ALCOHOLS SANTOSH CHEMISTRY DEPT Introduction Alcohols constitute a


















- Slides: 39

ALCOHOLS SANTOSH CHEMISTRY DEPT

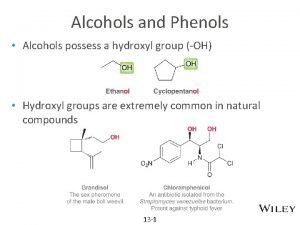
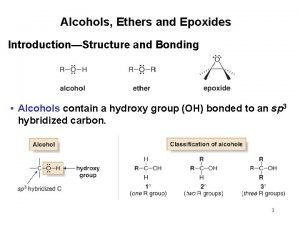
Introduction • Alcohols constitute a class of compounds which are regarded as hydroxy derivatives of hydrocarbons. • Based on the no. of hydroxyl groups the alcohols are classified as mono-, di- and trihydric accordingly, if the molecules contain 1, 2 or 3 –OH groups respectively. The alcohols which contain four or more no. of –OH groups are called polyhydric alcohols.

Classification CH 3, 1 o, 2 o, 3 o • Primary: carbon with –OH is bonded to one other carbon. • Secondary: carbon with –OH is bonded to two other carbons. • Tertiary: carbon with –OH is bonded to three other carbons. • Aromatic (phenol): -OH is bonded to a benzene ring.

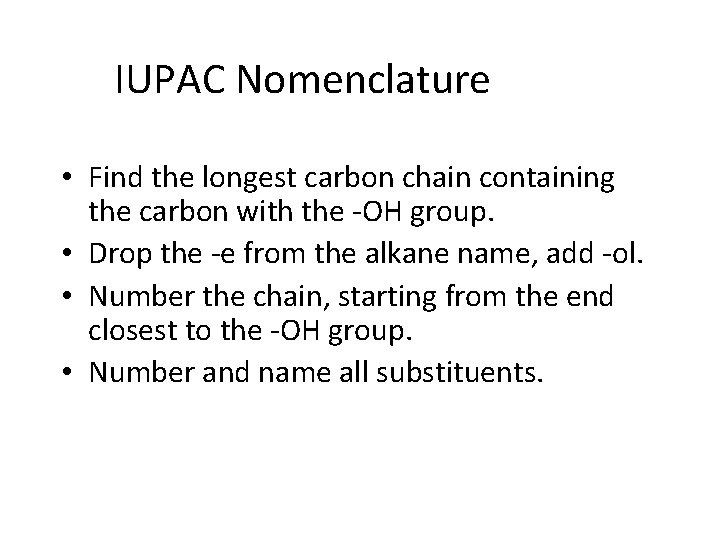
IUPAC Nomenclature • Find the longest carbon chain containing the carbon with the -OH group. • Drop the -e from the alkane name, add -ol. • Number the chain, starting from the end closest to the -OH group. • Number and name all substituents.

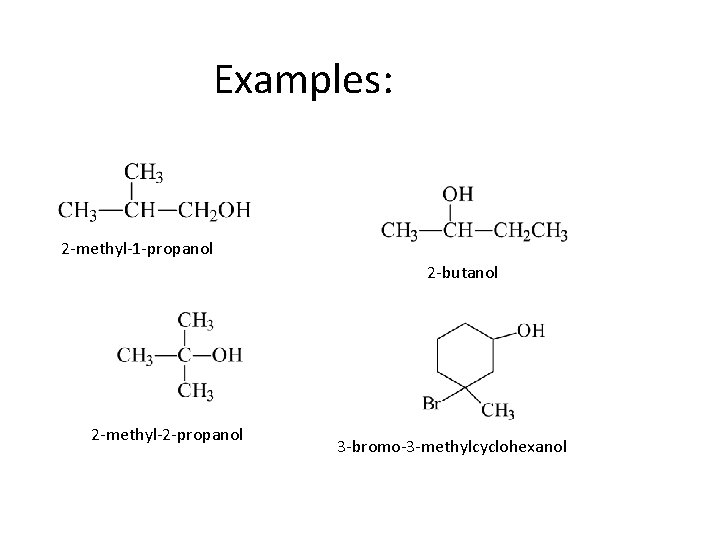
Examples: 2 -methyl-1 -propanol 2 -butanol 2 -methyl-2 -propanol 3 -bromo-3 -methylcyclohexanol

Unsaturated Alcohols • Hydroxyl group takes precedence. Assign that carbon the lowest number. • Use alkene or alkyne name. pent-4 -ene-2 -ol

Hydroxy Substituent • When -OH is part of a higher priority class of compound, it is named as hydroxy. • Example: 4 -hydroxybutanoic acid 7

Common Names • Alcohol can be named as alkyl alcohol. • Useful only for small alkyl groups. • Examples: isobutyl alcohol sec-butyl alcohol

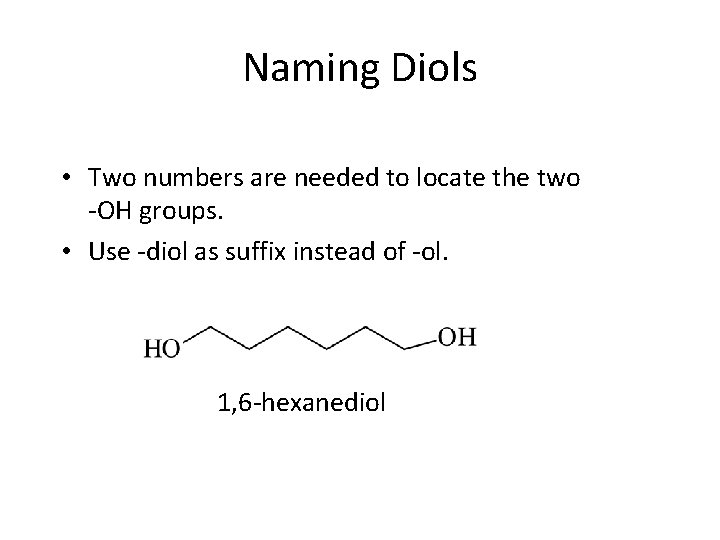
Naming Diols • Two numbers are needed to locate the two -OH groups. • Use -diol as suffix instead of -ol. 1, 6 -hexanediol

Glycols • 1, 2 diols (vicinal diols) are called glycols. • Common names for glycols use the name of the alkene from which they were made. 1, 2 -ethanediol 1, 2 -propanediol ethylene glycol propylene glycol

Preparation Reactions �Reduction of carbonyl compounds �Hydration of Alkenes �Grignard reactions

Reduction of Carbonyl Compounds • Reduction of Aldehydes/ketones • Reduction of Carboxylic acids/Esters

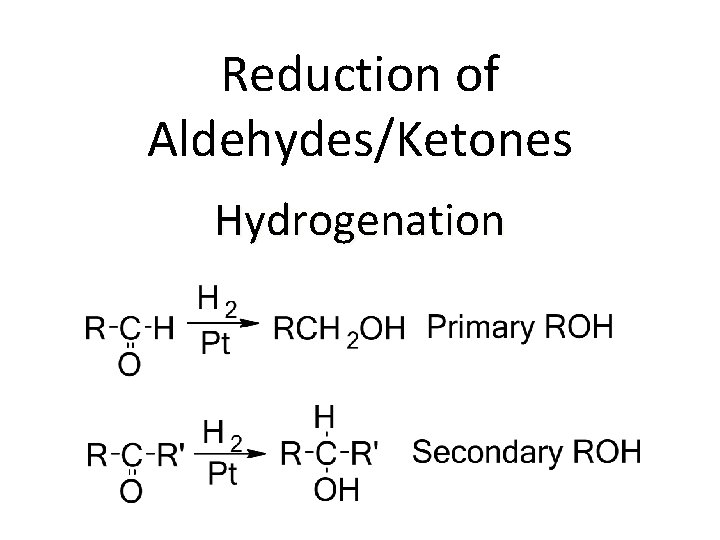
Reduction of Aldehydes/Ketones Hydrogenation

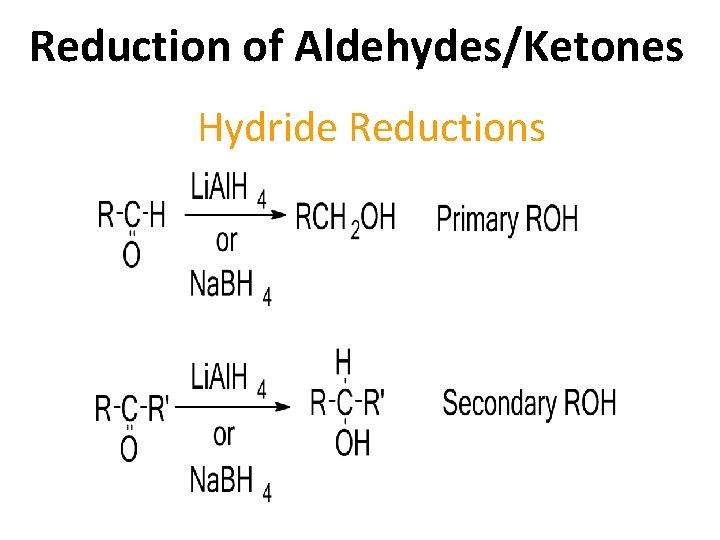
Reduction of Aldehydes/Ketones Hydride Reductions

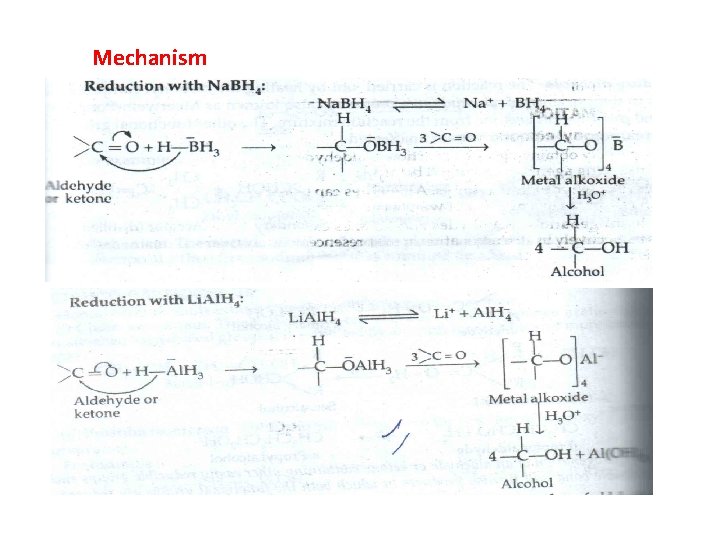
Mechanism

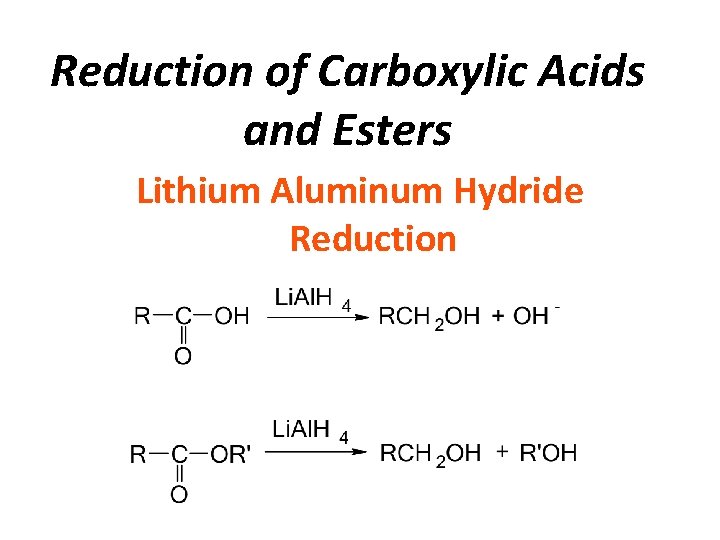
Reduction of Carboxylic Acids and Esters Lithium Aluminum Hydride Reduction

Hydration of Alkenes �Acid catalyzed Hydration �Oxymercuration-Demercuration �Hydroboration-Oxidation

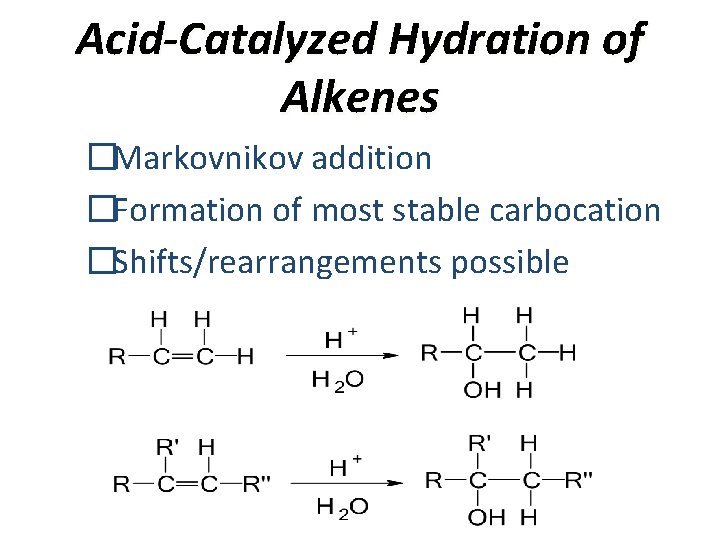
Acid-Catalyzed Hydration of Alkenes �Markovnikov addition �Formation of most stable carbocation �Shifts/rearrangements possible

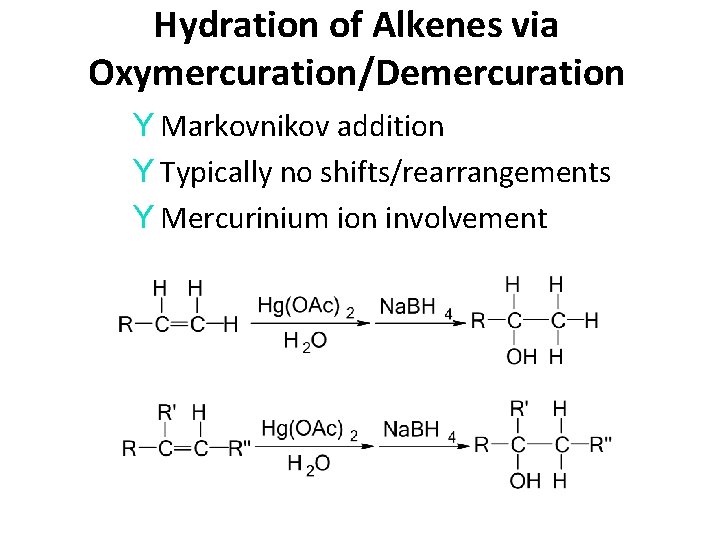
Hydration of Alkenes via Oxymercuration/Demercuration Y Markovnikov addition Y Typically no shifts/rearrangements Y Mercurinium ion involvement

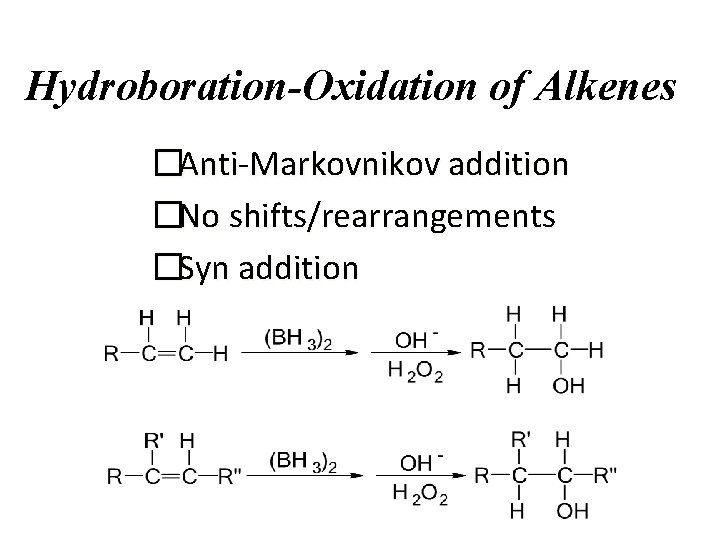
Hydroboration-Oxidation of Alkenes �Anti-Markovnikov addition �No shifts/rearrangements �Syn addition

Grignard Addition Reactions • • • Addition to Aldehydes/Ketones Addition to Esters Addition to Epoxides

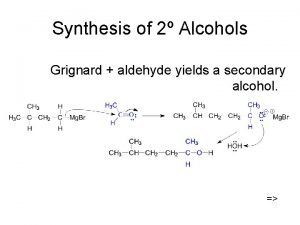
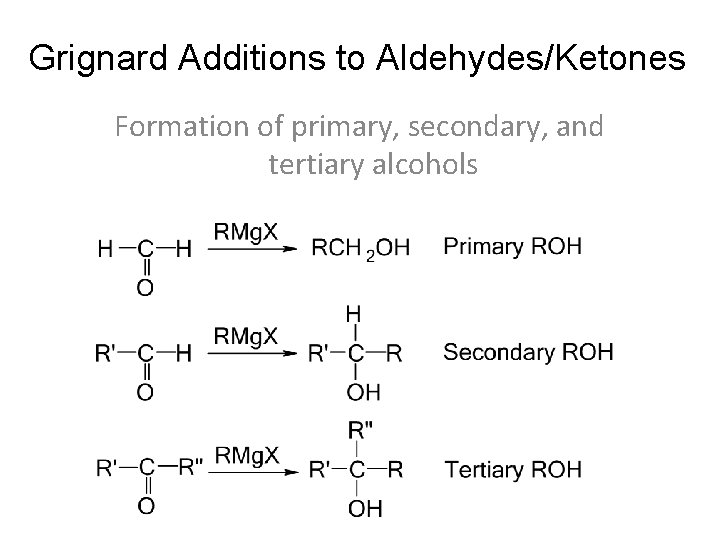
Grignard Additions to Aldehydes/Ketones Formation of primary, secondary, and tertiary alcohols

Grignard Additions to Esters Formation of secondary and tertiary alcohols

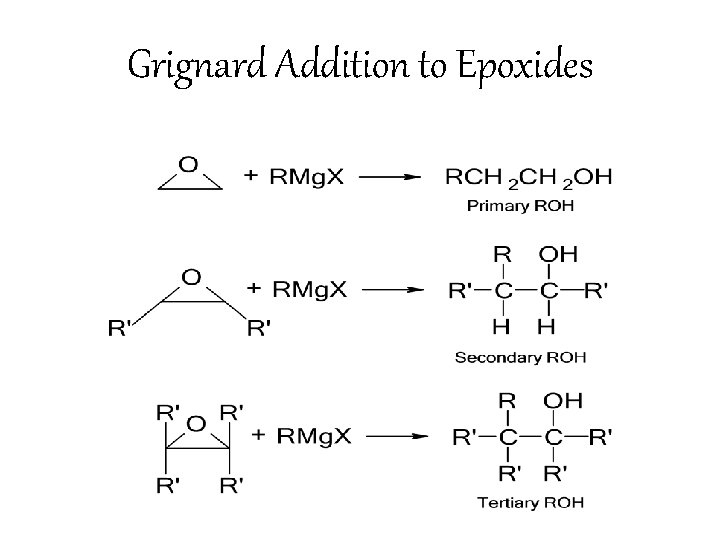
Grignard Addition to Epoxides

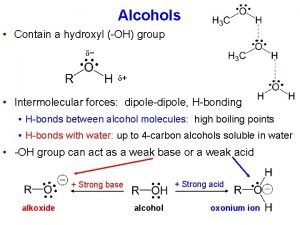
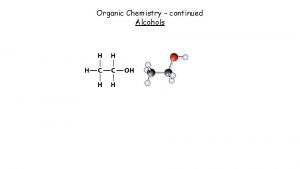
Physical Properties • Unusually high boiling points due to hydrogen bonding between molecules. • Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases.

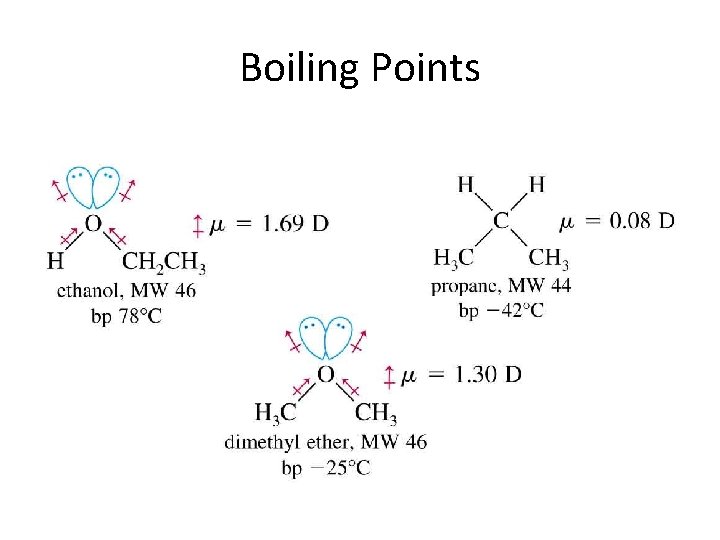
Boiling Points

Solubility in Water Solubility decreases as the size of the alkyl group increases.

CHEMICAL PROPERTIES The hydroxy gp present in alcohols is a very reactive gp and the characterstic rxns of alcohols are the rxns of –OH gp. In general, these are divided into 3 categories: I. Rxns involving the cleavage of O-H bond II. Rxns involving the cleavage of C-OH bond III. Rxns involving both alkyl and hydroxyl gps of the acohol molecules.

Typical Alcohol Reactions �Salt formation �Dehydration �Oxidation �Alkyl halide formation �Ester formation �Ether synthesis �Periodic acid cleavage of glycols �Haloform reaction of methyl carbinols �THP acetal formation

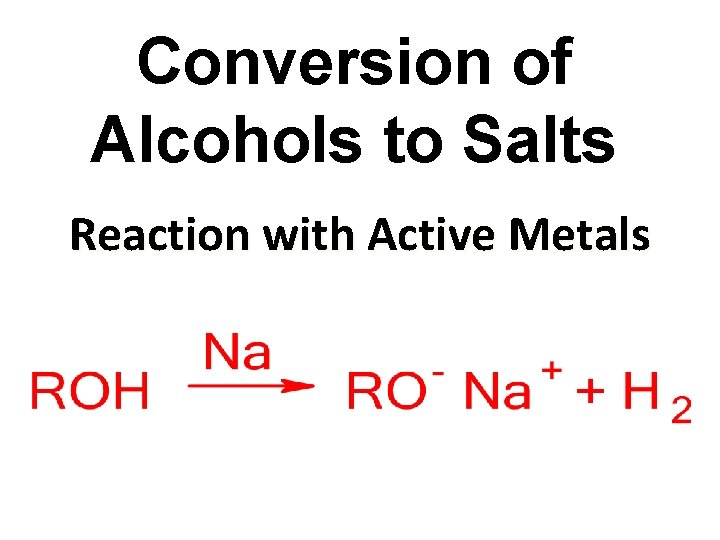
Conversion of Alcohols to Salts Reaction with Active Metals

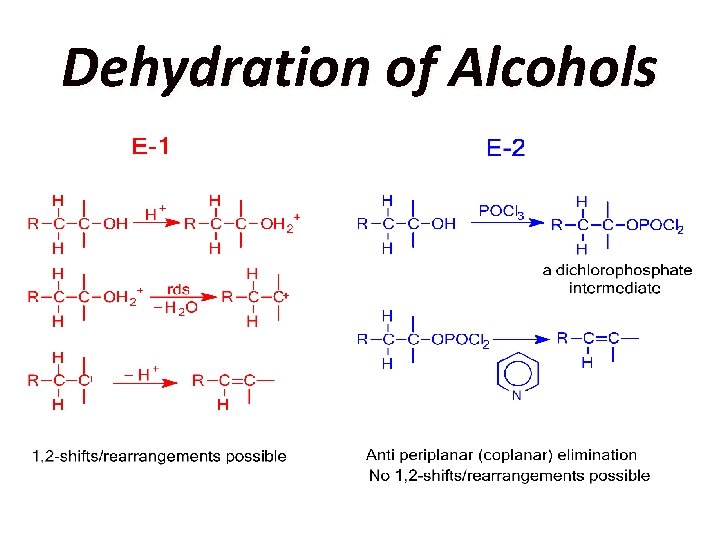
Dehydration of Alcohols

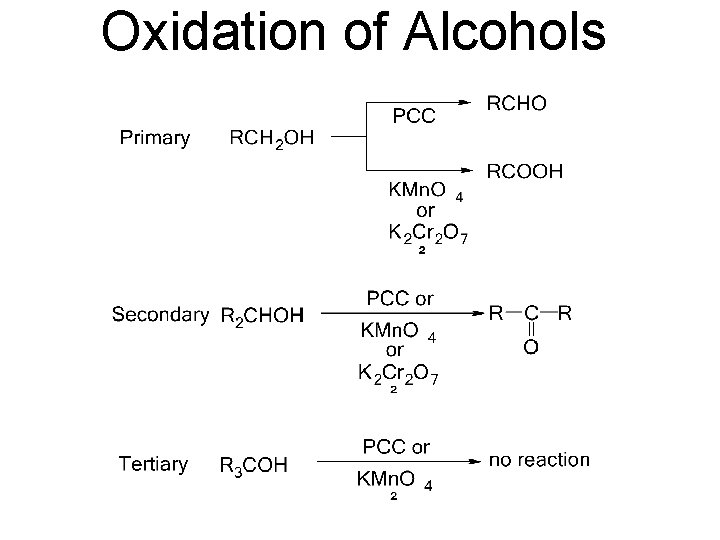
Oxidation of Alcohols

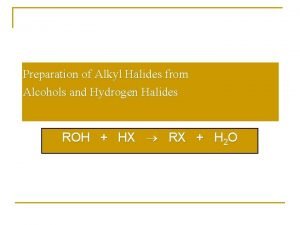
Alcohol Conversion to Alkyl Halides �Reaction with Hydrogen halides �Reaction with Thionyl chloride �Reaction with Phosphorus trihalides or pentahalides

Hydrogen Halide Conversion of Alcohols to Alkyl Halides

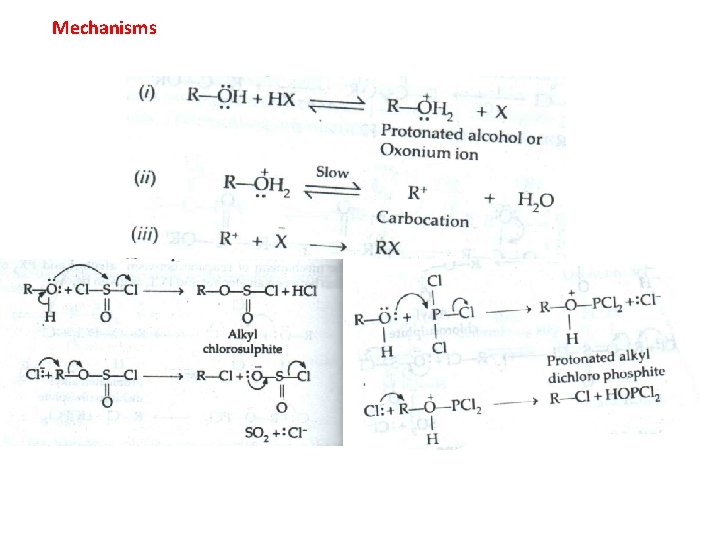
Mechanisms

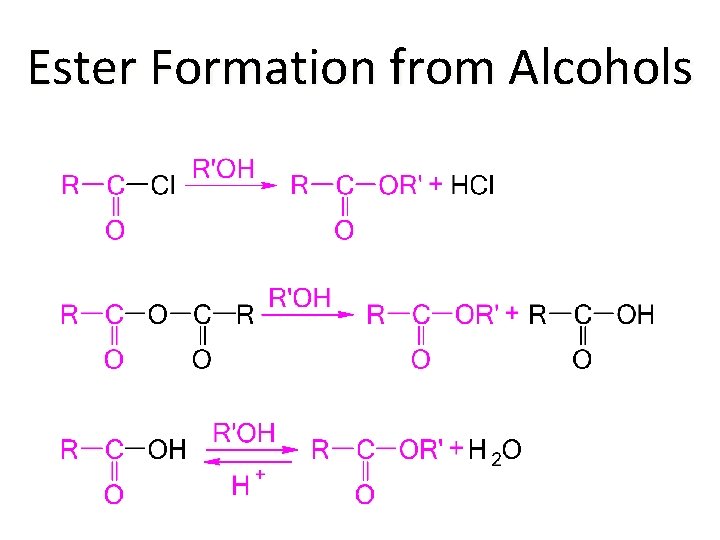
Ester Formation from Alcohols

Distinction b/w 1 o, 2 o & 3 o alcohols • LUCAS TEST Lucas reagent : equimolar mixture of c. HCl and anhyd. Zn. Cl 2 Appearance of cloudiness in the rxn mixture indicates the conversion of alcohol into alkyl halide. Observation 30 alcohol: - reacts immediately & cloudiness appears immediately. 20 alcohol: - reacts within about 5 minutes when the cloudiness appears. 10 alcohol: - does not react appreciably at room temp. & therefore no cloudiness appears. • An older method known as Victor Meyer’s test is seldom used these days.

Dihydric Alcohols Glycols

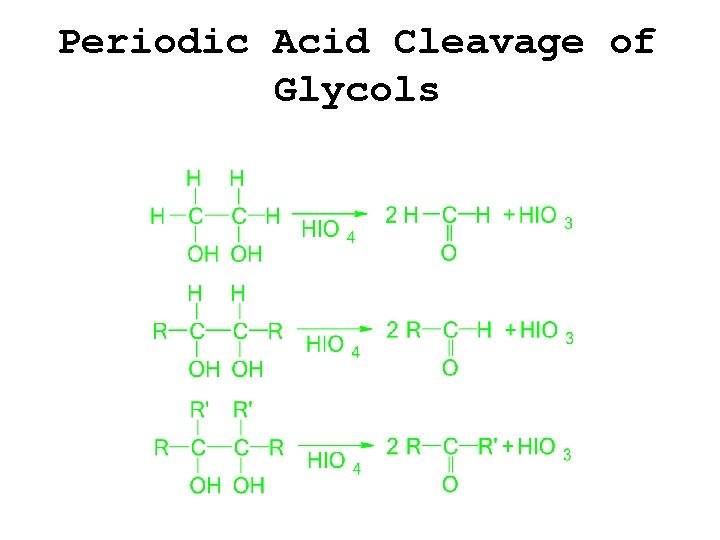
Periodic Acid Cleavage of Glycols
 Santosh vempala
Santosh vempala Dr santosh kumar swain
Dr santosh kumar swain Life of pi orange juice
Life of pi orange juice Santosh vempala
Santosh vempala Santosh thomas
Santosh thomas Job cost sheets constitute the subsidiary ledger for the
Job cost sheets constitute the subsidiary ledger for the The earths layer foldable
The earths layer foldable Define constitute
Define constitute Define constitute
Define constitute Data constitute the building blocks of information.
Data constitute the building blocks of information. Synthesis of alcohol
Synthesis of alcohol Alcohols containing cppp nucleus
Alcohols containing cppp nucleus What is secondary alcohol
What is secondary alcohol Ketone to tertiary alcohol
Ketone to tertiary alcohol What is the ester
What is the ester Lucas test reagent
Lucas test reagent Hydrogen halide
Hydrogen halide Chemsheets reactions of alcohols 1 answers
Chemsheets reactions of alcohols 1 answers Tscl py reaction
Tscl py reaction Acidity of alcohols
Acidity of alcohols Names of sugar alcohols
Names of sugar alcohols Alcohols nomenclature
Alcohols nomenclature Naming alkyl halides
Naming alkyl halides Ethers naming
Ethers naming 2-butanone functional group
2-butanone functional group Oh group in phenol is
Oh group in phenol is Lucas reagent is
Lucas reagent is Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Dept nmr spectroscopy
Dept nmr spectroscopy Department of agriculture consumer services
Department of agriculture consumer services Finance departments
Finance departments Department of inspectional services
Department of inspectional services Dept. name of organization (of affiliation)
Dept. name of organization (of affiliation) Mn dept of education
Mn dept of education Ms department of finance and administration
Ms department of finance and administration Dept. name of organization
Dept. name of organization Ohio dept of dd
Ohio dept of dd Affiliation poster presentation
Affiliation poster presentation Vaginal dept
Vaginal dept Gome dept
Gome dept