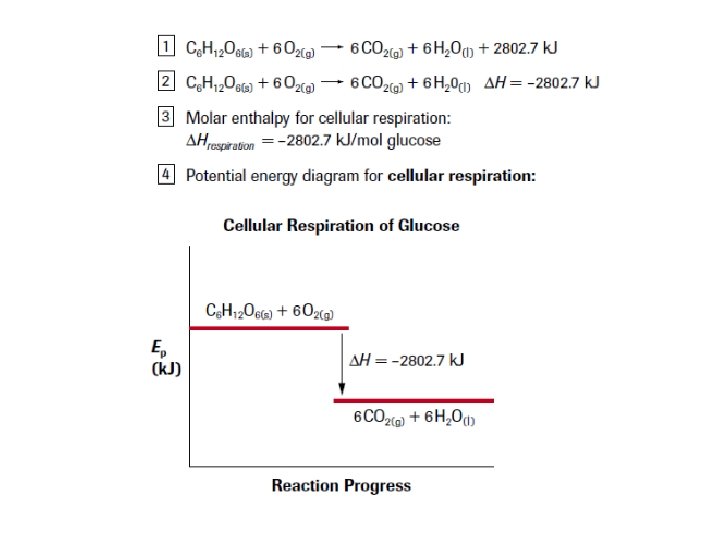
4. Potential Energy Diagrams CO(g)+2 H 2(g) ∆H°r = – 128. 6 k. J CH 3 OH(l)



Homework: Pg. 297 #1 -3 Pg. 301 #1 -4 Pg. 304 #1 -4