Structure of Alcohols The alcohols are a homologous



![Chemical Property 2 - Oxidation + 2 [O] + H 2 O C 2 Chemical Property 2 - Oxidation + 2 [O] + H 2 O C 2](https://slidetodoc.com/presentation_image_h/a1bb1b80d2d7d927758dab0b035b122a/image-8.jpg)

- Slides: 9

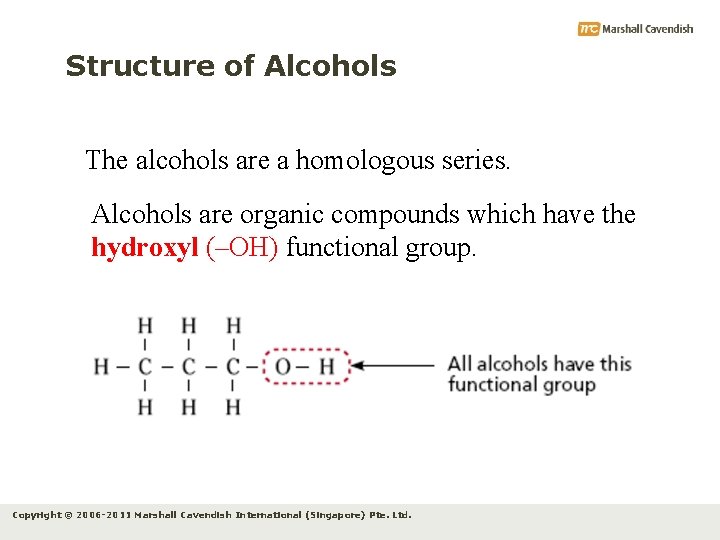
Structure of Alcohols The alcohols are a homologous series. Alcohols are organic compounds which have the hydroxyl (–OH) functional group. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

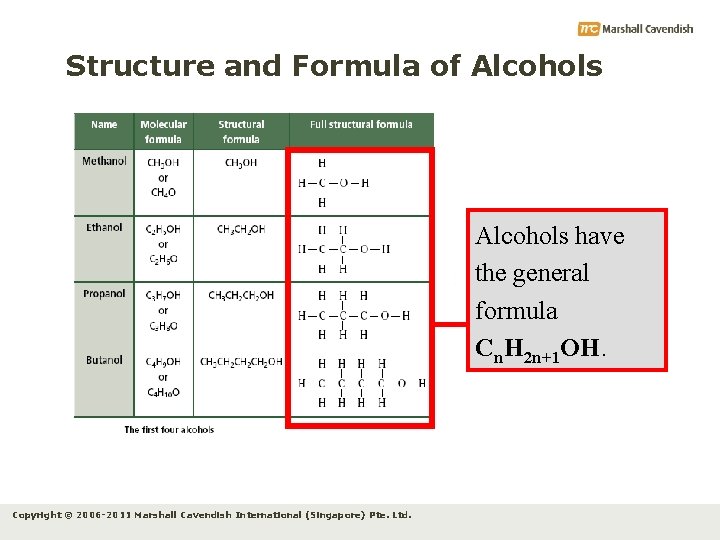
Structure and Formula of Alcohols have the general formula Cn. H 2 n+1 OH. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Properties of Alcohols are soluble in water, but their solubility decreases as their molecular size increases. For example, methanol is very soluble in water butanol is only slightly soluble in water. Unlike the alkanes and alkenes, the first four alcohols are liquids at room temperature and pressure. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

Properties of Alcohols Although alcohols contain the –OH group, they are not alkalis. In fact, they are all neutral. Alcohols can take part in these reactions: combustion and oxidation. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

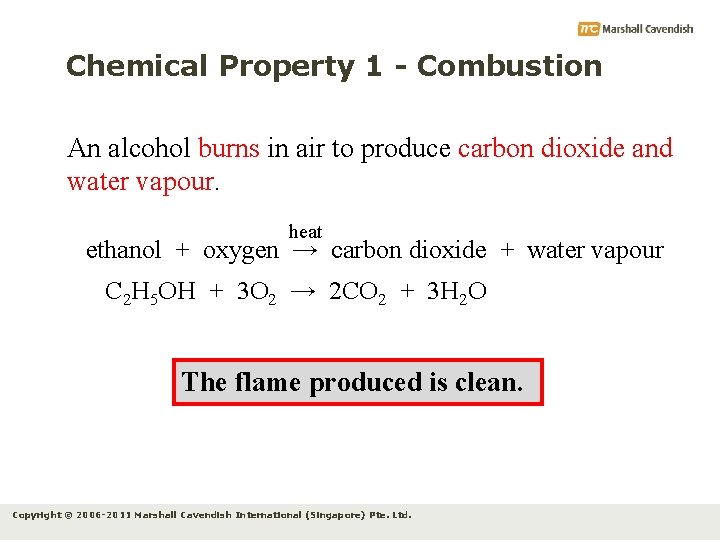
Chemical Property 1 - Combustion An alcohol burns in air to produce carbon dioxide and water vapour. heat ethanol + oxygen → carbon dioxide + water vapour C 2 H 5 OH + 3 O 2 → 2 CO 2 + 3 H 2 O The flame produced is clean. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

How is the combustion of alcohols useful to us? Alcohols can be used as a fuel because: Methanol is less likely than conventional fuels to explode in an accident. It is a clean fuel. When alcoholic beverage is burnt with food, it gives a distinct flavour. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.

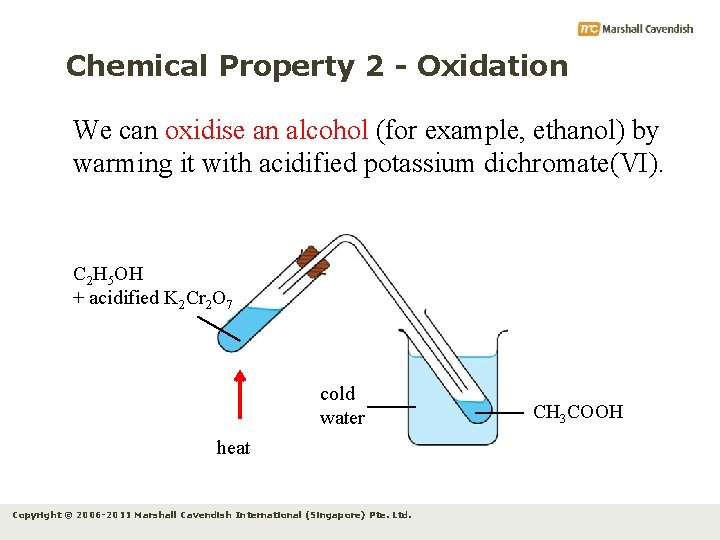
Chemical Property 2 - Oxidation We can oxidise an alcohol (for example, ethanol) by warming it with acidified potassium dichromate(VI). C 2 H 5 OH + acidified K 2 Cr 2 O 7 cold water heat Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd. CH 3 COOH
![Chemical Property 2 Oxidation 2 O H 2 O C 2 Chemical Property 2 - Oxidation + 2 [O] + H 2 O C 2](https://slidetodoc.com/presentation_image_h/a1bb1b80d2d7d927758dab0b035b122a/image-8.jpg)
Chemical Property 2 - Oxidation + 2 [O] + H 2 O C 2 H 5 OH + acidified K 2 Cr 2 O 7 The colour of potassium dichromate(VI) changes from orange to green. cold water heat Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd. CH 3 COOH

How is oxidation of alcohol useful? The police use a breathalyser to test the amount of alcohol consumed by drivers. A breathalyser contains potassium dichromate(VI). A colour change is registered if a certain amount of alcohol is present in the breath. The orange colour changes to green. Copyright © 2006 -2011 Marshall Cavendish International (Singapore) Pte. Ltd.