Aqueous Reactions and Solution Stoichiometry Chapter 4 1












- Slides: 28

Aqueous Reactions and Solution Stoichiometry Chapter 4 1

Chapter 4 Topics • Properties of aqueous solutions • Types of reactions – – Precipitation Metathesis Acid-Base Oxidation – Reduction (skip this section=pages 137144) • Concentrations of solutions – Molarity Chapter 4 2

Solution • A homogeneous mixture of two or more substances • The compound that is present in the greater quantity is called the solvent • The other compound is called the solute • In an aqueous solution, the solvent is water. Chapter 4 3

Salt water • Sodium chloride dissolved in water. • Na. Cl is the solute • Water is the solvent Chapter 4 4

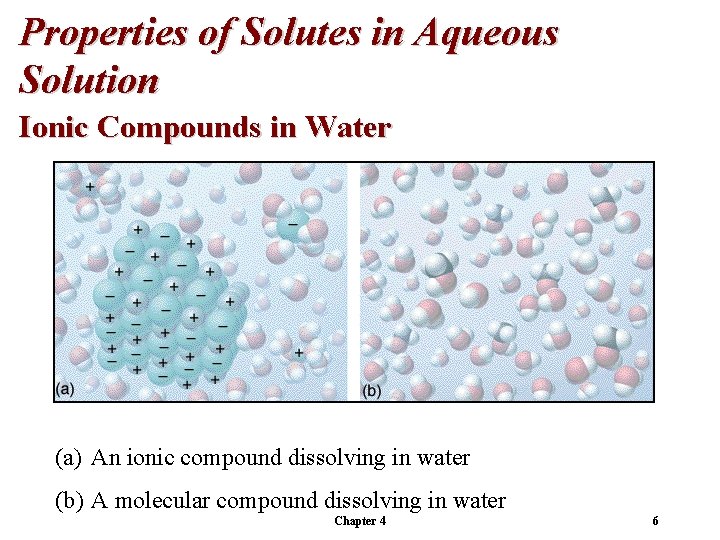
Properties of Solutes in Aqueous Solution Ionic Compounds in Water Ions dissociate in water. In solution, each ion is surrounded by water molecules. Transport of ions through solution causes flow of current. Chapter 4 5

Properties of Solutes in Aqueous Solution Ionic Compounds in Water (a) An ionic compound dissolving in water (b) A molecular compound dissolving in water Chapter 4 6

Properties of Solutes in Aqueous Solution Molecular Compounds in Water Molecular compounds in water (e. g. , CH 3 OH): no ions are formed. If there are no ions in solution, there is nothing to transport electric charge. Chapter 4 7

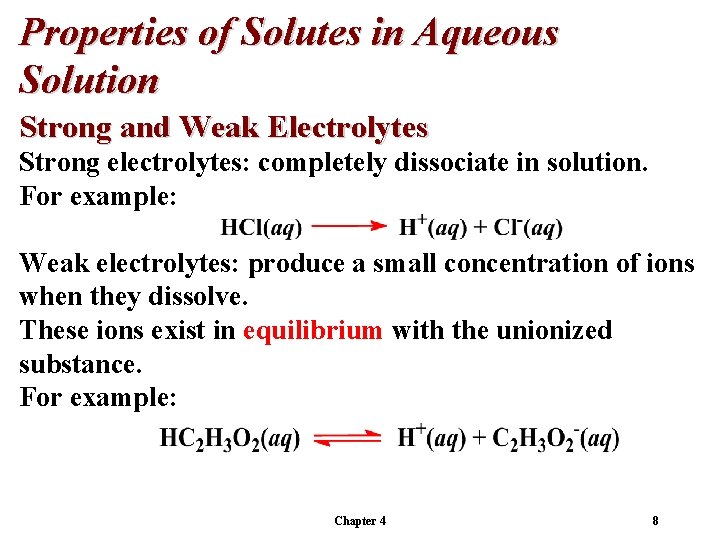
Properties of Solutes in Aqueous Solution Strong and Weak Electrolytes Strong electrolytes: completely dissociate in solution. For example: Weak electrolytes: produce a small concentration of ions when they dissolve. These ions exist in equilibrium with the unionized substance. For example: Chapter 4 8

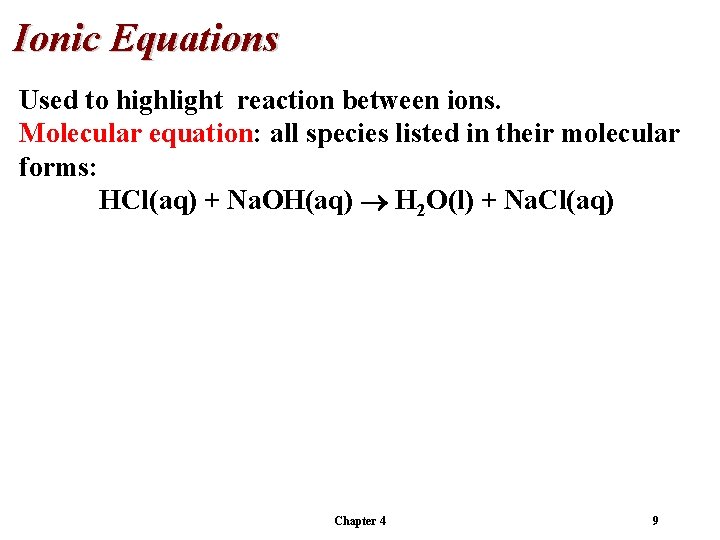
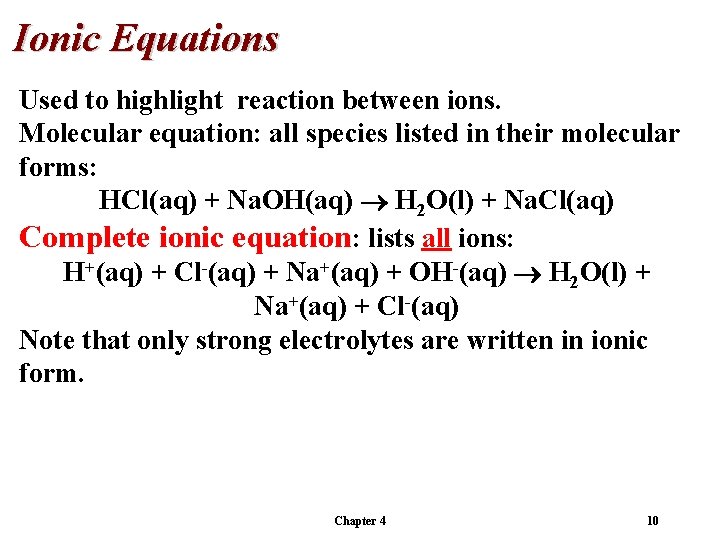
Ionic Equations Used to highlight reaction between ions. Molecular equation: all species listed in their molecular forms: HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) Chapter 4 9

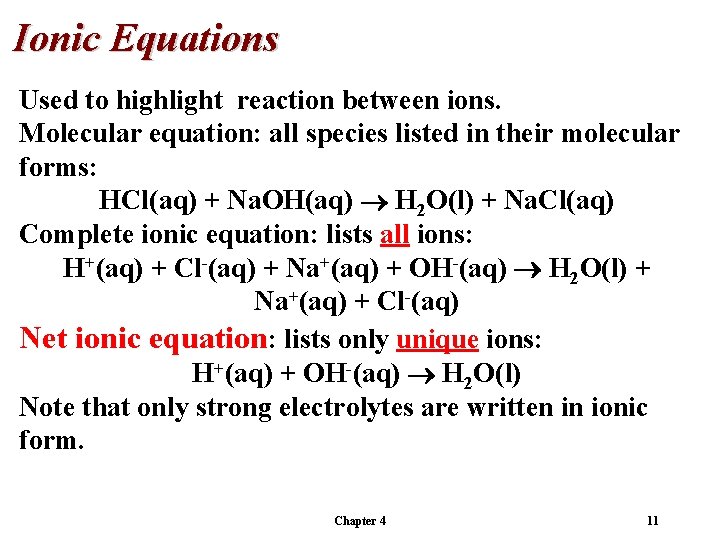
Ionic Equations Used to highlight reaction between ions. Molecular equation: all species listed in their molecular forms: HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) Complete ionic equation: lists all ions: H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) H 2 O(l) + Na+(aq) + Cl-(aq) Note that only strong electrolytes are written in ionic form. Chapter 4 10

Ionic Equations Used to highlight reaction between ions. Molecular equation: all species listed in their molecular forms: HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) Complete ionic equation: lists all ions: H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) H 2 O(l) + Na+(aq) + Cl-(aq) Net ionic equation: lists only unique ions: H+(aq) + OH-(aq) H 2 O(l) Note that only strong electrolytes are written in ionic form. Chapter 4 11

Metathesis Reactions Metathesis reactions involve swapping ions in solution: AX + BY AY + BX. Metathesis reactions will lead to a change in solution if one of three things occurs: • an insoluble solid is formed (precipitate), • weak or nonelectrolytes are formed, or • an insoluble gas is formed. Chapter 4 12

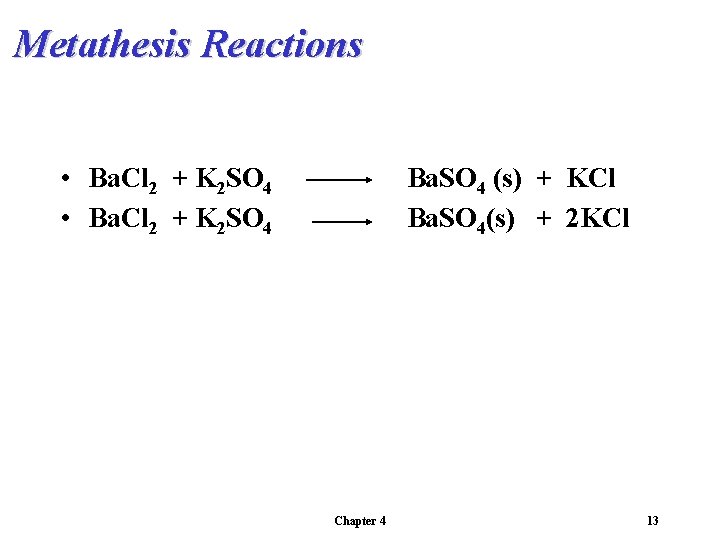
Metathesis Reactions • Ba. Cl 2 + K 2 SO 4 Ba. SO 4 (s) + KCl Ba. SO 4(s) + 2 KCl Chapter 4 13

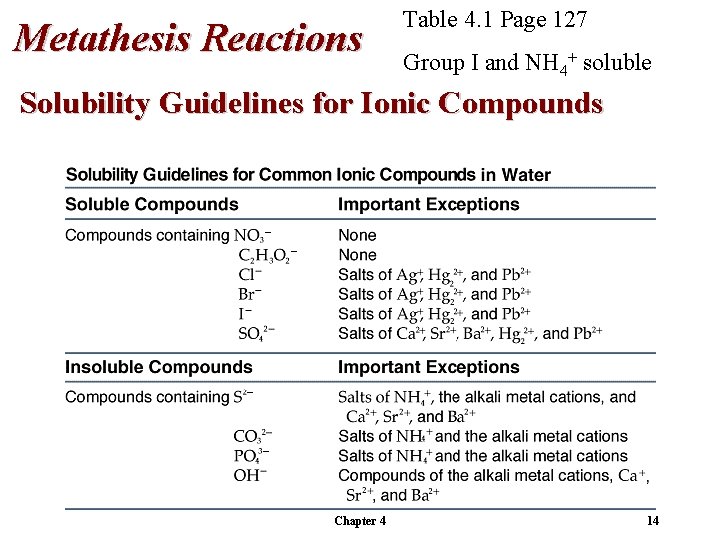
Metathesis Reactions Table 4. 1 Page 127 Group I and NH 4+ soluble Solubility Guidelines for Ionic Compounds Chapter 4 14

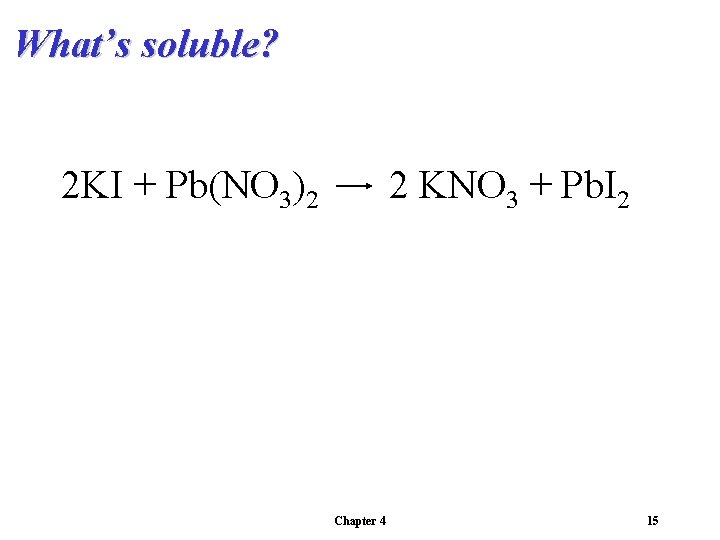
What’s soluble? 2 KI + Pb(NO 3)2 2 KNO 3 + Pb. I 2 Chapter 4 15

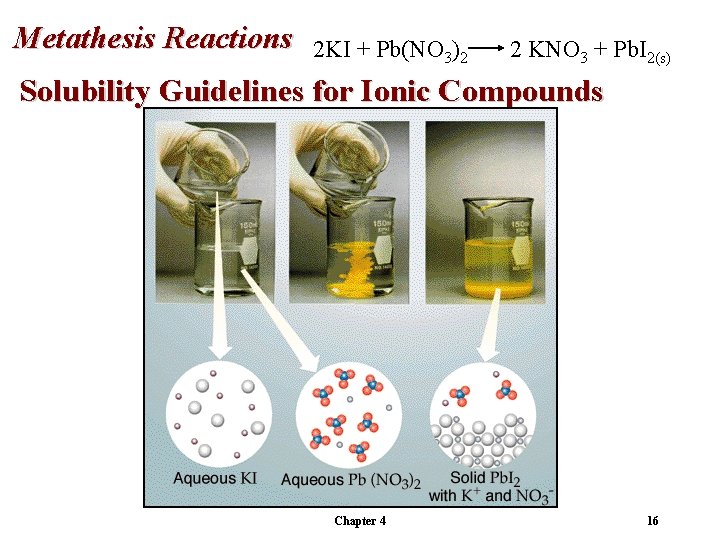
Metathesis Reactions 2 KI + Pb(NO 3)2 2 KNO 3 + Pb. I 2(s) Solubility Guidelines for Ionic Compounds Chapter 4 16

Acids, Bases, and Salts Acids Dissociation = pre-formed ions in solid move apart in solution. Ionization = neutral substance forms ions in solution. Acid = substances that ionizes to form H+ in solution (e. g. HCl, HNO 3, CH 3 CO 2 H, lemon, lime, vitamin C). Bases = substances that react with the H+ ions formed by acids (e. g. NH 3, Drano™, Milk of Magnesia™). Chapter 4 17

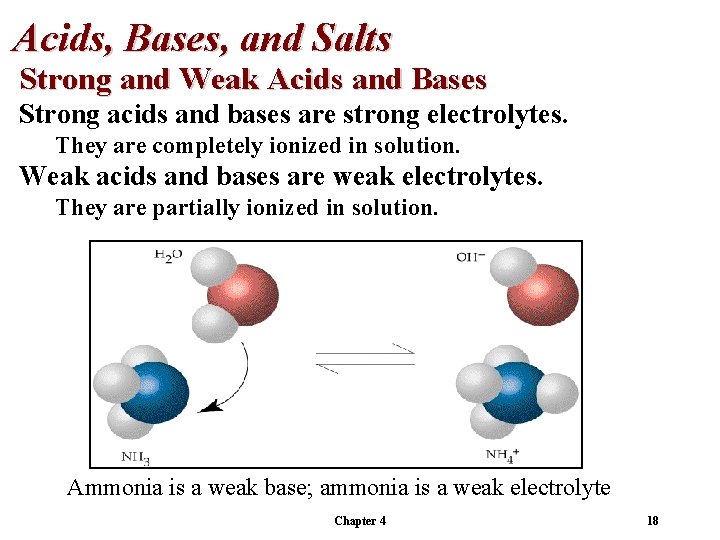
Acids, Bases, and Salts Strong and Weak Acids and Bases Strong acids and bases are strong electrolytes. They are completely ionized in solution. Weak acids and bases are weak electrolytes. They are partially ionized in solution. Ammonia is a weak base; ammonia is a weak electrolyte Chapter 4 18

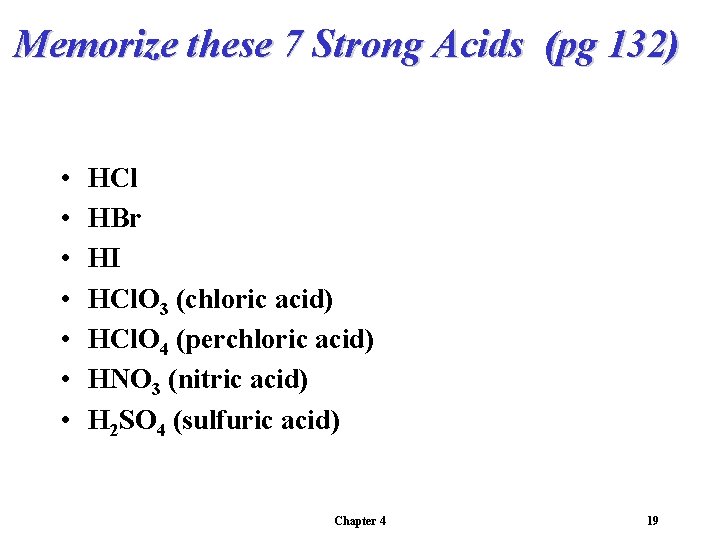
Memorize these 7 Strong Acids (pg 132) • • HCl HBr HI HCl. O 3 (chloric acid) HCl. O 4 (perchloric acid) HNO 3 (nitric acid) H 2 SO 4 (sulfuric acid) Chapter 4 19

And the strong bases (pg 132) • Group I hydroxides • Heavy Group II hydroxides ( Ca & down) Chapter 4 20

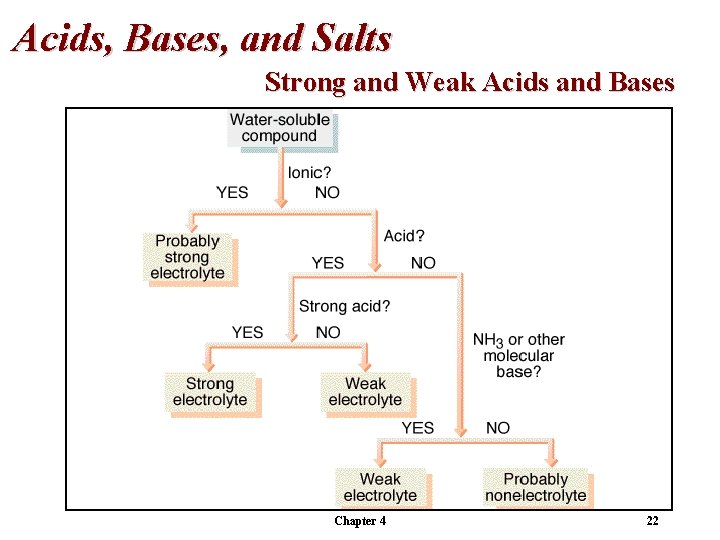
Acids, Bases, and Salts Strong and Weak Acids and Bases Water soluble and ionic = strong electrolyte (probably). Water soluble and is a strong acid (or base) = strong electrolyte. Water soluble and is a weak acid or base = weak electrolyte. Otherwise, the compound is probably a nonelectrolyte. Chapter 4 21

Acids, Bases, and Salts Strong and Weak Acids and Bases Chapter 4 22

Acids, Bases, and Salts Neutralization Reactions and Salts Neutralization occurs when a solution of an acid and a base are mixed: HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) Notice we form a salt (Na. Cl) and water. Salt = ionic compound whose cation comes from a base and anion from an acid. Neutralization between acid and metal hydroxide produces water and a salt. Chapter 4 23

Solution Composition Molarity • • Solution = solute dissolved in solvent. Solute: present in smallest amount. Water as solvent = aqueous solutions. Change concentration by using different amounts of solute and solvent. Molarity: Moles of solute per liter of solution. • If we know: molarity and liters of solution, we can calculate moles (and mass) of solute. Chapter 4 24

Solution Composition Molarity: Moles of solute per liter of solution. Chapter 4 25

Solution Composition • Molarity: Moles of solute per liter of solution. • What is the Molarity of 20 grams of Na. Cl dissolved in water to a volume of 1 L? • 20 grams x (1 mole Na. Cl / 58 grams) = 0. 34 moles per liter. • Therefore 0. 34 M Na. Cl Chapter 4 26

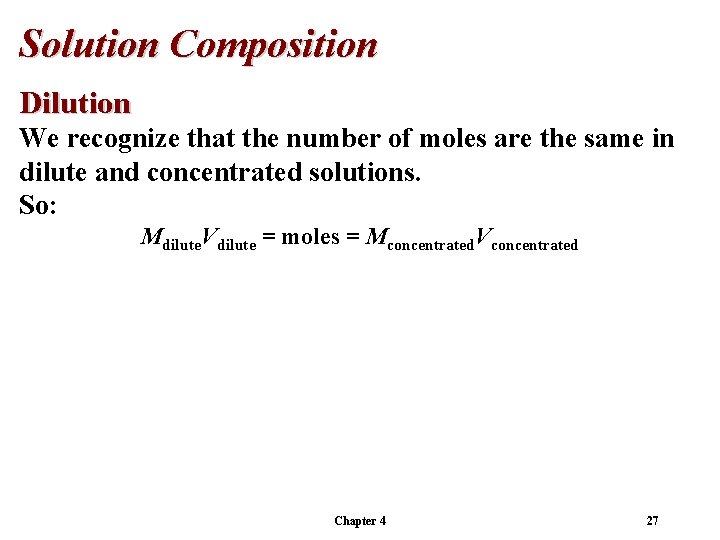
Solution Composition Dilution We recognize that the number of moles are the same in dilute and concentrated solutions. So: Mdilute. Vdilute = moles = Mconcentrated. Vconcentrated Chapter 4 27

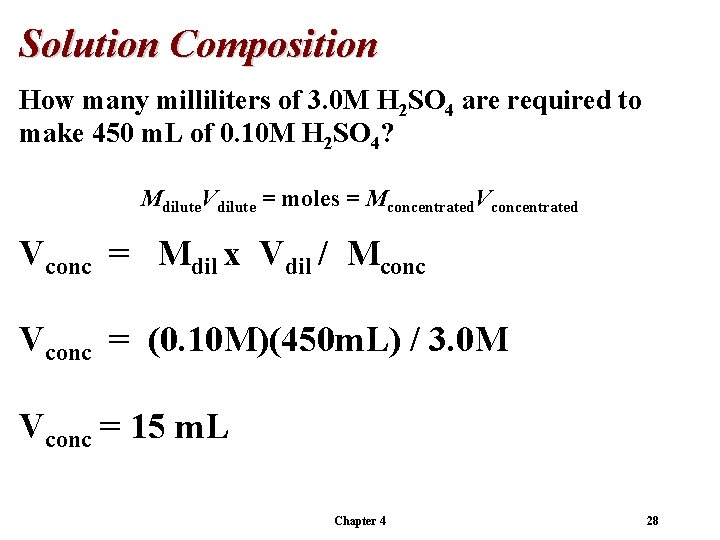
Solution Composition How many milliliters of 3. 0 M H 2 SO 4 are required to make 450 m. L of 0. 10 M H 2 SO 4? Mdilute. Vdilute = moles = Mconcentrated. Vconcentrated Vconc = Mdil x Vdil / Mconc Vconc = (0. 10 M)(450 m. L) / 3. 0 M Vconc = 15 m. L Chapter 4 28