Chapter 4 Aqueous Reactions and Solution Stoichiometry Properties


































- Slides: 42

Chapter 4 Aqueous Reactions and Solution Stoichiometry

Properties of Aqueous Solutions § Weak electrolytes exist mostly as molecules

Precipitation Reactions • Precipitation reactions are those that result in the formation of an insoluble product

Precipitation Reactions • Precipitation reactions occur when certain pairs of oppositely charged ions attract to each other so strongly that they form an insoluble ionic solid

Precipitation Reactions • Solubility of a substance is the amount of that substance that can be dissolved in a given quantity of solvent

Precipitation Reactions • Any substance with a solubility less than 0. 01 mol/L will be referred to as insoluble

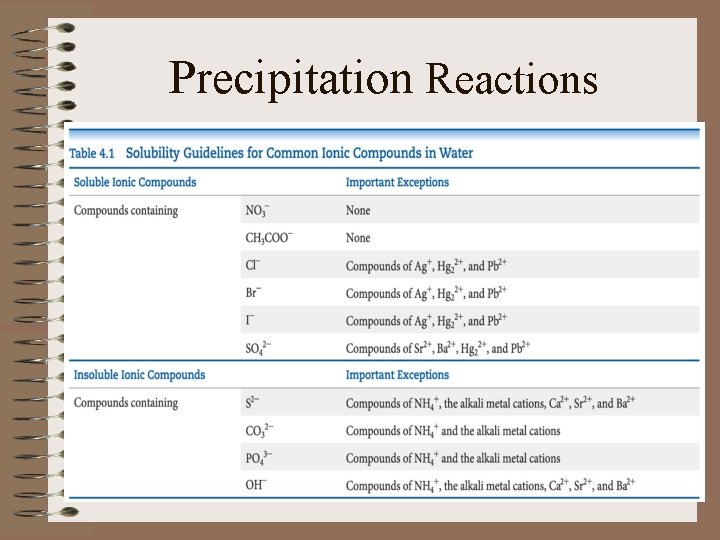
Precipitation Reactions • The solubility guidelines for common ionic compounds in water is organized by anions

Precipitation Reactions

Precipitation Reactions • To predict whether a precipitate will form when we mix aqueous solutions of electrolytes …

Precipitation Reactions • (1)note the ions present in the reactants • (2) consider combinations of anions and cations • (3) use the table to predict if any of the combinations are insoluble

Precipitation Reactions • Will a precipitate form when aqueous solution of magnesium nitrate and sodium hydroxide are mixed?

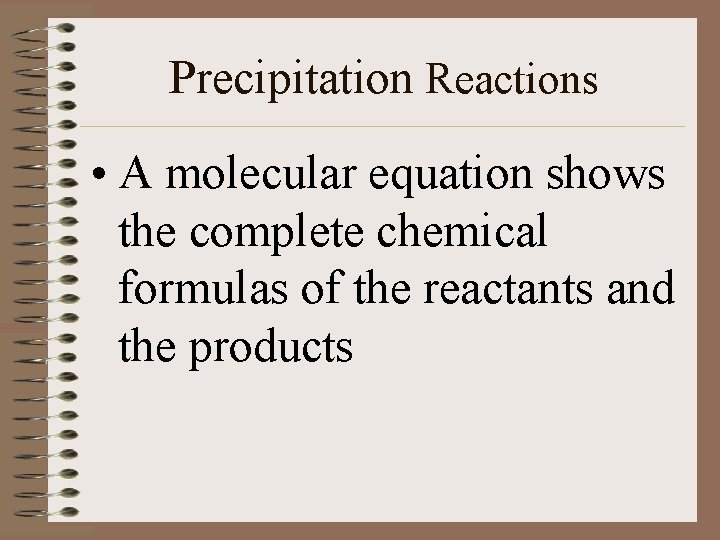
Precipitation Reactions • A molecular equation shows the complete chemical formulas of the reactants and the products

Precipitation Reactions • A complete ionic equation shows all strong electrolytes as ions rather than as compounds

Precipitation Reactions • Spectator ions are ions that appear as identical forms on both sides of the equation; they are present but don’t play a direct role in the reaction

Precipitation Reactions • Net ionic equations do not show spectator ions

Writing Net Ionic Equations • Write a balanced mol. E. q • Rewrite to show ions that form in solution • Cancel spectator ions

Acid-Base Reactions • Acids produce H+ when dissolved in water • Acids are proton donors

Acid-Base Reactions • Bases accept H+ions • Bases produce OH when they dissolve in water

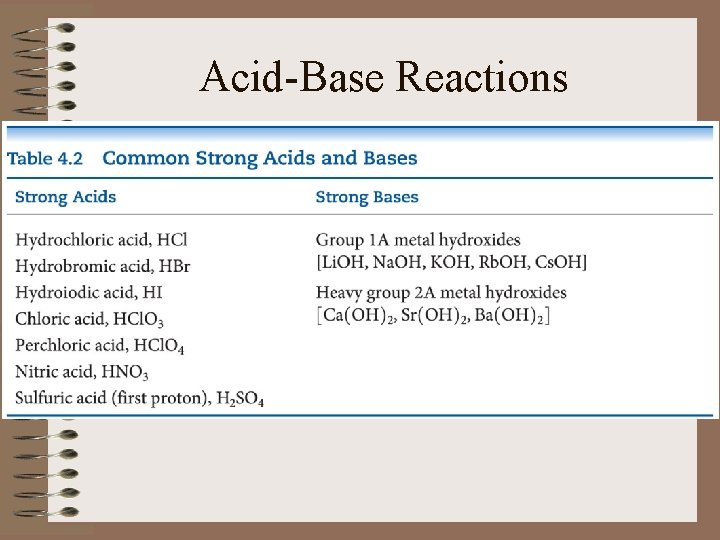
Acid-Base Reactions • Strong acids and bases are strong electrolytes – ionize completely

Acid-Base Reactions • Weak acids and bases are weak electrolytes – partially ionize ( do not write in ionized form)

Acid-Base Reactions • List Strong Acids and Bases

Acid-Base Reactions

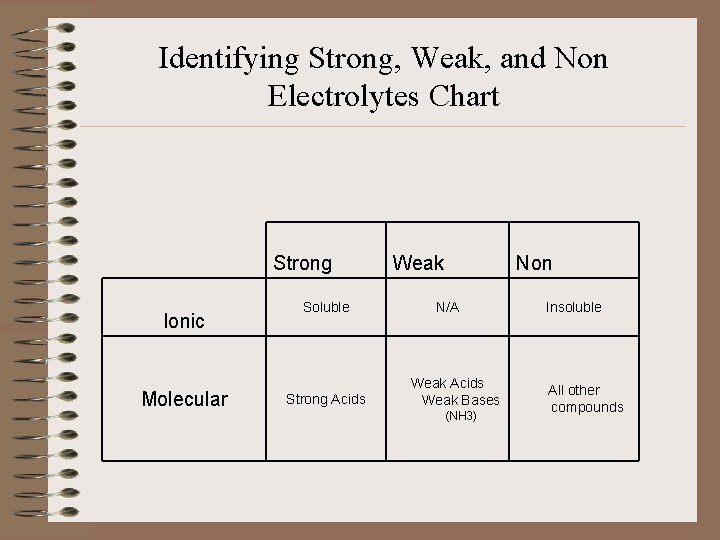
Identifying Strong, Weak, and Non Electrolytes Chart Strong Ionic Molecular Soluble Strong Acids Weak Non N/A Weak Acids Weak Bases (NH 3) Insoluble All other compounds

Classifying SE, WE, or NE • 1. Is it ionic or molecular? • 2. If ionic – is it soluble? • 3. If molecular – is it an acid? • 4. If an acid – strong or weak? • 5. Is it weak base NH 3? • 6. Everything else is a non electrolyte

Acid-Base Reactions • A neutralization reaction occurs when an acid and base are mixed producing water and salt • Salt means any ionic compound whose cation comes from the base and anion from the acid

Acid-Base Reactions • Reactions with the S 2 - ion or 2 CO 3 ion with acids will form gases with low solubility in water

Oxidation Numbers Oxidation numbers of an atom in a substance are a hypothetical charge based on a set of rules. 1. For an atom in its elemental form the oxidation number is always zero 2. For any monatomic ion the oxidation number equals the charge on the ion

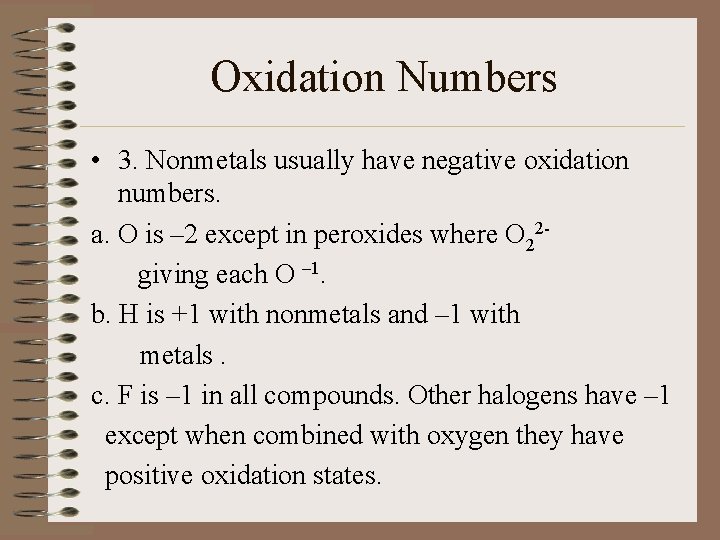
Oxidation Numbers • 3. Nonmetals usually have negative oxidation numbers. a. O is – 2 except in peroxides where O 22 giving each O – 1. b. H is +1 with nonmetals and – 1 with metals. c. F is – 1 in all compounds. Other halogens have – 1 except when combined with oxygen they have positive oxidation states.

Oxidation Numbers • 4. The sum of oxidation numbers of all atoms in a neutral compound is 0. • The sum of oxidation numbers in a polyatomic ion is equal to the charge of the ion.

Oxidation-Reduction Reactions • Reactions where electrons are transferred between reactants

Oxidation-Reduction Reactions • Oxidized – when an atom, ion, or molecule becomes more positively charged (lost e-) • Reducing Agent = the substance that is oxidized

Oxidation-Reduction Reactions • Reduced – when an atom, ion, or molecule has become more negative (gain e-) • Oxidizing agent = the substance that is reduced

Oxidation-Reduction Reactions • Determine oxidation numbers, then identify the oxidizing agent and reducing agent in the rxn below. • 2 Ca(s) + O 2(g) 2 Ca. O(s)

Oxidation-Reduction Reactions • A single replacement rxn is when an ion in solution is replaced through oxidation of an element

Oxidation-Reduction Reactions • Activity series is a list of metals arranged in order of decreasing ease of oxidation • Any metal on the list can be oxidized by the ions of elements below it.


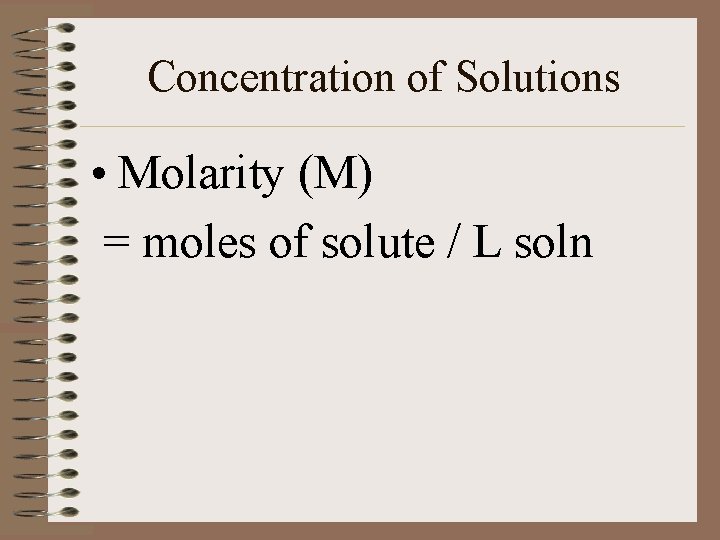
Concentration of Solutions • Molarity (M) = moles of solute / L soln

Making a Solution: How do you make a 250. 0 m. L soln of 0. 1 M Cu. SO 4?

Concentration of Solutions • Electrolytes and Concentration: When an ionic compound dissolves, the relative concentration of ion depends on the chemical formula

Concentration of Solutions • A dilution can be made to concentrated stock solutions by adding water to the solutions, and therefore making them less concentrated. M 1 V 1 = M 2 V 2

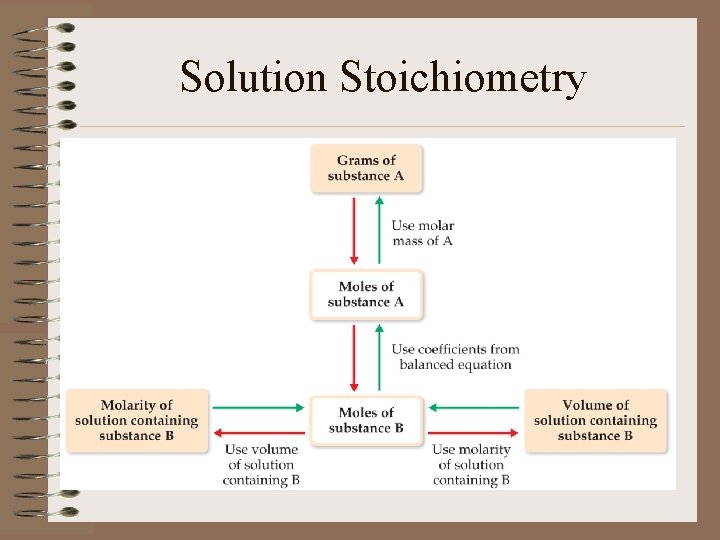
Solution Stoichiometry • Use mole conversions (molarity, molar mass, etc. ) and mole ratios to solve stoichiometry problems through DA

Solution Stoichiometry