AZIENDA OSPEDALIEROUNIVERSITARIA DI MODENA Evoluzione del trattamento del















- Slides: 47

AZIENDA OSPEDALIERO-UNIVERSITARIA DI MODENA Evoluzione del trattamento del tumore mammario: dalla ricerca alla clinica Nonantola, 18 Novembre, 2011 Pier. Franco Conte Dipartimento di Oncologia, Ematologia e Malattie dell’ Apparato Respiratorio Università di Modena e Reggio Emilia

The Conquest of Breast Cancer: a few more steps ahead…. – Where we are now – How we got here – Standard of Care for EBC – Remaining questions – The Challenges ahead

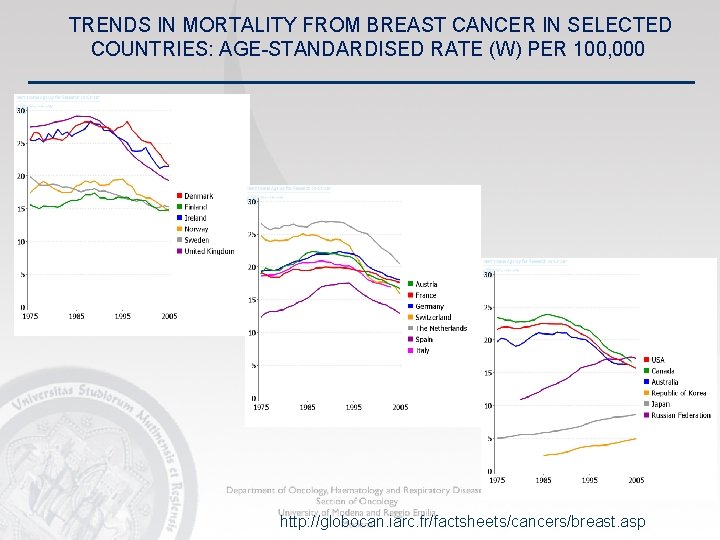
TRENDS IN MORTALITY FROM BREAST CANCER IN SELECTED COUNTRIES: AGE-STANDARDISED RATE (W) PER 100, 000 http: //globocan. iarc. fr/factsheets/cancers/breast. asp

The Conquest of Breast Cancer: a few more steps ahead…. – Where we are now – How we got here – Standard of Care for EBC – Remaining questions – The Challenges ahead

Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database • Breast cancer mortality in three pairs of countries (North Ireland vs Rep. of Ireland, the Netherlands vs Belgium and Sweden vs Norway) from 1989 to 2006 • Countries in each pair had similar healthcare services, but differing implementation of mammography screening, with a gap of about 10 -15 years • No correlation between the introduction of screeening and reduction in breast cancer mortality • The steepest fall in mortality observed was among the women under 50 who had not been invited for screening • Screening did not play a direct role in the reductions of breast cancer mortality • Improvements in adjuvant treatment may be a more plausible explanation. P Autier et al; BMJ 2011

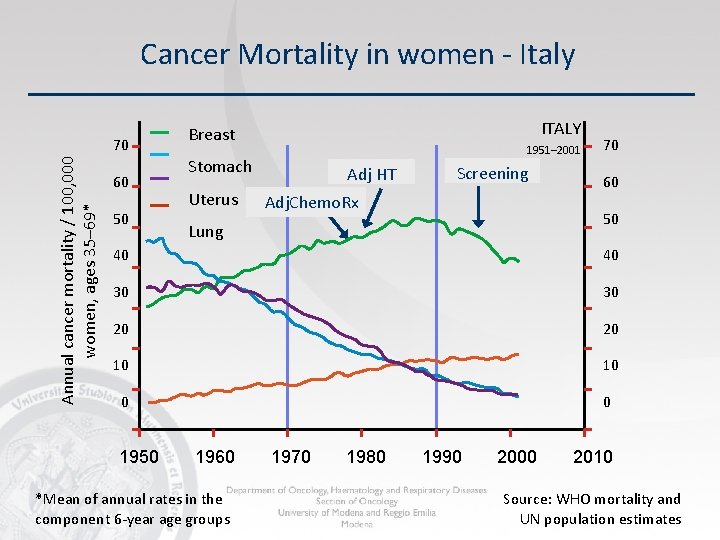
Cancer Mortality in women - Italy Annual cancer mortality / 100, 000 women, ages 35– 69* 70 60 50 ITALY Breast 1951– 2001 Stomach Uterus Adj HT Screening Adj. Chemo. Rx 70 60 50 Lung 40 40 30 30 20 20 10 10 0 0 1950 1960 *Mean of annual rates in the component 6 -year age groups 1970 1980 1990 2000 2010 Source: WHO mortality and UN population estimates

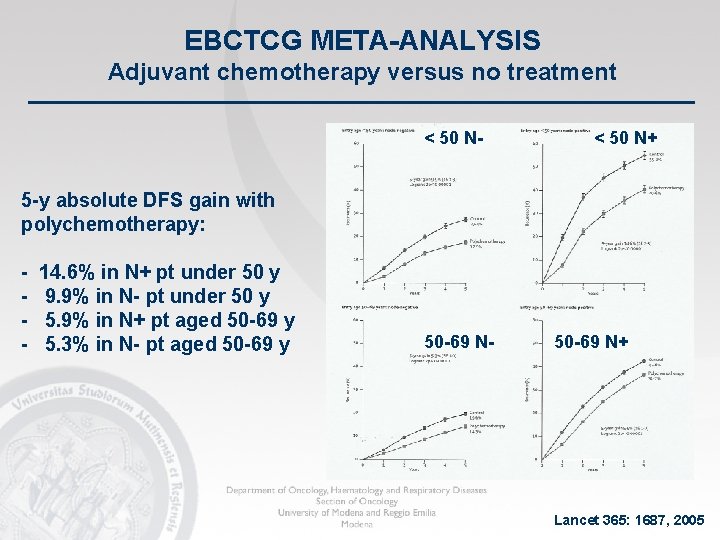
EBCTCG META-ANALYSIS Adjuvant chemotherapy versus no treatment < 50 N- < 50 N+ 5 -y absolute DFS gain with polychemotherapy: - 14. 6% in N+ pt under 50 y 9. 9% in N- pt under 50 y 5. 9% in N+ pt aged 50 -69 y 5. 3% in N- pt aged 50 -69 y 50 -69 N- 50 -69 N+ Lancet 365: 1687, 2005

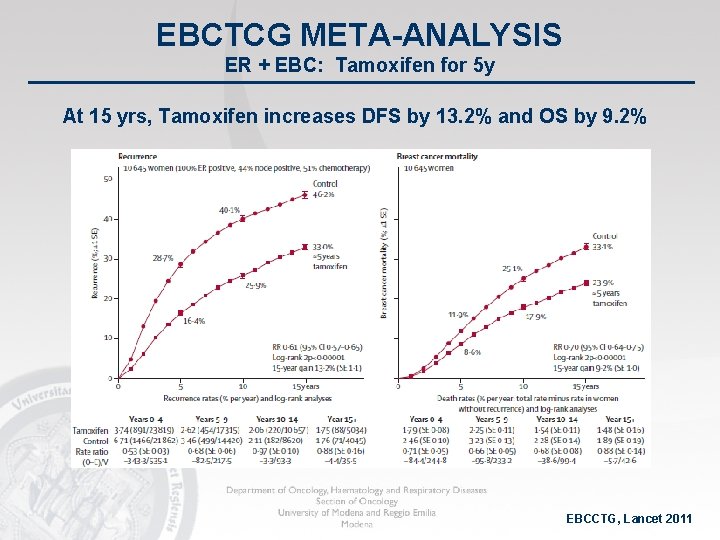
EBCTCG META-ANALYSIS ER + EBC: Tamoxifen for 5 y At 15 yrs, Tamoxifen increases DFS by 13. 2% and OS by 9. 2% EBCCTG, Lancet 2011

The Conquest of Breast Cancer: a few more steps ahead…. – Where we are now – How we got here – Standard of Care for EBC – Remaining questions – The Challenges ahead

Adjuvant Treatment of EBC: Refinement of Standard of Care 1990 on: increasing use of anthracyclines 2000 on: increasing use of taxanes increasing use of aromatase inhibitors 2005 on: increasing use of trastuzumab

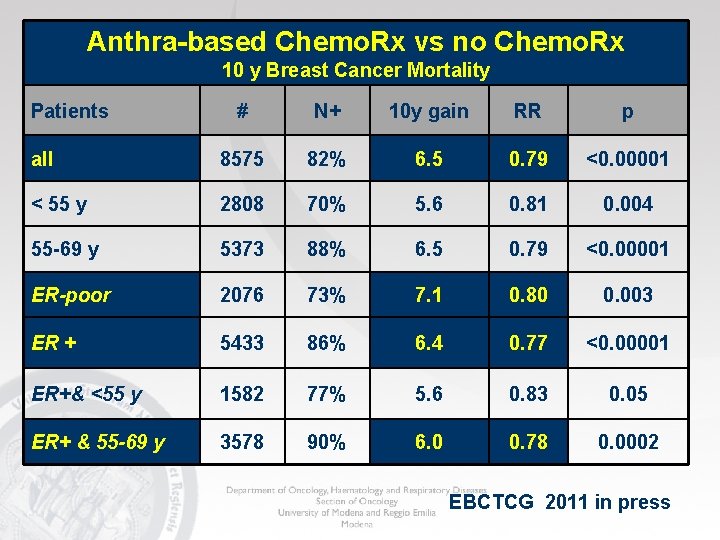
Anthra-based Chemo. Rx vs no Chemo. Rx 10 y Breast Cancer Mortality Patients # N+ 10 y gain RR p all 8575 82% 6. 5 0. 79 <0. 00001 < 55 y 2808 70% 5. 6 0. 81 0. 004 55 -69 y 5373 88% 6. 5 0. 79 <0. 00001 ER-poor 2076 73% 7. 1 0. 80 0. 003 ER + 5433 86% 6. 4 0. 77 <0. 00001 ER+& <55 y 1582 77% 5. 6 0. 83 0. 05 ER+ & 55 -69 y 3578 90% 6. 0 0. 78 0. 0002 EBCTCG 2011 in press

EBCTCG Meta-analysis Anthra + Taxane vs non Taxane Chemo. Rx (33, 084 pts; 82% N+) 5 y RR gain p recurrence 0. 86 2. 9 <0. 00001 BC mortality 0. 88 1. 4 0. 0001 EBCTCG 2011 in press

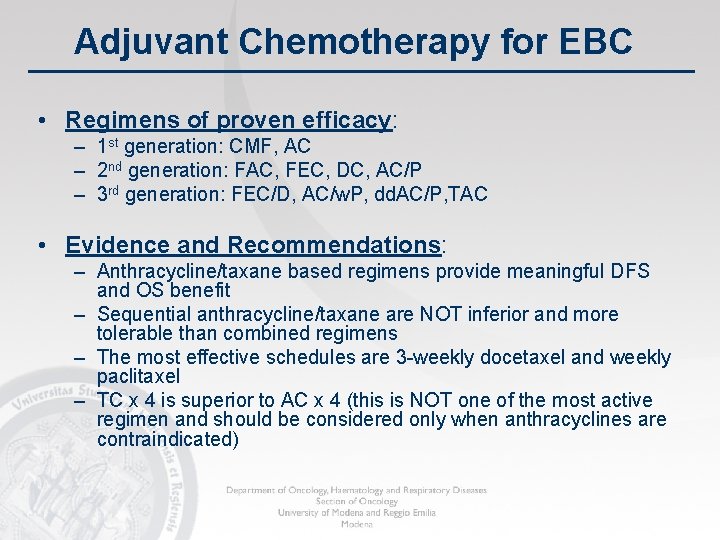
Adjuvant Chemotherapy for EBC • Regimens of proven efficacy: – 1 st generation: CMF, AC – 2 nd generation: FAC, FEC, DC, AC/P – 3 rd generation: FEC/D, AC/w. P, dd. AC/P, TAC • Evidence and Recommendations: – Anthracycline/taxane based regimens provide meaningful DFS and OS benefit – Sequential anthracycline/taxane are NOT inferior and more tolerable than combined regimens – The most effective schedules are 3 -weekly docetaxel and weekly paclitaxel – TC x 4 is superior to AC x 4 (this is NOT one of the most active regimen and should be considered only when anthracyclines are contraindicated)

Adjuvant Treatment of EBC: Refinement of Standard of Care 1990 on: increasing use of anthracyclines 2000 on: increasing use of taxanes increasing use of aromatase inhibitors 2005 on: increasing use of trastuzumab

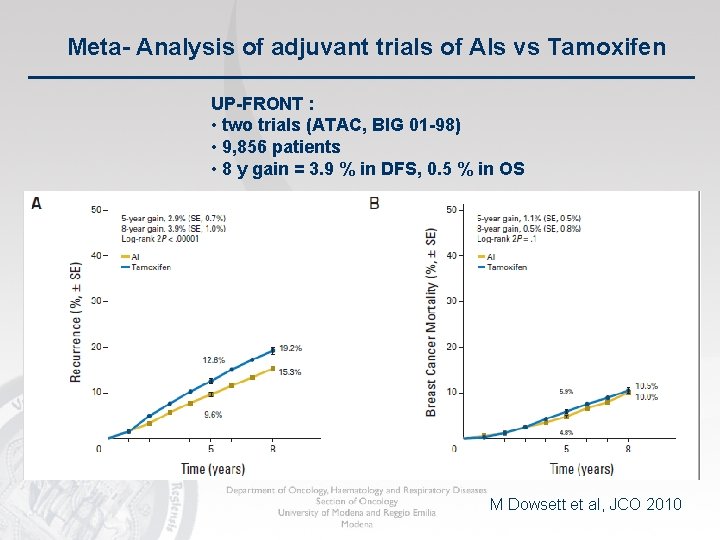
Meta- Analysis of adjuvant trials of AIs vs Tamoxifen UP-FRONT : • two trials (ATAC, BIG 01 -98) • 9, 856 patients • 8 y gain = 3. 9 % in DFS, 0. 5 % in OS M Dowsett et al, JCO 2010

Meta- Analysis of adjuvant trials of AIs vs Tamoxifen SWITCH : • four trials (ARNO, IES, ITA, ABCSG VIII) • 9, 015 patients • 6 y gain = 3. 6 % in DFS, 1. 7 % in OS M Dowsett et al, JCO 2010

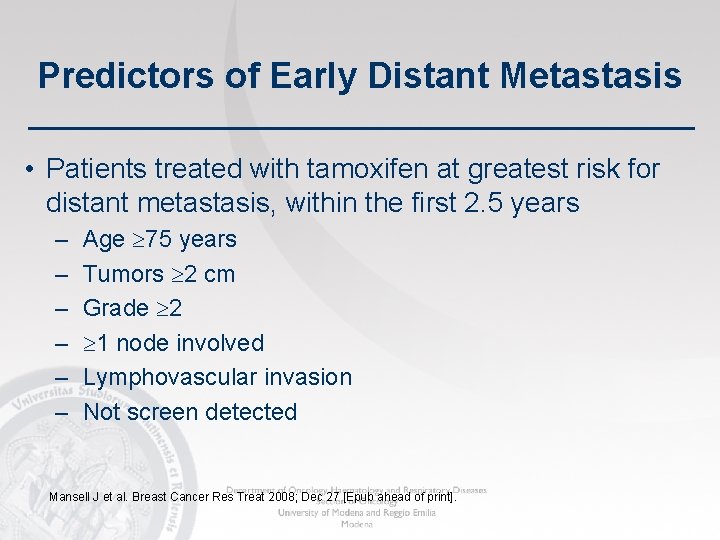
Predictors of Early Distant Metastasis • Patients treated with tamoxifen at greatest risk for distant metastasis, within the first 2. 5 years – – – Age 75 years Tumors 2 cm Grade 2 1 node involved Lymphovascular invasion Not screen detected Mansell J et al. Breast Cancer Res Treat 2008; Dec 27 [Epub ahead of print].

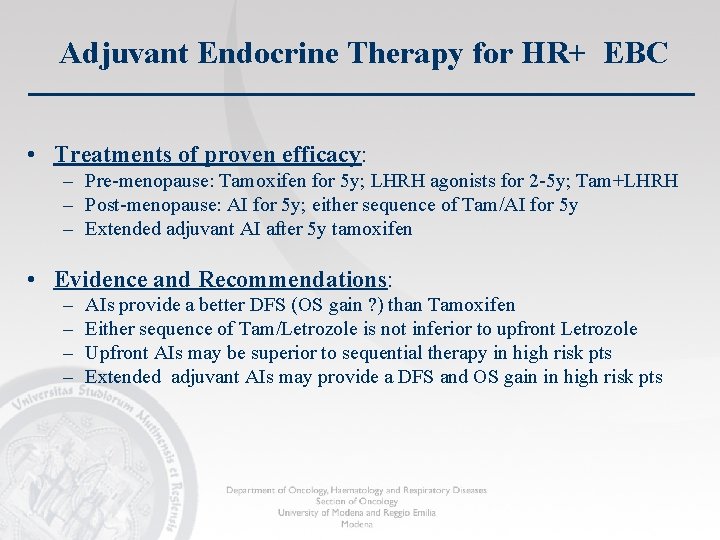
Adjuvant Endocrine Therapy for HR+ EBC • Treatments of proven efficacy: – Pre-menopause: Tamoxifen for 5 y; LHRH agonists for 2 -5 y; Tam+LHRH – Post-menopause: AI for 5 y; either sequence of Tam/AI for 5 y – Extended adjuvant AI after 5 y tamoxifen • Evidence and Recommendations: – – AIs provide a better DFS (OS gain ? ) than Tamoxifen Either sequence of Tam/Letrozole is not inferior to upfront Letrozole Upfront AIs may be superior to sequential therapy in high risk pts Extended adjuvant AIs may provide a DFS and OS gain in high risk pts

Adjuvant Treatment of EBC: Refinement of Standard of Care 1990 on: increasing use of anthracyclines 2000 on: increasing use of taxanes increasing use of aromatase inhibitors 2005 on: increasing use of trastuzumab

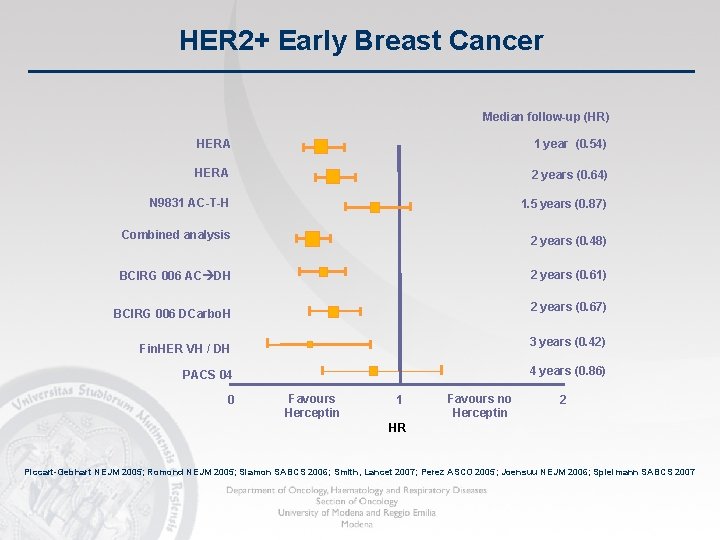
HER 2+ Early Breast Cancer Median follow-up (HR) HERA 1 year (0. 54) HERA 2 years (0. 64) N 9831 AC-T-H 1. 5 years (0. 87) Combined analysis 2 years (0. 48) BCIRG 006 AC DH 2 years (0. 61) 2 years (0. 67) BCIRG 006 DCarbo. H 3 years (0. 42) Fin. HER VH / DH 4 years (0. 86) PACS 04 0 Favours Herceptin 1 Favours no Herceptin 2 HR Piccart-Gebhart NEJM 2005; Romond NEJM 2005; Slamon SABCS 2006; Smith, Lancet 2007; Perez ASCO 2005; Joensuu NEJM 2006; Spielmann SABCS 2007

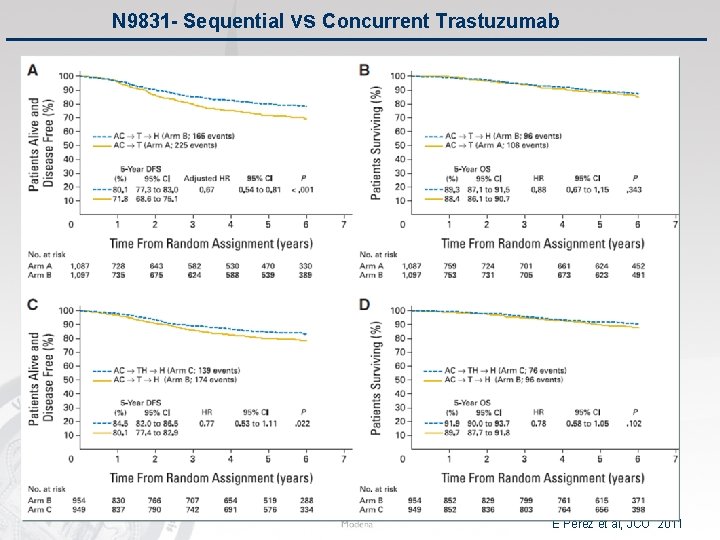
N 9831 - Sequential vs Concurrent Trastuzumab E Perez et al, JCO 2011

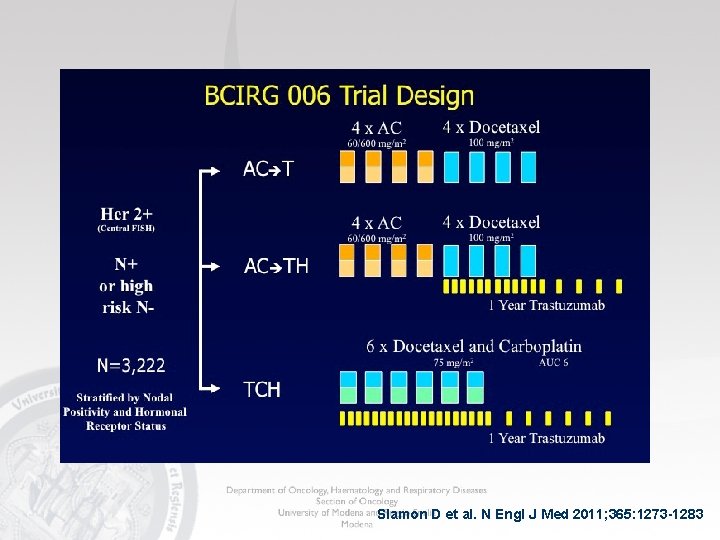
Slamon D et al. N Engl J Med 2011; 365: 1273 -1283

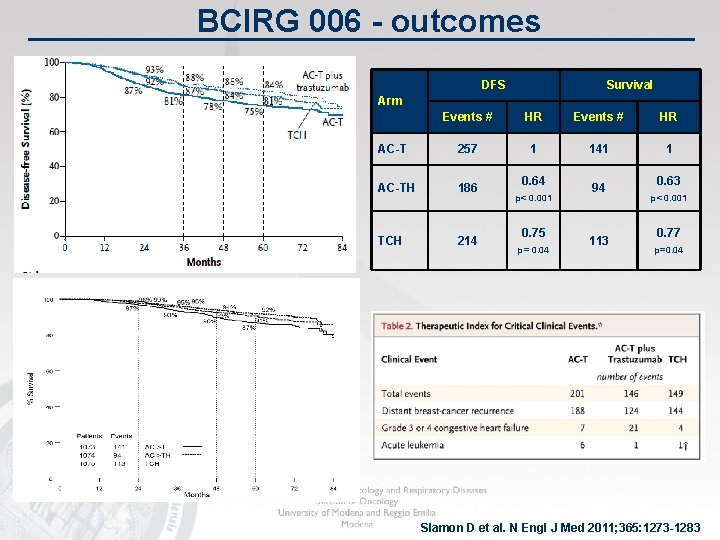
BCIRG 006 - outcomes DFS Survival Arm Events # HR AC-T 257 1 141 1 AC-TH 186 TCH 214 0. 64 p< 0. 001 0. 75 p= 0. 04 94 113 0. 63 p< 0. 001 0. 77 p=0. 04 Slamon D et al. N Engl J Med 2011; 365: 1273 -1283

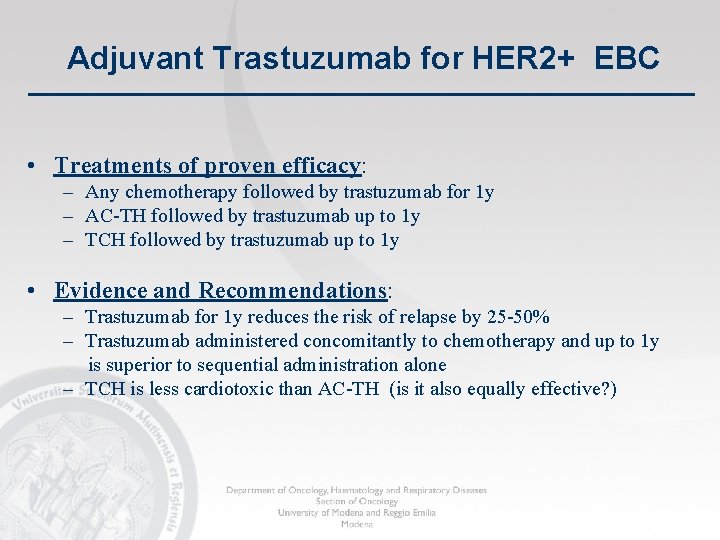
Adjuvant Trastuzumab for HER 2+ EBC • Treatments of proven efficacy: – Any chemotherapy followed by trastuzumab for 1 y – AC-TH followed by trastuzumab up to 1 y – TCH followed by trastuzumab up to 1 y • Evidence and Recommendations: – Trastuzumab for 1 y reduces the risk of relapse by 25 -50% – Trastuzumab administered concomitantly to chemotherapy and up to 1 y is superior to sequential administration alone – TCH is less cardiotoxic than AC-TH (is it also equally effective? )

The Conquest of Breast Cancer: a few more steps ahead…. – Where we are now – How we got here – Standard of Care for EBC – Remaining questions – The Challenges ahead

Adjuvant treatments of EBC Remaining questions – Genomic assays to predict outcome and chemosensitivity of HR+ tumors – Trastuzumab for small, N-ve HER 2+ tumors

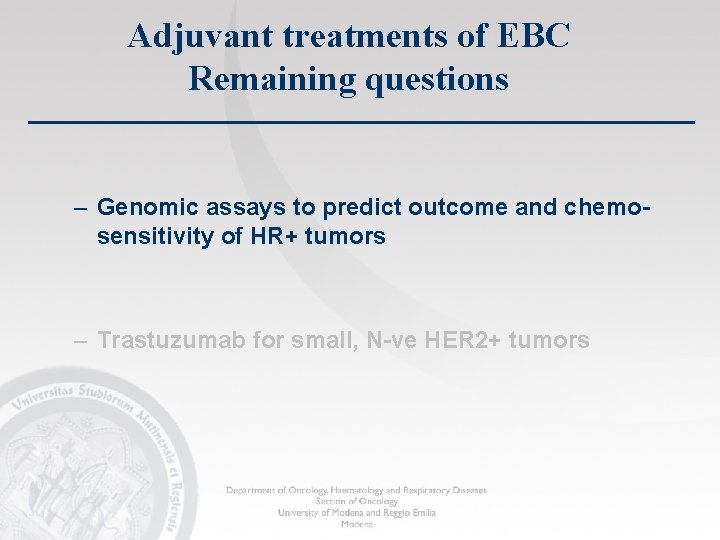
Genomic assays to predict clinical outcome C Sotiriou & L Pusztai

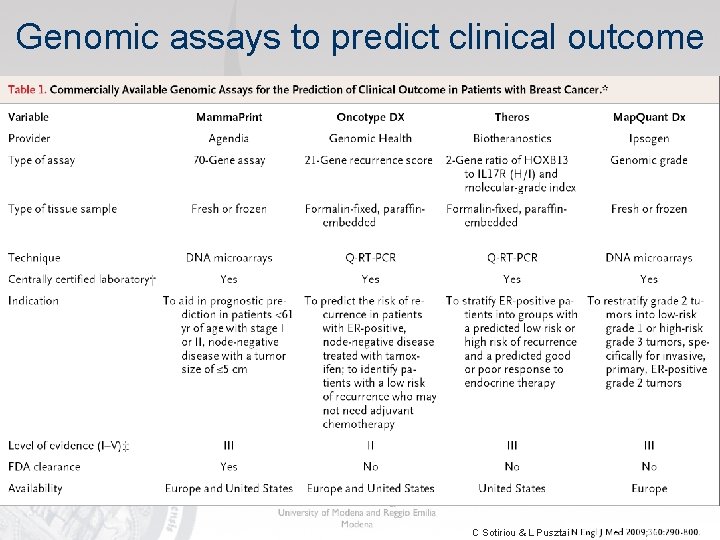
Benefit of Tamoxifen or Chemotherapy by RS NSABP B-20 NSABP B-14 Low Risk (RS<18) Int Risk (RS 18 -30) High Risk (RS≥ 31)

Genomic Health Web Site (november 2011) • Oncotype. Dx testing requests: - more than 10, 000 doctors - 55 countries - 175, 000 patients

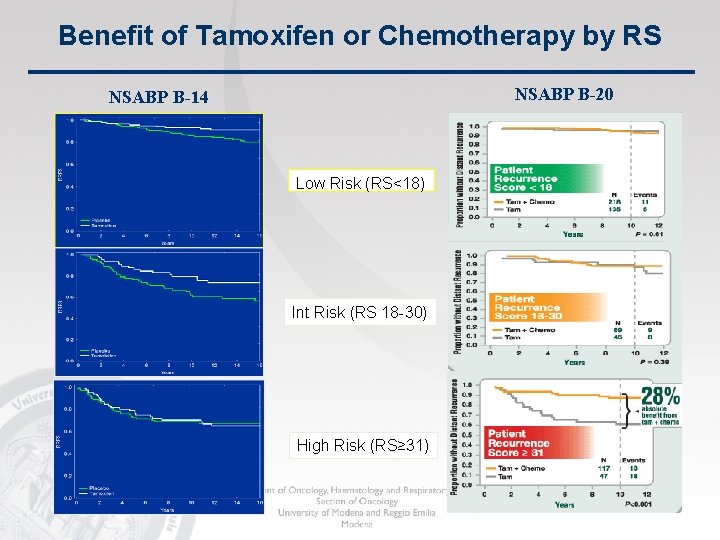
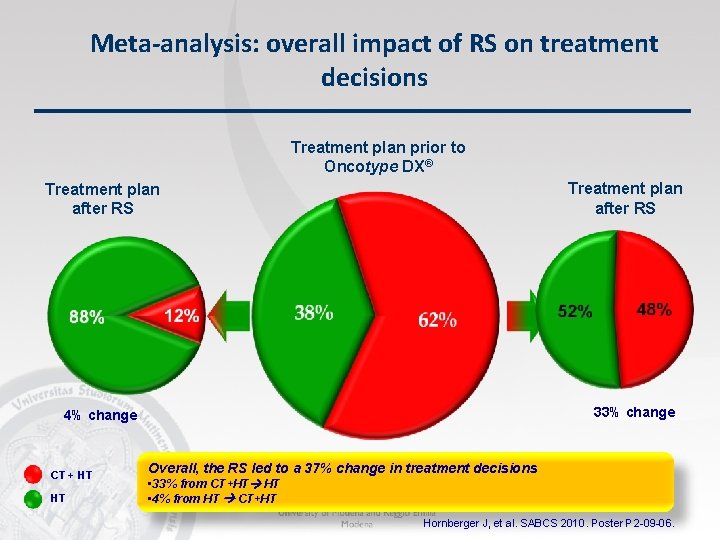
Meta-analysis: overall impact of RS on treatment decisions Treatment plan prior to Oncotype DX® Treatment plan after RS 33% change 4% change CT + HT HT Overall, the RS led to a 37% change in treatment decisions • 33% from CT+HT HT • 4% from HT CT+HT Hornberger J, et al. SABCS 2010. Poster P 2 -09 -06.

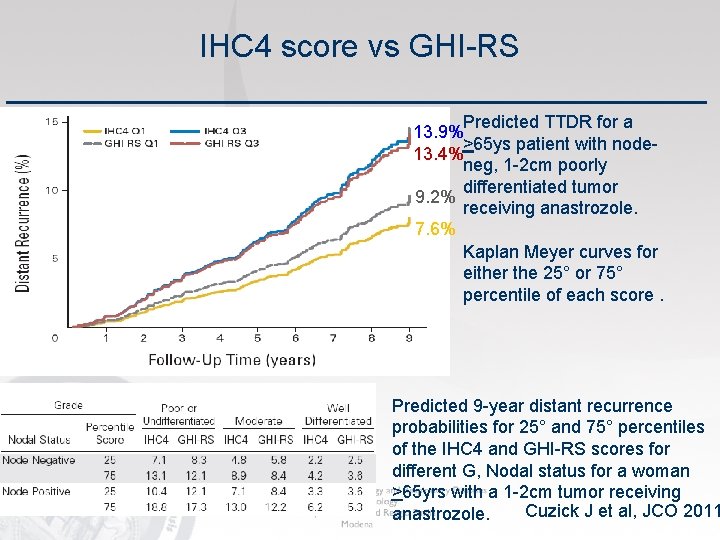
IHC 4 score vs GHI-RS Predicted TTDR for a 13. 9% >65 ys patient with node 13. 4% neg, 1 -2 cm poorly differentiated tumor 9. 2% receiving anastrozole. 7. 6% Kaplan Meyer curves for either the 25° or 75° percentile of each score. Predicted 9 -year distant recurrence probabilities for 25° and 75° percentiles of the IHC 4 and GHI-RS scores for different G, Nodal status for a woman >65 yrs with a 1 -2 cm tumor receiving Cuzick J et al, JCO 2011 anastrozole.

Adjuvant treatments of EBC Remaining questions – Genomic assays to predict outcome and chemosensitivity of HR+ tumors – Trastuzumab for small, N-ve HER 2+ tumors

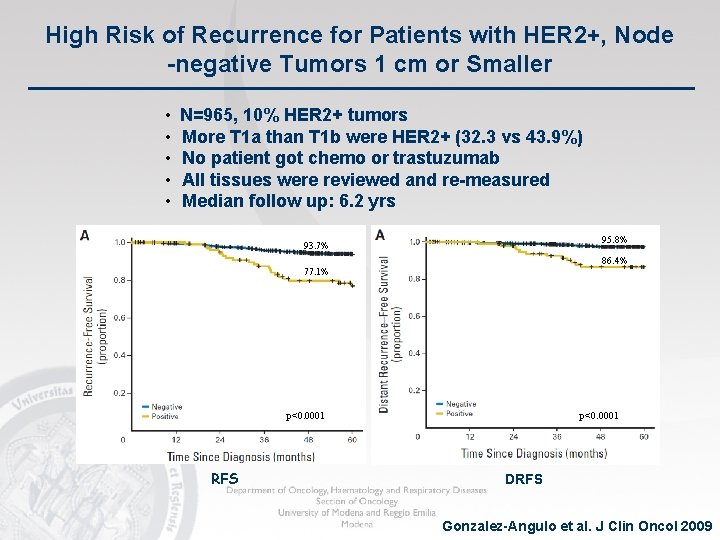
High Risk of Recurrence for Patients with HER 2+, Node -negative Tumors 1 cm or Smaller • • • N=965, 10% HER 2+ tumors More T 1 a than T 1 b were HER 2+ (32. 3 vs 43. 9%) No patient got chemo or trastuzumab All tissues were reviewed and re-measured Median follow up: 6. 2 yrs 95. 8% 93. 7% 86. 4% 77. 1% p<0. 0001 RFS p<0. 0001 DRFS Gonzalez-Angulo et al. J Clin Oncol 2009

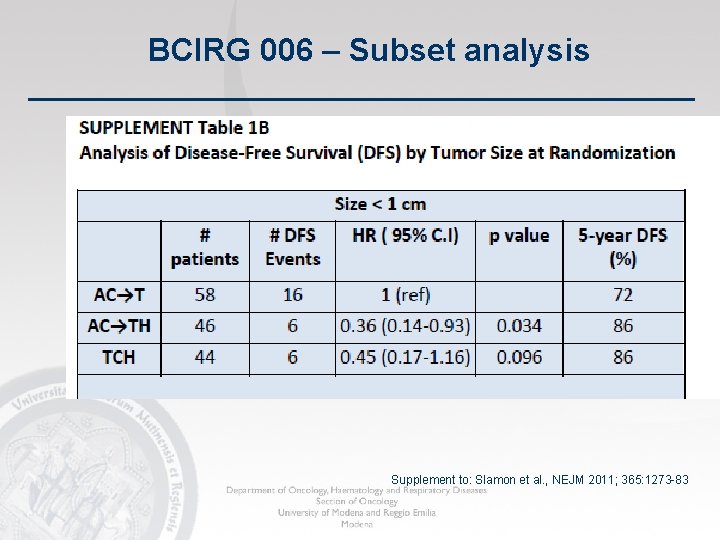
BCIRG 006 – Subset analysis Supplement to: Slamon et al. , NEJM 2011; 365: 1273 -83

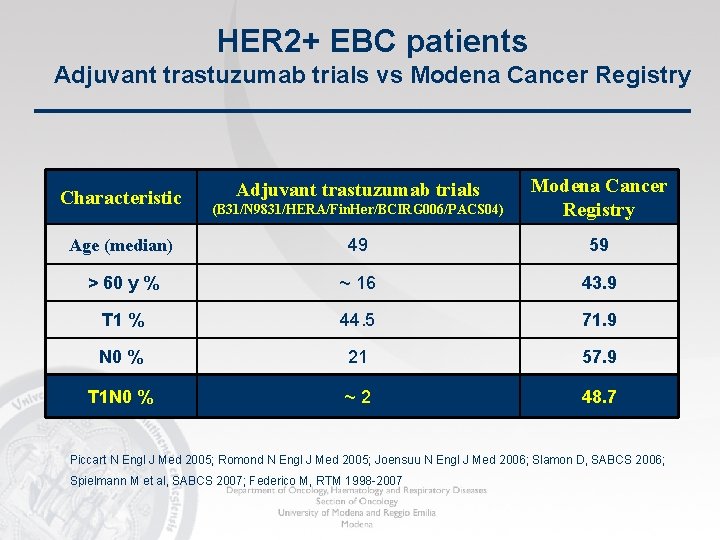
HER 2+ EBC patients Adjuvant trastuzumab trials vs Modena Cancer Registry (B 31/N 9831/HERA/Fin. Her/BCIRG 006/PACS 04) Modena Cancer Registry Age (median) 49 59 > 60 y % ~ 16 43. 9 T 1 % 44. 5 71. 9 N 0 % 21 57. 9 T 1 N 0 % ~ 2 48. 7 Characteristic Adjuvant trastuzumab trials Piccart N Engl J Med 2005; Romond N Engl J Med 2005; Joensuu N Engl J Med 2006; Slamon D, SABCS 2006; Spielmann M et al, SABCS 2007; Federico M, RTM 1998 -2007

Adjuvant Trastuzumab for T 1 N 0 HER 2+ EBC • Prognosis of small, N 0, HER 2+ tumors is poorer • Trastuzumab has shown efficacy in these patients • Small, node negative tumors as well as elderly patients, are underepresented in clinical trials • Cardiac safety and cardiac recovery in elderly patients treated with trastuzumab are basically unknown • In these patients, competitive deaths are prevalent, and the decision to treat should be based on a careful evaluation of the risk/benefit ratio • Trials designed for frail and low risk patients are warranted

The Conquest of Breast Cancer: a few more steps ahead…. – Where we are now – How we got here – Standard of Care for EBC – Remaining questions – The Challenges ahead

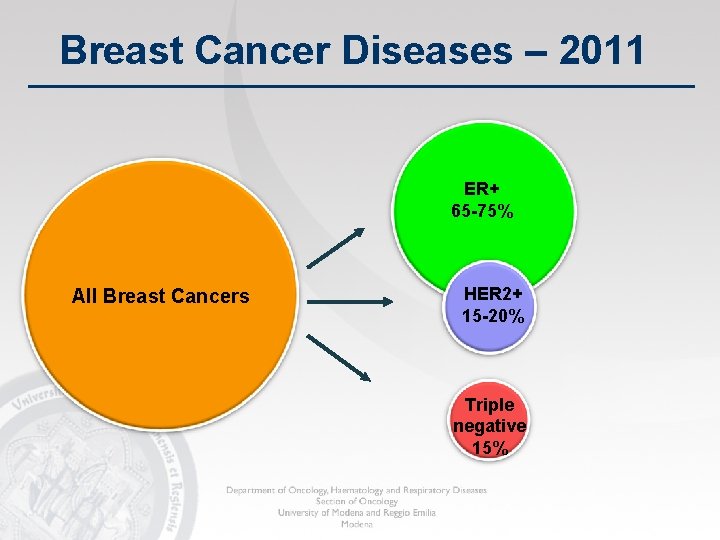
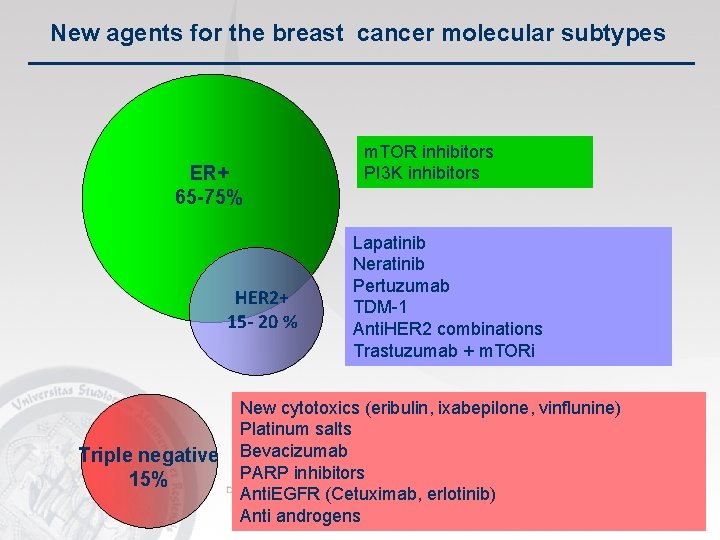
Breast Cancer Diseases – 2011 ER+ 65 -75% All Breast Cancers HER 2+ 15 -20% Triple negative 15%

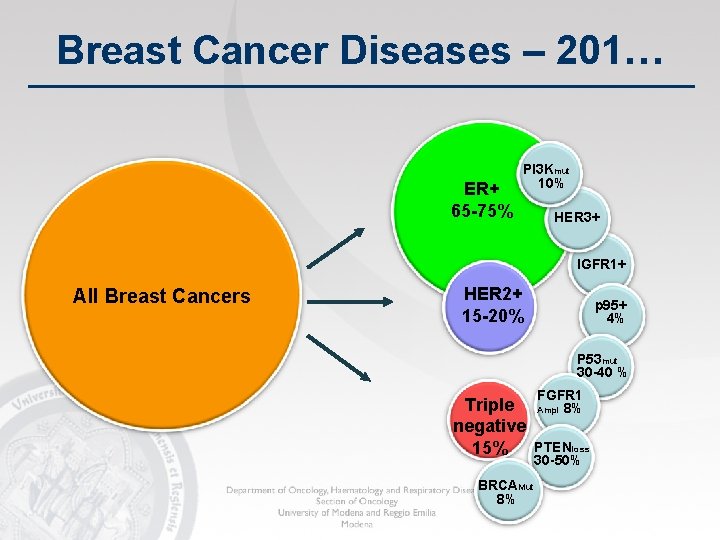
Breast Cancer Diseases – 201… ER+ 65 -75% PI 3 Kmut 10% HER 3+ IGFR 1+ All Breast Cancers HER 2+ 15 -20% p 95+ 4% P 53 mut 30 -40 % Triple negative 15% BRCAMut 8% FGFR 1 Ampl 8% PTENloss 30 -50%

Clinical Genomics: The Next Frontier Stratton, Campbell and Futreal Nature 2009 Ph. RMA Report 2011, Medicines in development for cancer

New agents for the breast cancer molecular subtypes ER+ 65 -75% HER 2+ 15 - 20 % Triple negative 15% m. TOR inhibitors PI 3 K inhibitors Lapatinib Neratinib Pertuzumab TDM-1 Anti. HER 2 combinations Trastuzumab + m. TORi New cytotoxics (eribulin, ixabepilone, vinflunine) Platinum salts Bevacizumab PARP inhibitors Anti. EGFR (Cetuximab, erlotinib) Anti androgens

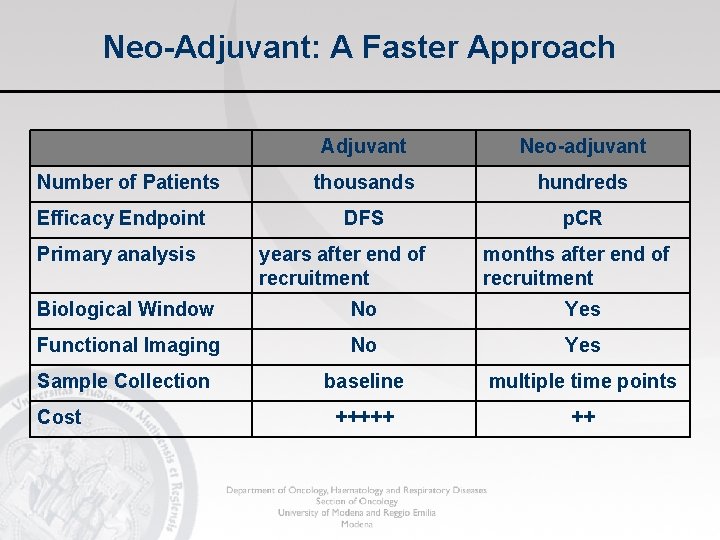
Neo-Adjuvant: A Faster Approach Number of Patients Efficacy Endpoint Primary analysis Adjuvant Neo-adjuvant thousands hundreds DFS p. CR years after end of recruitment months after end of recruitment Biological Window No Yes Functional Imaging No Yes Sample Collection baseline multiple time points +++++ ++ Cost

HER 2+ EBC RCTs of PCT + dual anti. HER 2 blockade Trial pts # Regimen p. CR % (breast & N) Neo-ALLTO 1 455 w. Pac+T/L/TL 20 /27. 6/46. 9* Neo. Sphere 2 417 DT/DTP/TP/DP 21. 5/39. 3*/11. 2/17. 7 Cher. Lob 3 121 T = trastuzumab L = Lapatinib P = Pertuzumab w. P-FECT/L/TL 25. 7/27. 8/43. 1* * p value < 0. 05 1 Baselga J et al, SABCS 2010; 2 Gianni L et al, SABCS 2010; 3 Guarneri V et al, ASCO 2011 43

ALTTO study design: use of lapatinib in adjuvant setting for Erb. B 2 -positive breast cancer 1 Completion of all (neo) adjuvant anthracyclinebased chemotherapy (≥ 4 cycles) No taxane Design 2 Concomitant paclitaxel Randomisation Surgery Trastuzumab Design 1 Lapatinib Trastuzumab Wash out Lapatinib 12 wks 6 wks 34 wks (12 wks) Lapatinib + trastuzumab 52 wks N = 8000 (2000 patients per treatment arm) 1. www. clinicaltrial. gov NCT 00490139


The Conquest of Breast Cancer: from Evidence Based to Personalized Medicine • Breast Cancer mortality is declining because of earlier diagnosis and effective adjuvant treatments • Randomized clinical trials have allowed to define the appropriate therapy for the average patient population • The backbone of adjuvant treatments is based on the molecular characterization of the disease: • • • HR+ Endocrine therapy HER 2+ Anti. HER 2 therapy Triple negative Chemotherapy • Clinical genomics will allow to move forward personalized cancer medicine: • • • More refined prognosticators to spare unecessary therapy More reliable predictors of sensitivity to administer individualized therapy More reliable predictors of resistance to develop innovative therapies

 Vain 1 trattamento
Vain 1 trattamento Decoupage tecnico cinema
Decoupage tecnico cinema Trattamento qqn controindicazioni
Trattamento qqn controindicazioni Classificazione di hinchey
Classificazione di hinchey Ulcera forrest iii
Ulcera forrest iii Classificazione forrest
Classificazione forrest Frattura di monteggia e galeazzi
Frattura di monteggia e galeazzi Verifica homo erectus scuola primaria
Verifica homo erectus scuola primaria Anno sidereo
Anno sidereo Impianto autodiegetico
Impianto autodiegetico Evoluzione normativa diritto del lavoro
Evoluzione normativa diritto del lavoro Ufficio scolastico provinciale di modena
Ufficio scolastico provinciale di modena Imprese industriali esempi
Imprese industriali esempi Asl novara
Asl novara Azienda ospedaliera rummo
Azienda ospedaliera rummo Organigramma azienda sanitaria
Organigramma azienda sanitaria Organigramma azienda sanitaria
Organigramma azienda sanitaria Istituti clinici di perfezionamento
Istituti clinici di perfezionamento L'azienda è un sistema dinamico perchè
L'azienda è un sistema dinamico perchè Gerarchia aziendale
Gerarchia aziendale Gmail azienda ospedaliera verona
Gmail azienda ospedaliera verona Getis azienda zero
Getis azienda zero Elementi costitutivi dell azienda
Elementi costitutivi dell azienda Analisi swot azienda vitivinicola
Analisi swot azienda vitivinicola Fiscalità latente valutazione azienda
Fiscalità latente valutazione azienda Azienda frareg
Azienda frareg Compravendita economia aziendale
Compravendita economia aziendale Azienda
Azienda Aopd veneto referti
Aopd veneto referti Uonpia paderno dugnano
Uonpia paderno dugnano Valerio parisi genetica ed evoluzione
Valerio parisi genetica ed evoluzione Nulla ha senso in biologia se non alla luce dell'evoluzione
Nulla ha senso in biologia se non alla luce dell'evoluzione Embriologia comparata evoluzione
Embriologia comparata evoluzione Evoluzione prebiologica
Evoluzione prebiologica Evoluzione a cespuglio scuola primaria
Evoluzione a cespuglio scuola primaria Cladogramma uomo
Cladogramma uomo Pubblica amministrazione esempi scuola
Pubblica amministrazione esempi scuola Evoluzione storica della normativa sull'inclusione
Evoluzione storica della normativa sull'inclusione Evoluzione dei telefoni
Evoluzione dei telefoni Evoluzione
Evoluzione Sao ko kelle terre
Sao ko kelle terre Rutherford esperimento
Rutherford esperimento Modena analisi consulenza e gestione finanziaria
Modena analisi consulenza e gestione finanziaria Sistemi informativi aziendali modena
Sistemi informativi aziendali modena 10 ic modena
10 ic modena Hera via razzaboni
Hera via razzaboni Scuola media calvino modena
Scuola media calvino modena Registro elettronico tassoni
Registro elettronico tassoni