Atomic Theory Terms to Know Atom the smallest












- Slides: 26

Atomic Theory

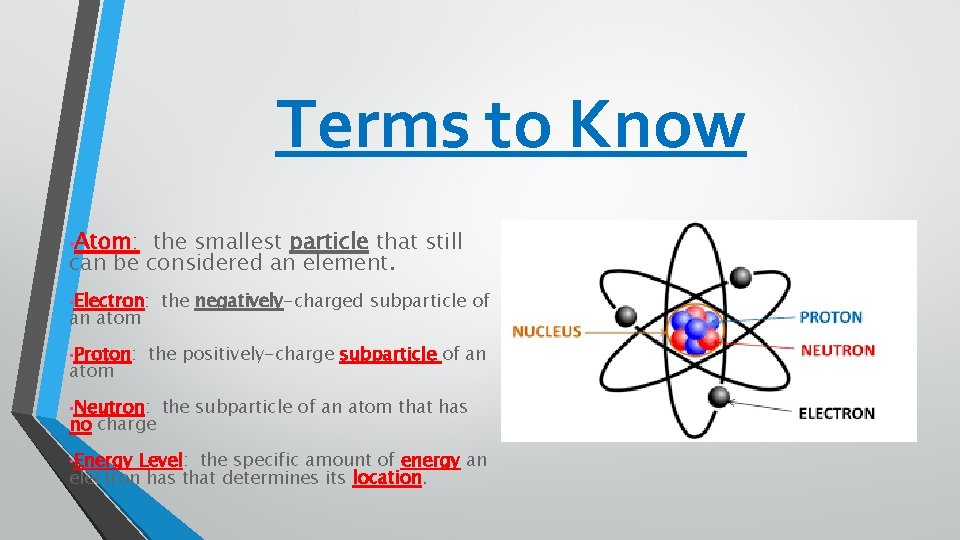
Terms to Know • Atom: the smallest particle that still can be considered an element. • Electron: an atom • Proton: atom the positively-charge subparticle of an • Neutron: no charge • Energy the negatively-charged subparticle of the subparticle of an atom that has Level: the specific amount of energy an electron has that determines its location.

Atomic Theory • The theory that all matter is made up of tiny indivisible particles (atoms). • According to the modern version, the atoms of each element are effectively identical, but differ from those of other elements, and unite to form compounds in fixed proportions.

Discovering the Atom How did we find the atom? It’s too small to see with the naked eye. Even the smallest visible speck of dust is made up of approximately 10 million billion atoms. The idea of an atom started waaaaaaay back in 430 BC. That seems unfathomable … but it is true!

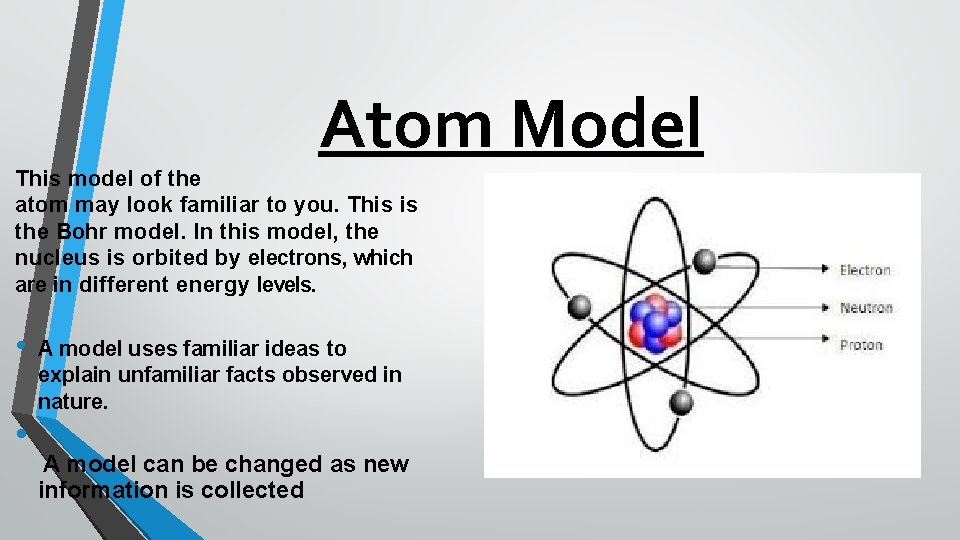
Atom Model This model of the atom may look familiar to you. This is the Bohr model. In this model, the nucleus is orbited by electrons, which are in different energy levels. • • A model uses familiar ideas to explain unfamiliar facts observed in nature. A model can be changed as new information is collected

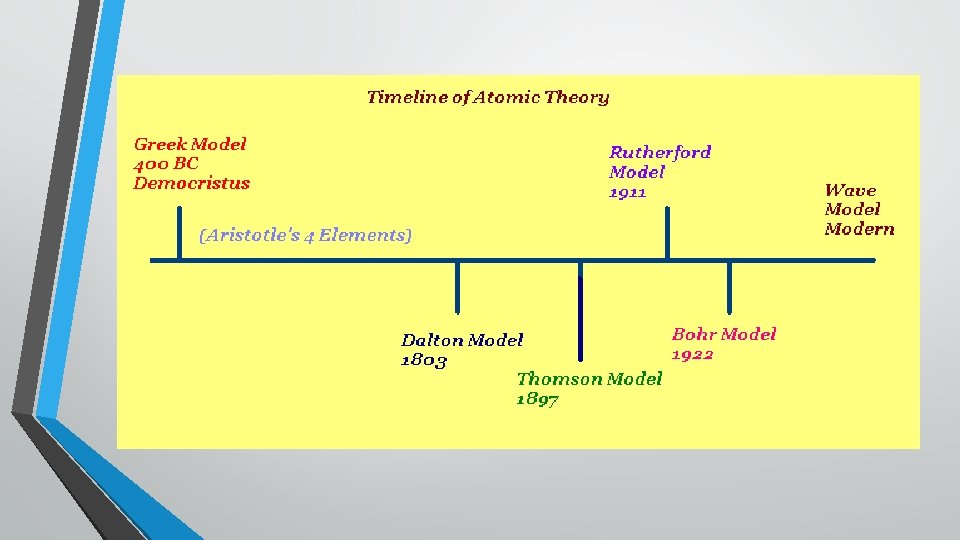
Atom Model • The atomic model has changed throughout the centuries, starting in 400 BC, when it looked like a billiard ball →

Discovering the Atom In 430 BC, a Greek philosopher named Democritus proposed an idea that matter was formed from the same small pieces that could be cut and cut, until eventually you get to a piece that is “uncuttable. ” He called this uncuttable pieces atomos. The term atom comes from this and an atom is plainly the smallest particle that can still be considered an element, or that same thing.


This theory was ignored and forgotten for more than 2000 years!

WHY? ? ? • The eminent philosophers of the time, Aristotle and Plato, had a more respected, (and ultimately wrong) theory. Aristotle and Plato favored the earth, fire, air and water approach to the nature of matter. Their ideas held sway because of their eminence as philosophers. The atomos idea was buried for approximately 2000 years.


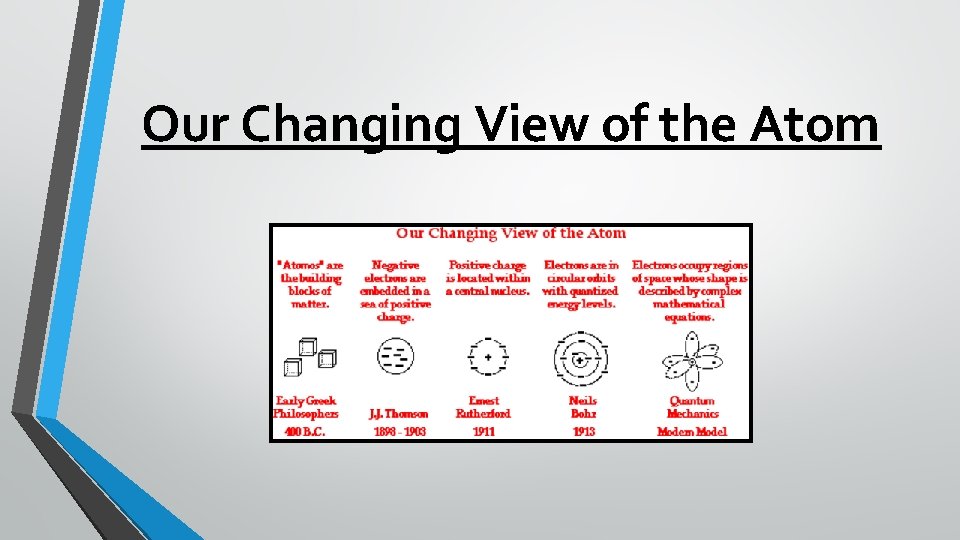
Our Changing View of the Atom

John Dalton- 1803 While experimenting with the mass of different elements, John Dalton inferred that atoms had certain characteristics. Through the evidence he gathered, he theorized that: 1. 2. 3. 4. Atoms had certain characteristics. Atoms were smooth, hard balls that could not be broken into smaller pieces. All atoms of the same element are exactly alike and have the same mass. He also said that elements cannot change into other elements through chemical reactions.

John Dalton “Solid Sphere”

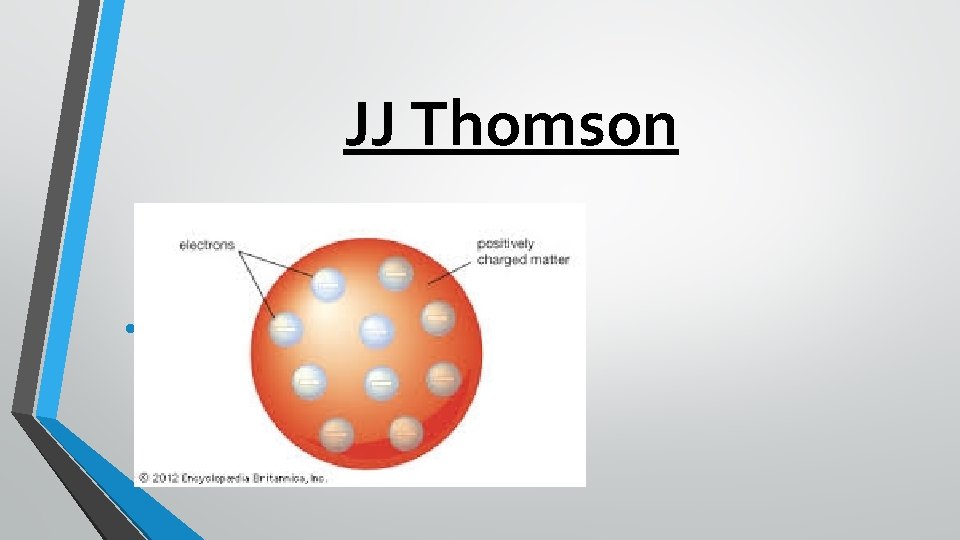
J. J. Thomson-1897 While some of Dalton’s theory still holds true, in 1897 JJ Thomson discovered that atoms have negative and positive charges, that he named electrons and protons. His study of atoms within a cathode ray tube led him to develop his model, which looked like a watermelon (the positive charge) with electrons scattered throughout like the seeds. However, he was British, so he called his model the “ Plum Pudding” model, with the pudding as the + (pos) charge and the raisins as the – (neg) charge.

JJ Thomson • “Plum Pudding Model”

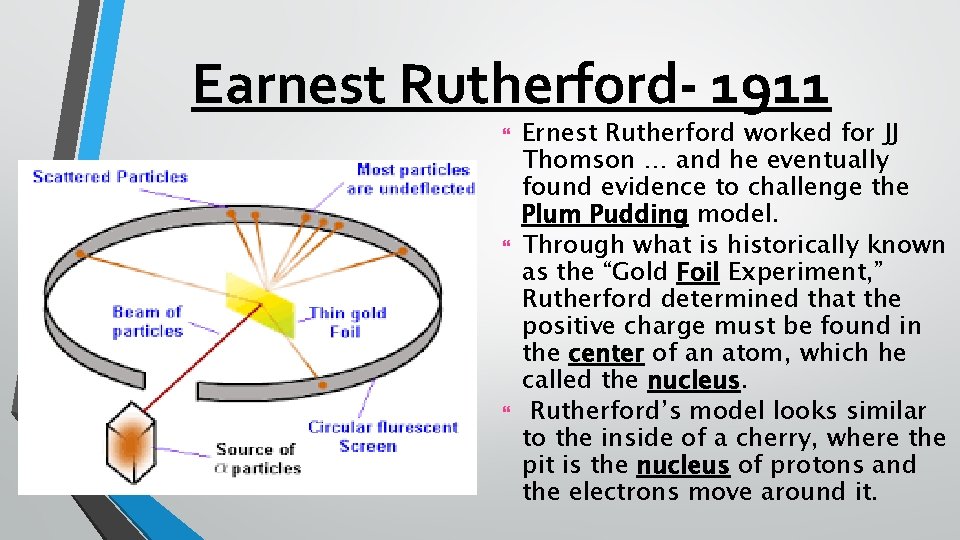
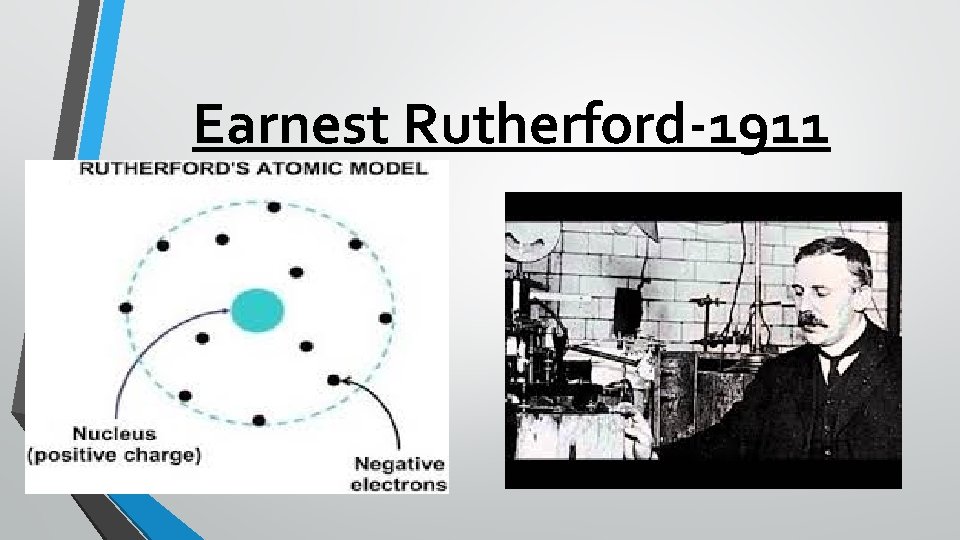
Earnest Rutherford- 1911 Ernest Rutherford worked for JJ Thomson … and he eventually found evidence to challenge the Plum Pudding model. Through what is historically known as the “Gold Foil Experiment, ” Rutherford determined that the positive charge must be found in the center of an atom, which he called the nucleus. Rutherford’s model looks similar to the inside of a cherry, where the pit is the nucleus of protons and the electrons move around it.

Earnest Rutherford-1911

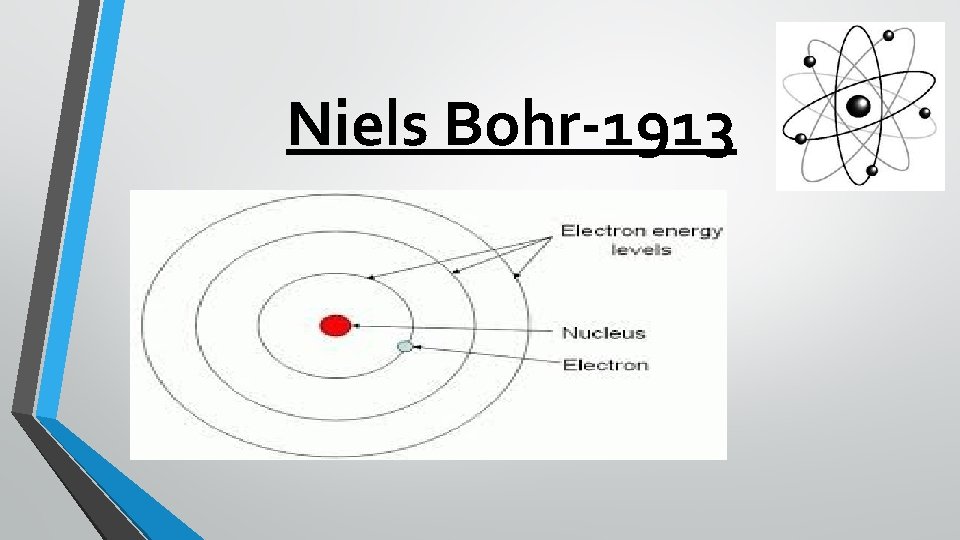
Niels Bohr-1913 Niels Bohr was a student of Rutherford, and while he believed Rutherford’s model to be accurate, he suggested that electrons are found only in specific orbit or energy levels around the nucleus, like planets in the solar system orbiting the Sun. Bohr’s model looked similar to the rings in a tree, the proton nucleus and the electrons in rings moving away from the center.

Niels Bohr-1913

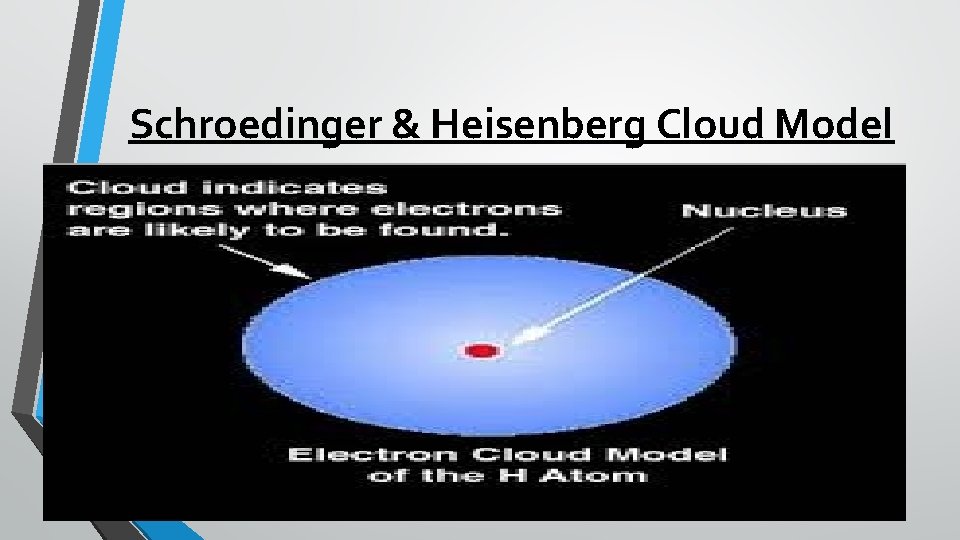
Schroedinger & Heisenberg Cloud Model

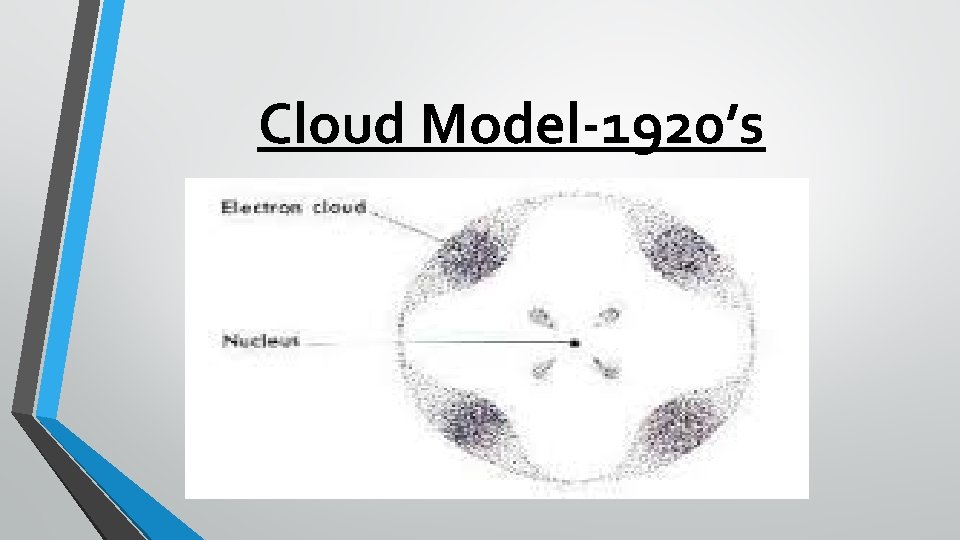
Cloud Model-1920’s A team of scientists in the 1920 s changed the model of the atom again. They determined that electrons do not orbit the nucleus in fixed spots around the nucleus; instead, electrons move rapidly within a cloudlike region around the nucleus. Similar to Bohr, these scientists agreed that there are energy levels, but the electrons can move from level to level depending on the amount of energy they have at a given time.

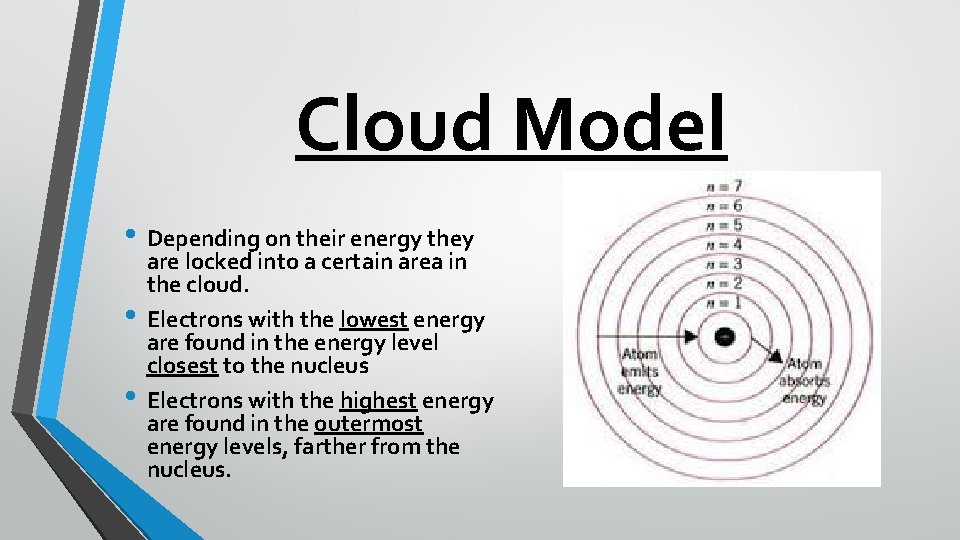
Cloud Model • Depending on their energy they • • are locked into a certain area in the cloud. Electrons with the lowest energy are found in the energy level closest to the nucleus Electrons with the highest energy are found in the outermost energy levels, farther from the nucleus.

Cloud Model-1920’s

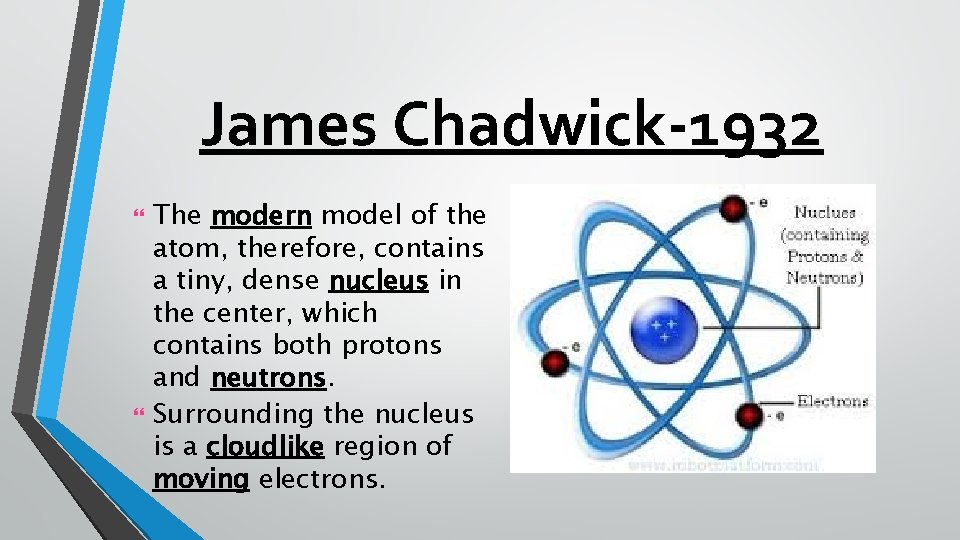
James Chadwick-1932 The cloud model was agreed upon by scientists in the movement of electrons, but James Chadwick researched the mass of an atom and determined that there must be another subatomic particle that gave the atom its “weight. ” He theorized that we couldn’t detect it, however, because it has no charge. He called this subatomic particle the neutron.

James Chadwick-1932 The modern model of the atom, therefore, contains a tiny, dense nucleus in the center, which contains both protons and neutrons. Surrounding the nucleus is a cloudlike region of moving electrons.