Announcements Now is the time to master quiz










- Slides: 39

Announcements • Now is the time to master quiz game and get highest score. • I specifically designed levels to help you practice these concepts • You can do each level over and over until you understand every answer • Honestly, I cannot possibly tell you how much you really ought to do this. • I expect you to have mastered this stuff for the exam after thanksgiving, and I will write all the questions from this perspective

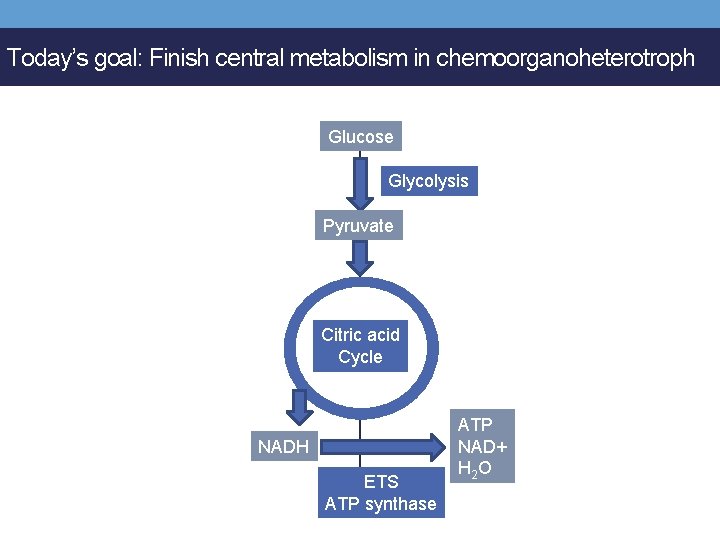
Today’s goal: Finish central metabolism in chemoorganoheterotroph Glucose Glycolysis Pyruvate Citric acid Cycle NADH ETS ATP synthase ATP NAD+ H 2 O

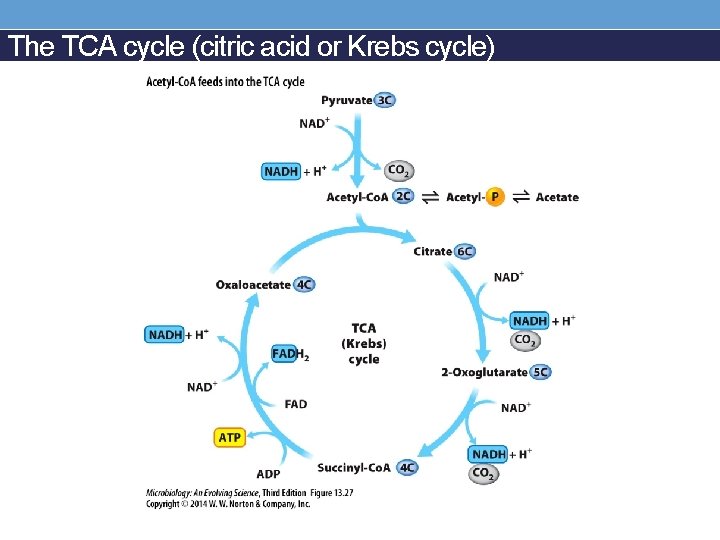
The TCA cycle (citric acid or Krebs cycle)

PRINCIPLES OF OXIDATIVE PHOSPHORYLATION

Learning outcomes • Be able to calculate the free energy of a reaction based on the • • • change in reduction potential Be able to identify potential electron donors and acceptors for metabolism based on reduction potential Apply understanding of reduction potential to determine how electron transfer releases energy in oxidative phosphorylation Explain the elements of proton-motive force and how it powers the ATP synthase and flagella Be able to compare and contrast oxidative phosphorylation and substrate-level phosphorylation Use redox potential to make predictions about how and why oxidative phosphorylation varies, resulting in alternative metabolisms and acclimation to the environment. Compare and contrast aerobic and anaerobic respiration

REDUCTION POTENTIAL Fundamental concept affecting everything else you learn today and Thursday.

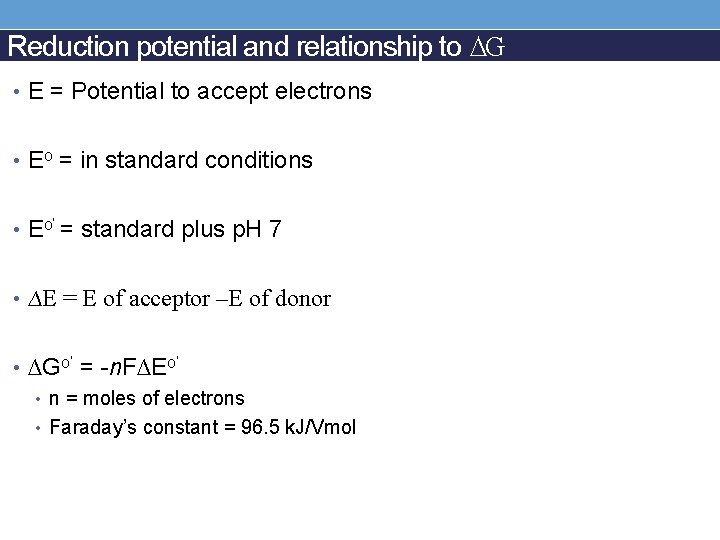
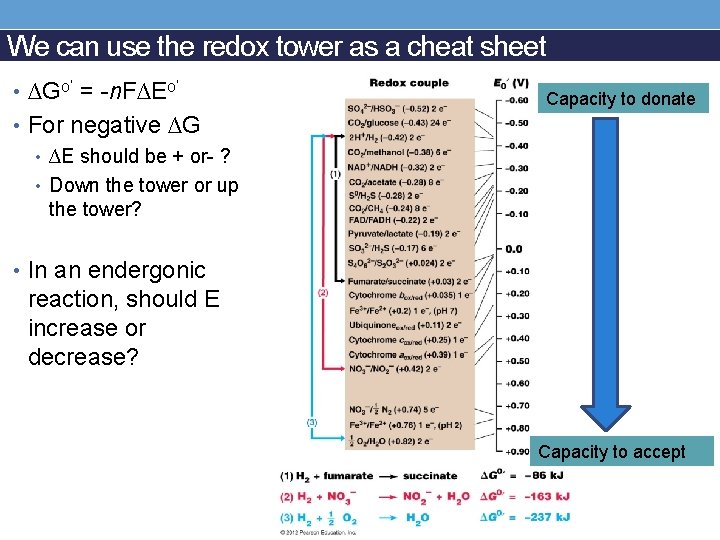
Reduction potential and relationship to ∆G • E = Potential to accept electrons • Eo = in standard conditions • Eo’ = standard plus p. H 7 • ∆E = E of acceptor –E of donor • ∆Go’ = -n. F∆Eo’ • n = moles of electrons • Faraday’s constant = 96. 5 k. J/Vmol

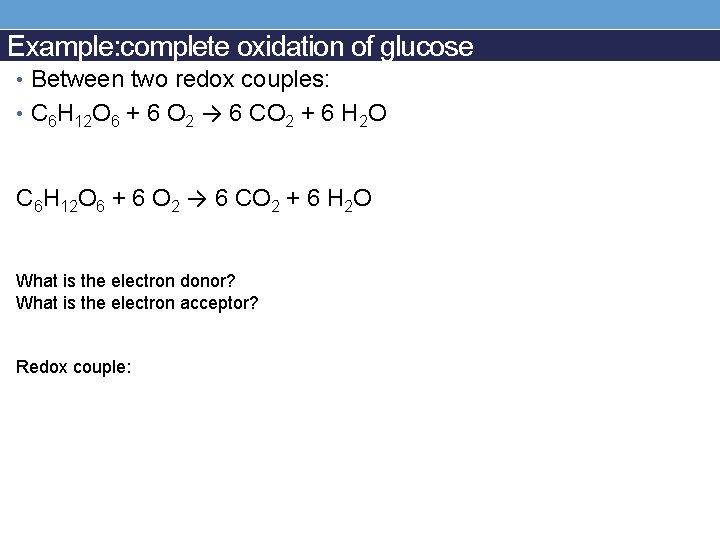
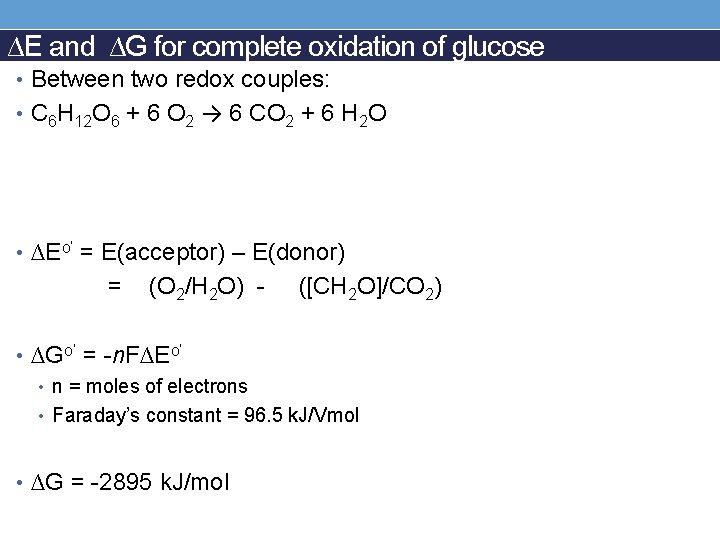
Example: complete oxidation of glucose • Between two redox couples: • C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O What is the electron donor? What is the electron acceptor? Redox couple:

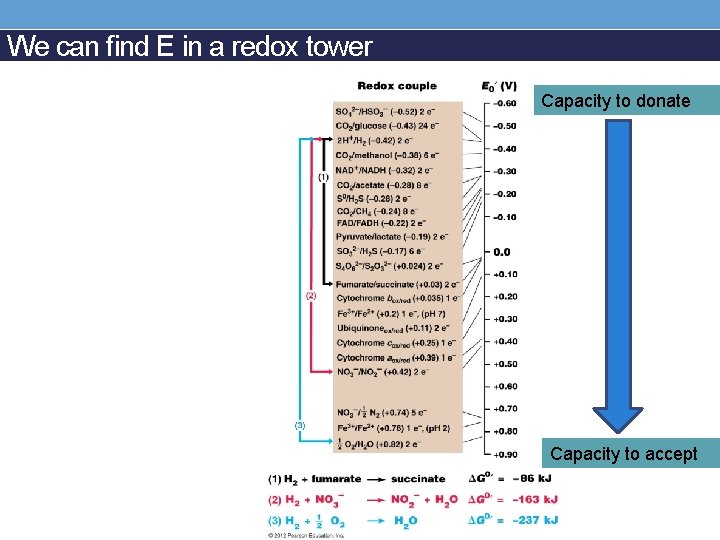
We can find E in a redox tower Capacity to donate Capacity to accept

∆E and ∆G for complete oxidation of glucose • Between two redox couples: • C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O • ∆Eo’ = E(acceptor) – E(donor) = (O 2/H 2 O) - ([CH 2 O]/CO 2) • ∆Go’ = -n. F∆Eo’ • n = moles of electrons • Faraday’s constant = 96. 5 k. J/Vmol • ∆G = -2895 k. J/mol

We can use the redox tower as a cheat sheet • ∆Go’ = -n. F∆Eo’ Capacity to donate • For negative ∆G • ∆E should be + or- ? • Down the tower or up the tower? • In an endergonic reaction, should E increase or decrease? Capacity to accept

A SERIES OF REDOX REACTIONS IN CELL MEMBRANE GENERATES PROTON-MOTIVE FORCE How the reservoir above the dam gets filled up with water….

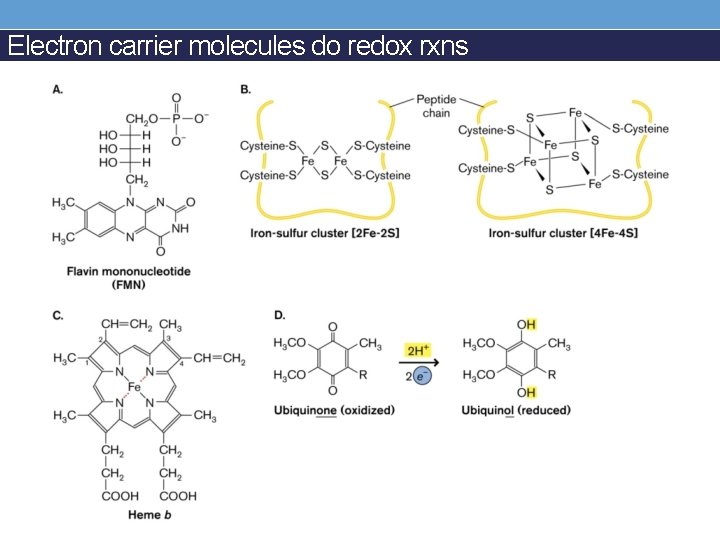
Electron carrier molecules do redox rxns

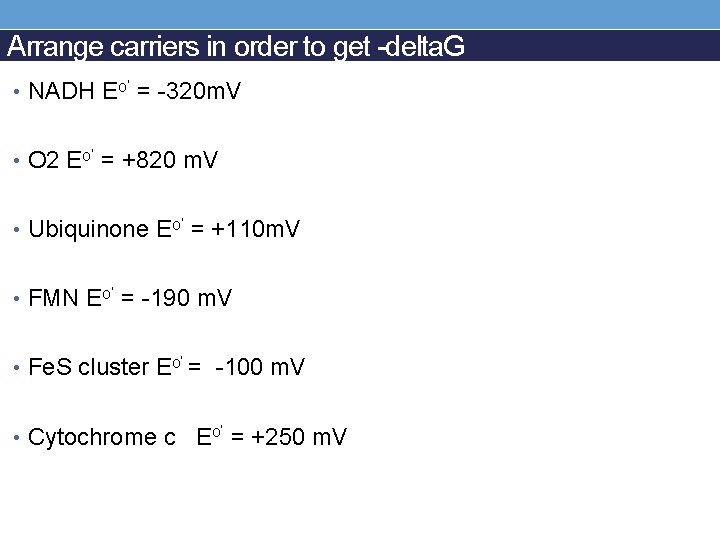
Arrange carriers in order to get -delta. G • NADH Eo’ = -320 m. V • O 2 Eo’ = +820 m. V • Ubiquinone Eo’ = +110 m. V • FMN Eo’ = -190 m. V • Fe. S cluster Eo’ = -100 m. V • Cytochrome c Eo’ = +250 m. V

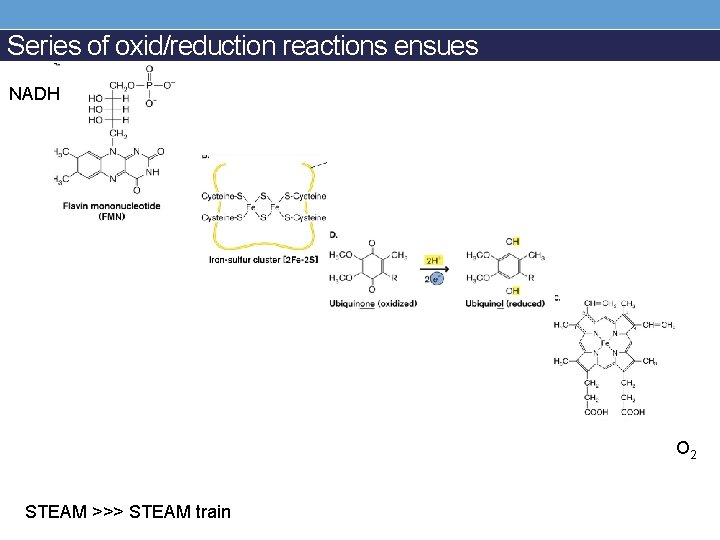
Series of oxid/reduction reactions ensues NADH O 2 STEAM >>> STEAM train

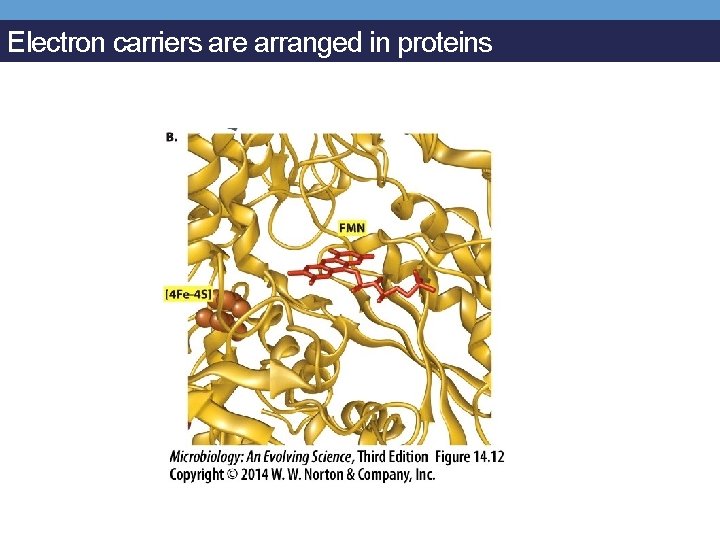
Electron carriers are arranged in proteins

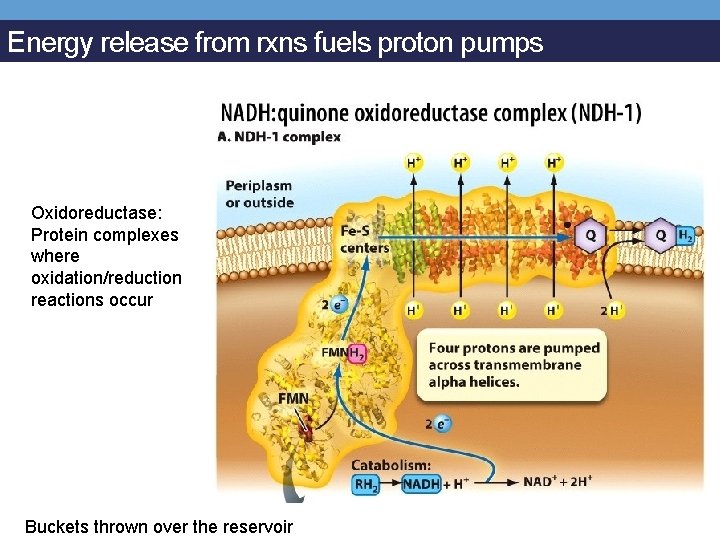
Energy release from rxns fuels proton pumps Oxidoreductase: Protein complexes where oxidation/reduction reactions occur Buckets thrown over the reservoir

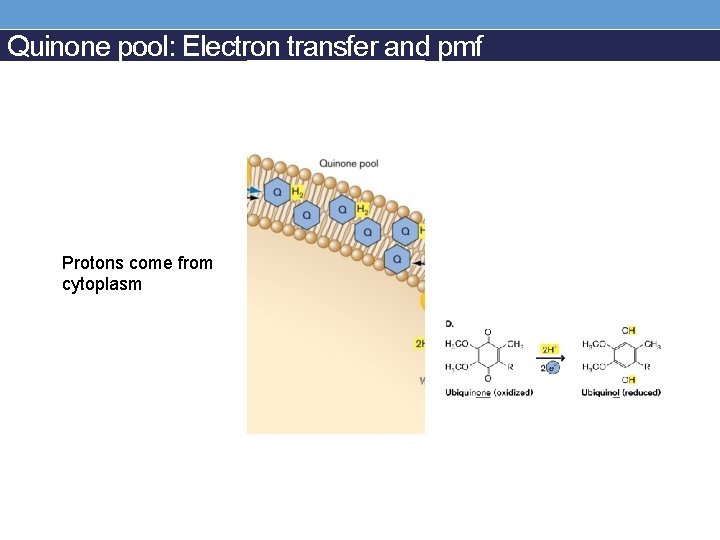
Quinone pool: Electron transfer and pmf Protons come from cytoplasm

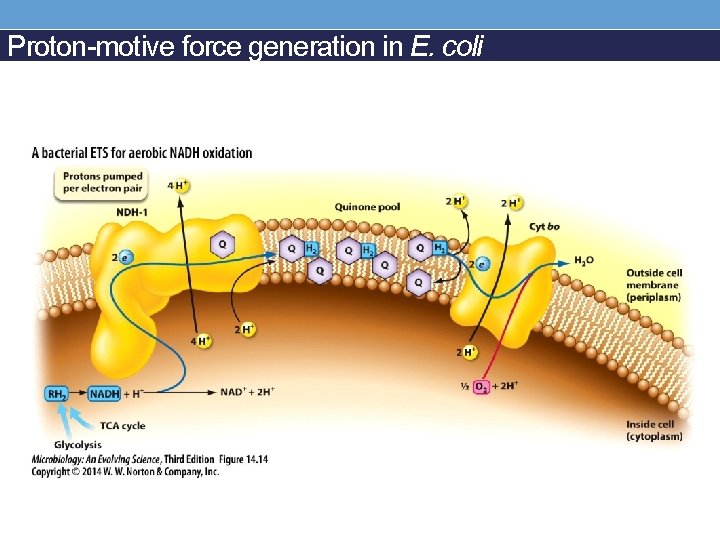
Proton-motive force generation in E. coli

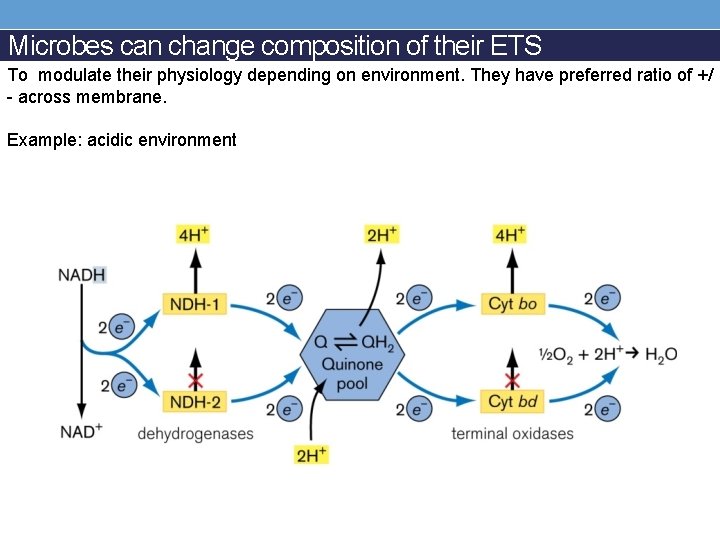
Microbes can change composition of their ETS To modulate their physiology depending on environment. They have preferred ratio of +/ - across membrane. Example: acidic environment

WHAT PROTON-MOTIVE FORCE IS AND HOW IT DRIVES ATP SYNTHESIS What is in the reservoir, and how that potential energy is converted into ATP

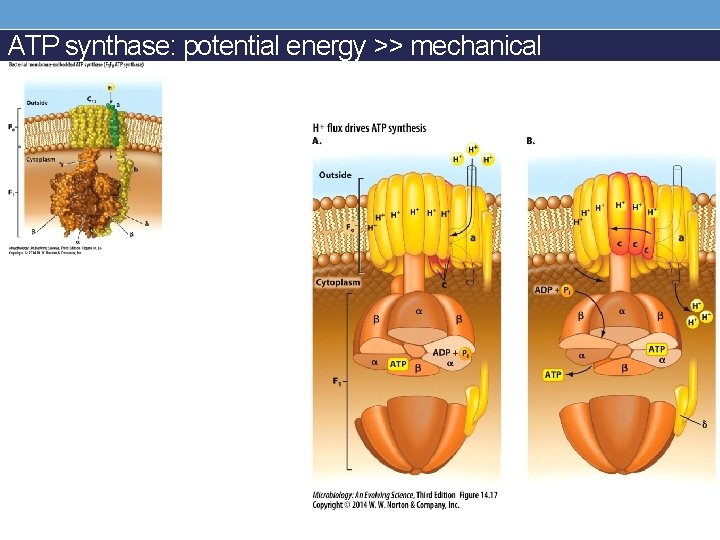
Potential energy → mechanical → ATP

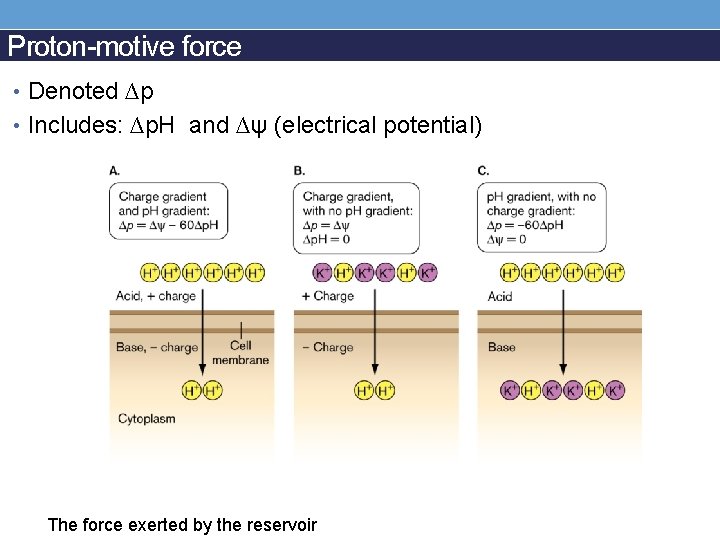
Proton-motive force • Denoted ∆p • Includes: ∆p. H and ∆ψ (electrical potential) The force exerted by the reservoir

ATP synthase: potential energy >> mechanical

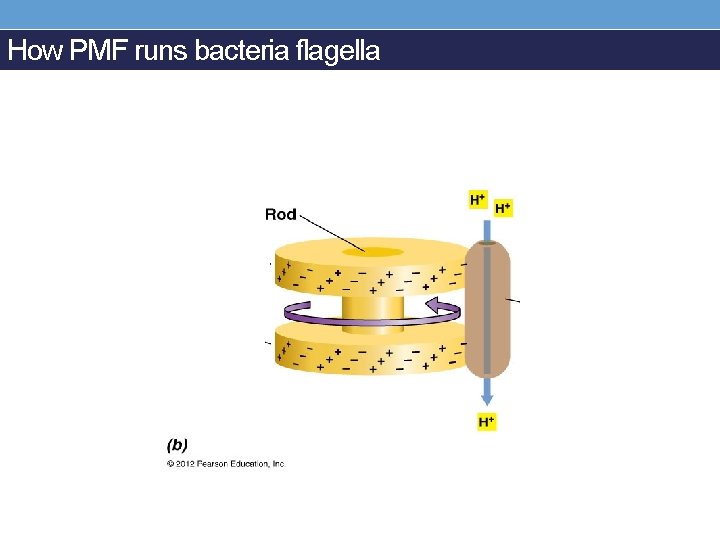
How PMF runs bacteria flagella

What is the 3 rd function of PMF in bacteria? • Follow-up: Then why might the cell want to use ATP to run ATP synthase backwards?

ANAEROBIC RESPIRATION What it is E of terminal acceptor structures communities Variation in e-donors and acceptors determines G for anaerobes Anaerobes may not use NADH

Anaerobic respiration • Electron acceptors other than oxygen • Examples include: • SO 4 • Mn • Fe • NO 3 • NO 2 • Uranium, Plutonium, Tc 99

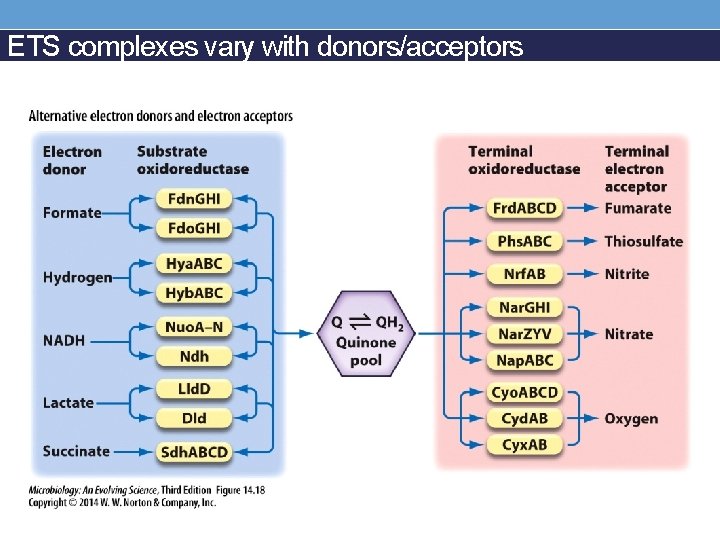
ETS complexes vary with donors/acceptors

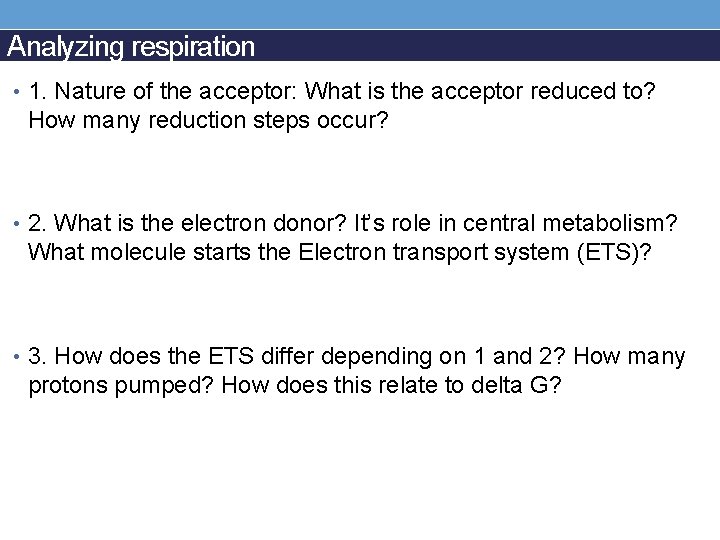
Analyzing respiration • 1. Nature of the acceptor: What is the acceptor reduced to? How many reduction steps occur? • 2. What is the electron donor? It’s role in central metabolism? What molecule starts the Electron transport system (ETS)? • 3. How does the ETS differ depending on 1 and 2? How many protons pumped? How does this relate to delta G?

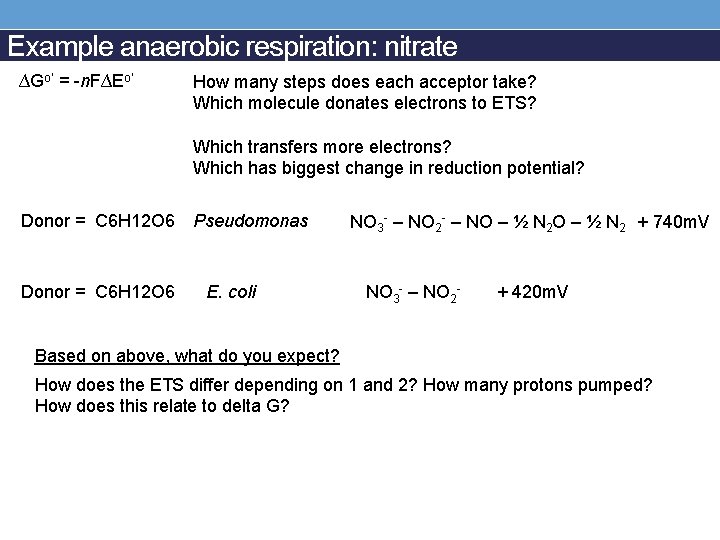
Example anaerobic respiration: nitrate ∆Go’ = -n. F∆Eo’ How many steps does each acceptor take? Which molecule donates electrons to ETS? Which transfers more electrons? Which has biggest change in reduction potential? Donor = C 6 H 12 O 6 Pseudomonas Donor = C 6 H 12 O 6 E. coli NO 3 - – NO 2 - – NO – ½ N 2 + 740 m. V NO 3 - – NO 2 - + 420 m. V Based on above, what do you expect? How does the ETS differ depending on 1 and 2? How many protons pumped? How does this relate to delta G?

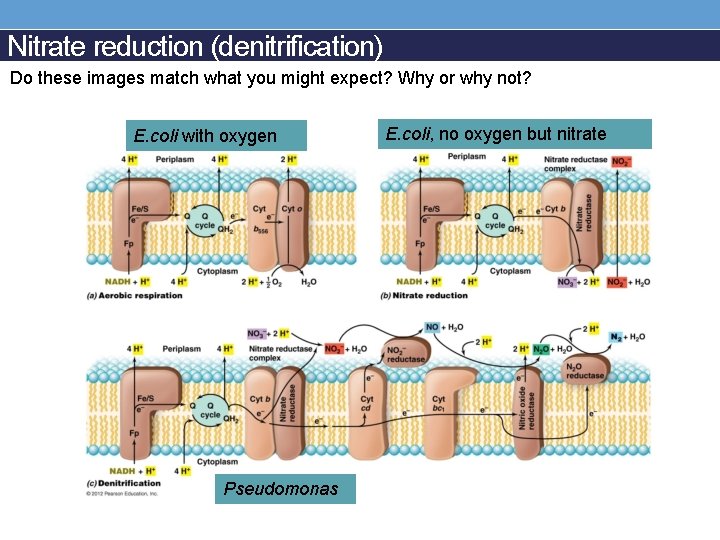
Nitrate reduction (denitrification) Do these images match what you might expect? Why or why not? E. coli with oxygen Pseudomonas E. coli, no oxygen but nitrate

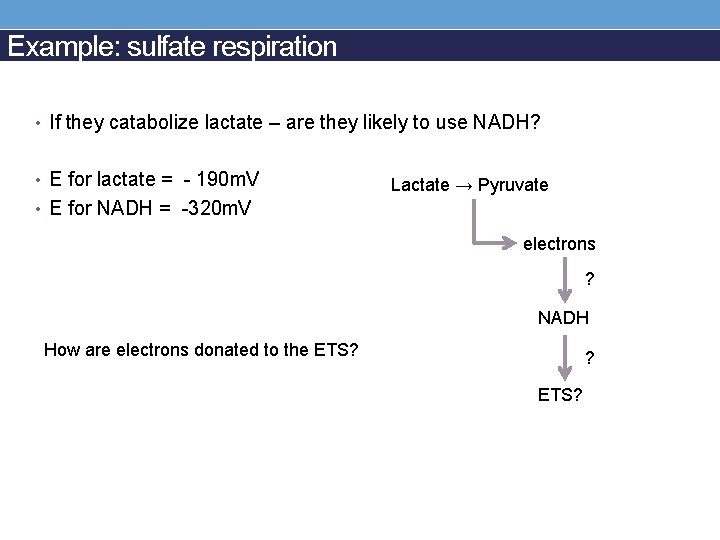
Example: sulfate respiration • If they catabolize lactate – are they likely to use NADH? • E for lactate = - 190 m. V Lactate → Pyruvate • E for NADH = -320 m. V electrons ? NADH How are electrons donated to the ETS? ? ETS?

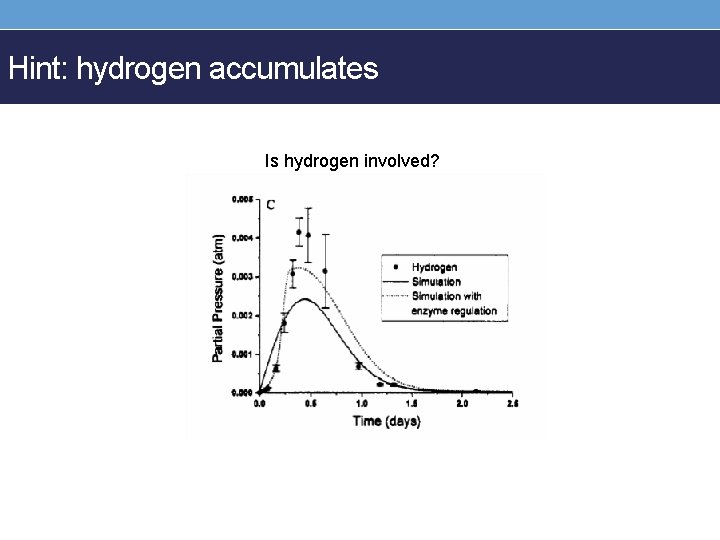
Hint: hydrogen accumulates Is hydrogen involved?

Central metabolism layout, sulfate reducers

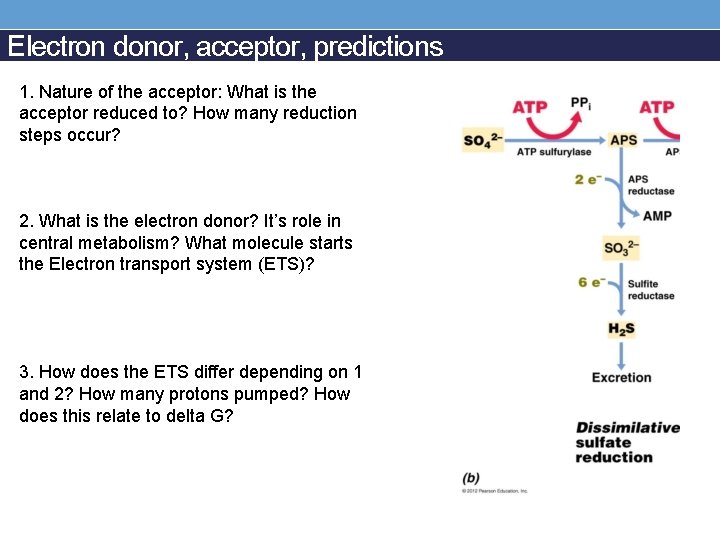
Electron donor, acceptor, predictions 1. Nature of the acceptor: What is the acceptor reduced to? How many reduction steps occur? 2. What is the electron donor? It’s role in central metabolism? What molecule starts the Electron transport system (ETS)? 3. How does the ETS differ depending on 1 and 2? How many protons pumped? How does this relate to delta G?

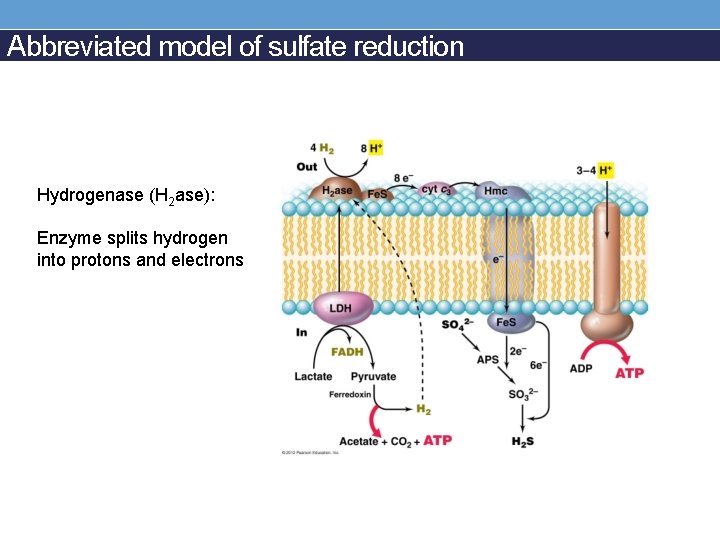
Abbreviated model of sulfate reduction Hydrogenase (H 2 ase): Enzyme splits hydrogen into protons and electrons

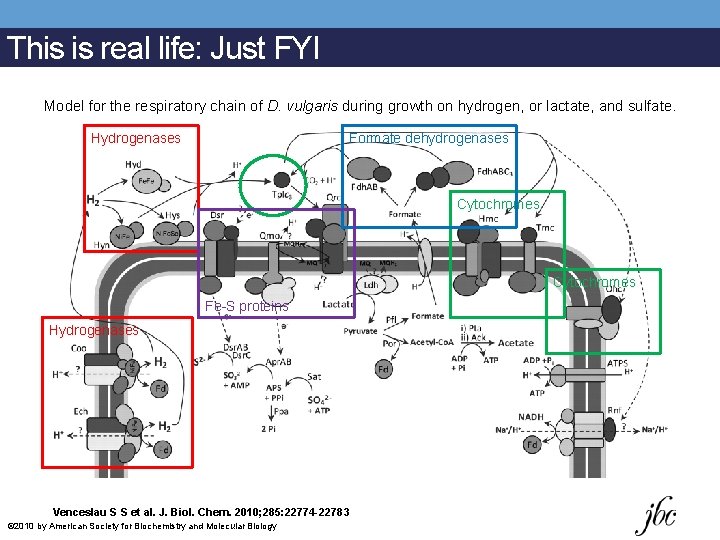
This is real life: Just FYI Model for the respiratory chain of D. vulgaris during growth on hydrogen, or lactate, and sulfate. Hydrogenases Formate dehydrogenases Cytochromes Fe-S proteins Hydrogenases Venceslau S S et al. J. Biol. Chem. 2010; 285: 22774 -22783 © 2010 by American Society for Biochemistry and Molecular Biology

What is oxidative phosphorylation?
 Pvu market cap
Pvu market cap /r/announcements
/r/announcements David ritthaler
David ritthaler What happened when montag crossed the ten-lane highway
What happened when montag crossed the ten-lane highway Kluver bucy syndrome
Kluver bucy syndrome General announcements
General announcements Now i see it now you don't
Now i see it now you don't Start time end time and elapsed time
Start time end time and elapsed time Quiz master introduction
Quiz master introduction Target master quiz
Target master quiz If + past perfect
If + past perfect James forten from now is your time
James forten from now is your time Hamlet's 5th soliloquy
Hamlet's 5th soliloquy Now is the time to repent
Now is the time to repent Time order words
Time order words Ca time now
Ca time now Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Thang điểm glasgow
Thang điểm glasgow Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Ví dụ giọng cùng tên
Ví dụ giọng cùng tên