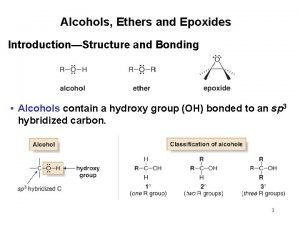
Alcohols Alcohols l l l Methanol CH 3









- Slides: 20

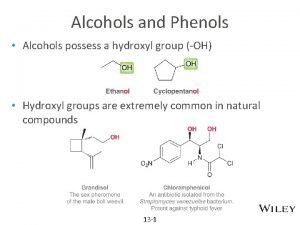
Alcohols

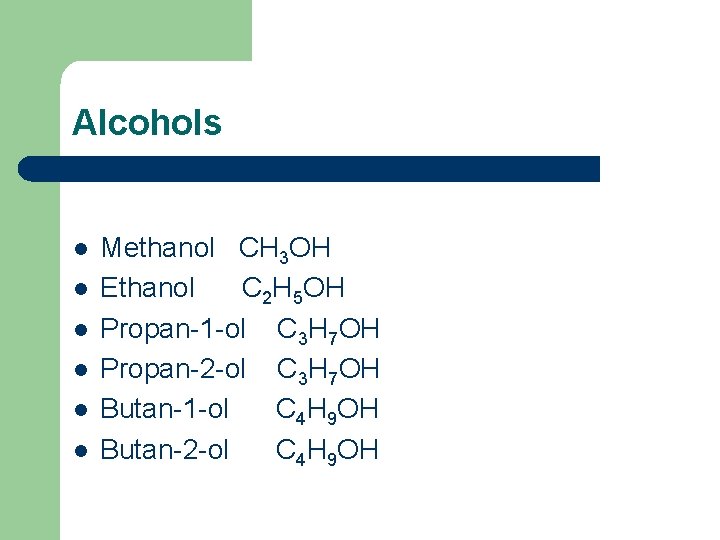
Alcohols l l l Methanol CH 3 OH Ethanol C 2 H 5 OH Propan-1 -ol C 3 H 7 OH Propan-2 -ol C 3 H 7 OH Butan-1 -ol C 4 H 9 OH Butan-2 -ol C 4 H 9 OH

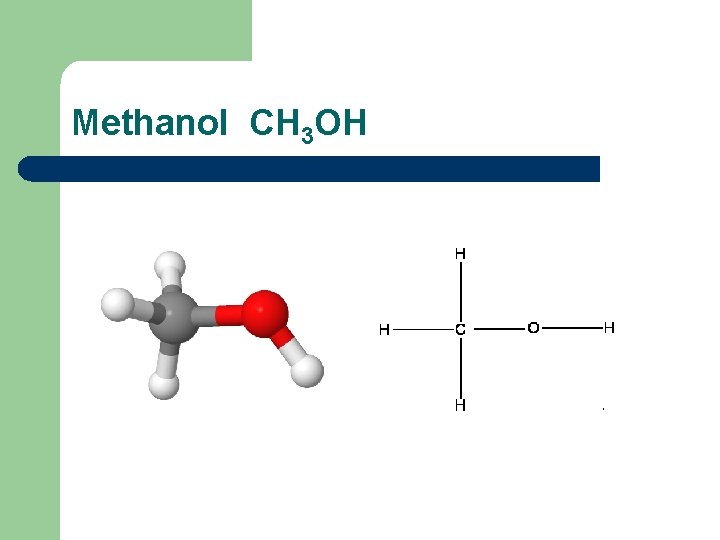
Methanol CH 3 OH

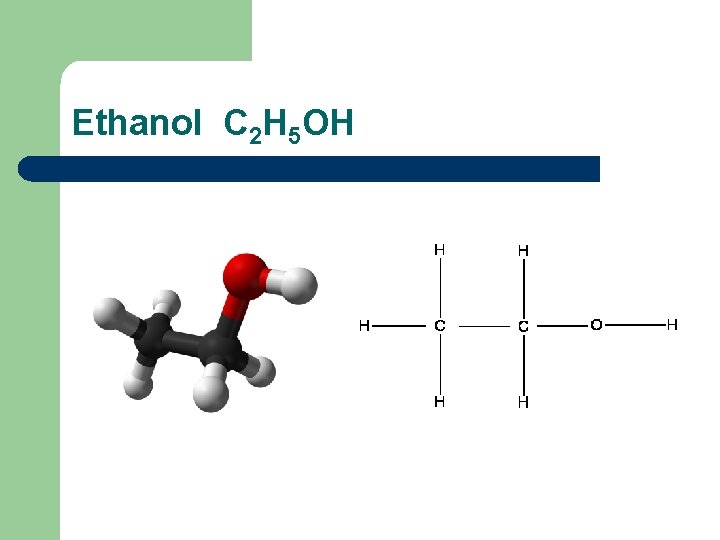
Ethanol C 2 H 5 OH

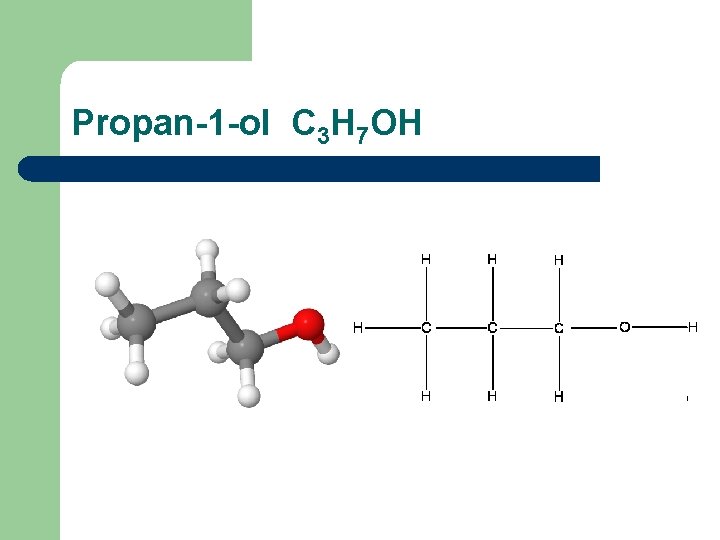
Propan-1 -ol C 3 H 7 OH

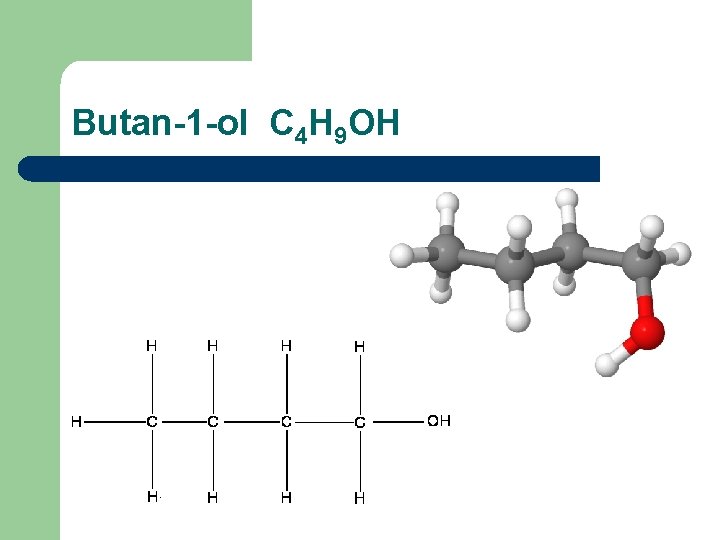
Butan-1 -ol C 4 H 9 OH

Propan-2 -ol CH 3 CH(OH)CH 3

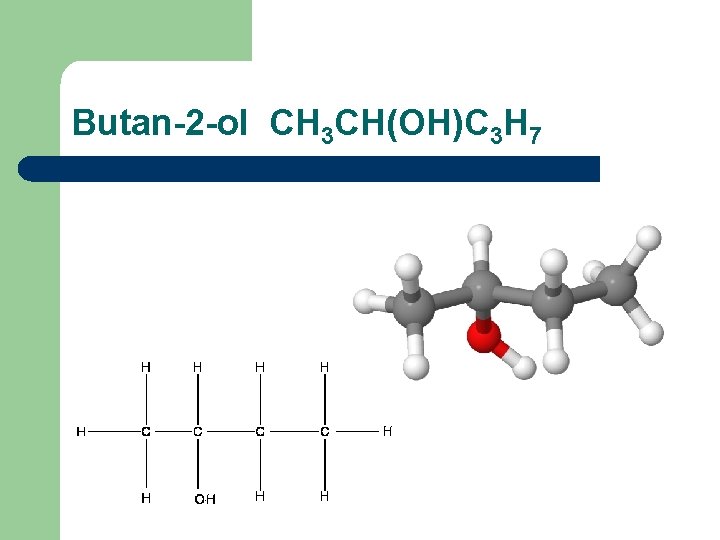
Butan-2 -ol CH 3 CH(OH)C 3 H 7

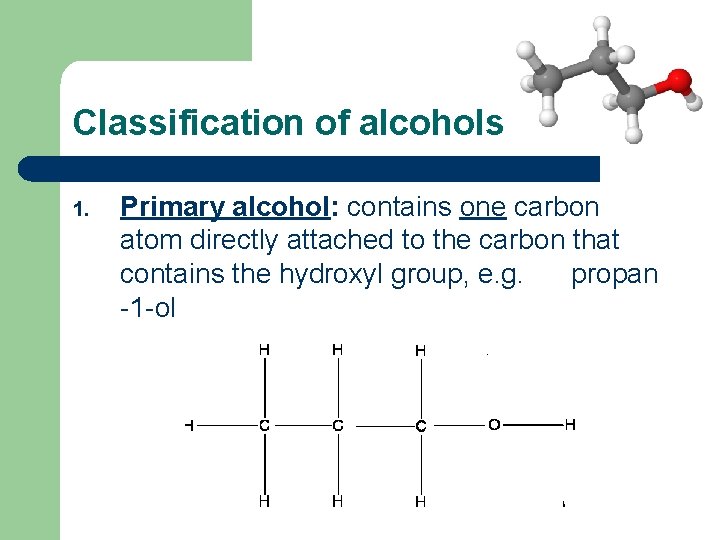
Classification of alcohols 1. Primary alcohol: contains one carbon atom directly attached to the carbon that contains the hydroxyl group, e. g. propan -1 -ol

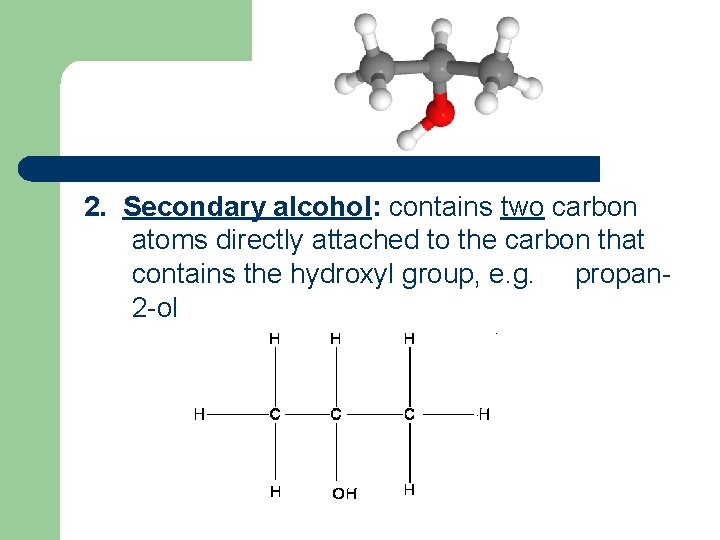
2. Secondary alcohol: contains two carbon atoms directly attached to the carbon that contains the hydroxyl group, e. g. propan 2 -ol

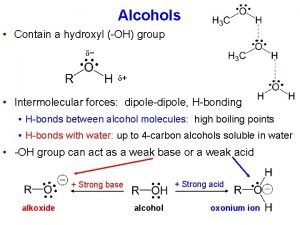
Physical properties l l Physical state: Liquid Boiling points much higher than the corresponding alkanes, due to polar OH group

Physical properties Solubility of methanol in l (i) cyclohexane – not very soluble methanol is polar cyclohexane is not l (ii) water - completely soluble because it is polar. l As alcohol molecule gets bigger the polar part becomes less significant so the alcohol becomes less soluble in water and more soluble in cyclohexane

Butan-1 -ol is – (i) soluble in cyclohexane – (ii) not very soluble in water l The polar OH group is becoming less significant as the molecule gets bigger

Comparison with water l l Both have polar OH groups Alcohols have a non-polar part Both form hydrogen bonds between their molecules Water is more polar and has a greater capacity to form hydrogen bonds and so has a higher boiling point than methanol or ethanol

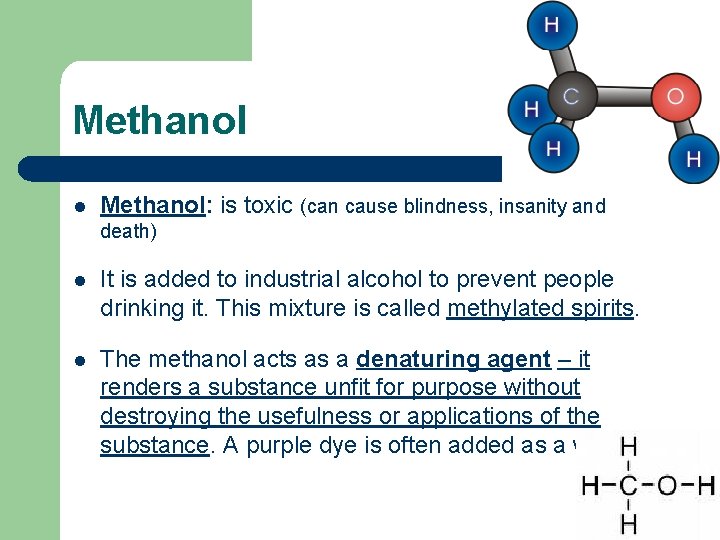
Methanol l Methanol: is toxic (can cause blindness, insanity and death) l It is added to industrial alcohol to prevent people drinking it. This mixture is called methylated spirits. l The methanol acts as a denaturing agent – it renders a substance unfit for purpose without destroying the usefulness or applications of the substance. A purple dye is often added as a warning.

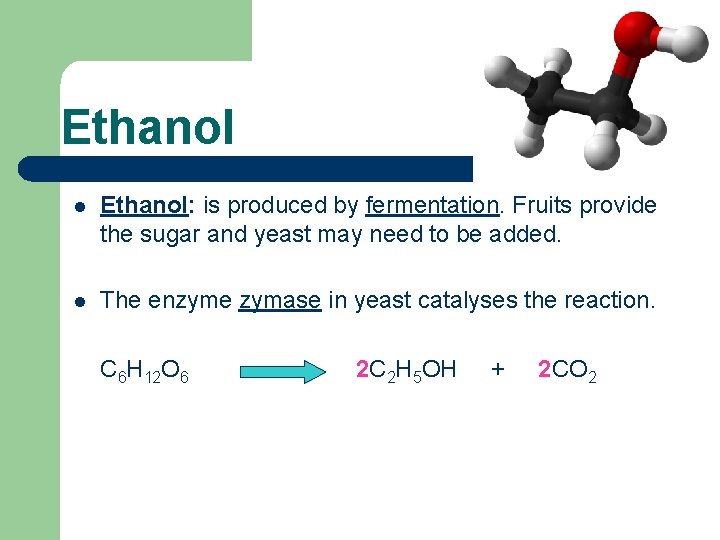
Ethanol l Ethanol: is produced by fermentation. Fruits provide the sugar and yeast may need to be added. l The enzyme zymase in yeast catalyses the reaction. C 6 H 12 O 6 2 C 2 H 5 OH + 2 CO 2

Alcoholic Drinks Ingredient Drink Grapes Wine % (v/v) alcohol 12 Apples Cider 4. 5 Malted grain Beer 5

Ethanol l To produce drinks of higher alcohol concentration the fermented liquids must be distilled. l Spirits (whiskey, brandy, gin, vodka) contain 40% alcohol.

Gasohol l Ethanol obtained from sugar cane is used for making gasohol in Brazil. This is then used as a fuel.

Uses of ethanol 1. Alcoholic drinks 2. Fuel 3. Solvent (can dissolve both polar and nonpolar solutes)
 Lucas reaction
Lucas reaction Acidity of alcohols
Acidity of alcohols These are alcohols containing cppp nucleus
These are alcohols containing cppp nucleus Alkoxide leaving group
Alkoxide leaving group Naming alkyl halides
Naming alkyl halides Chlorination
Chlorination Sp
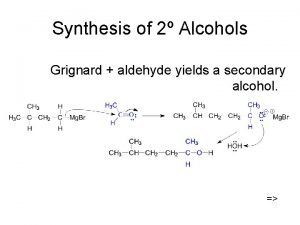
Sp Primary alcohol vs secondary alcohol
Primary alcohol vs secondary alcohol Swern oxidation
Swern oxidation Alcohols nomenclature
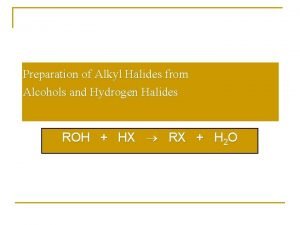
Alcohols nomenclature Hydrogen halide
Hydrogen halide Relative sweetness chart
Relative sweetness chart Oxidation of tertiary alcohol
Oxidation of tertiary alcohol Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Methyl propyl ether
Methyl propyl ether Reactions of alcohols 1 chemsheets answers
Reactions of alcohols 1 chemsheets answers Tertiary alcohol synthesis
Tertiary alcohol synthesis Alcohols nomenclature
Alcohols nomenclature Ethanol use
Ethanol use Transfer buffer methanol 역할
Transfer buffer methanol 역할 Aceetic acid
Aceetic acid