Organic Chemistry continued Alcohols Alcohols general formula Cn

- Slides: 8

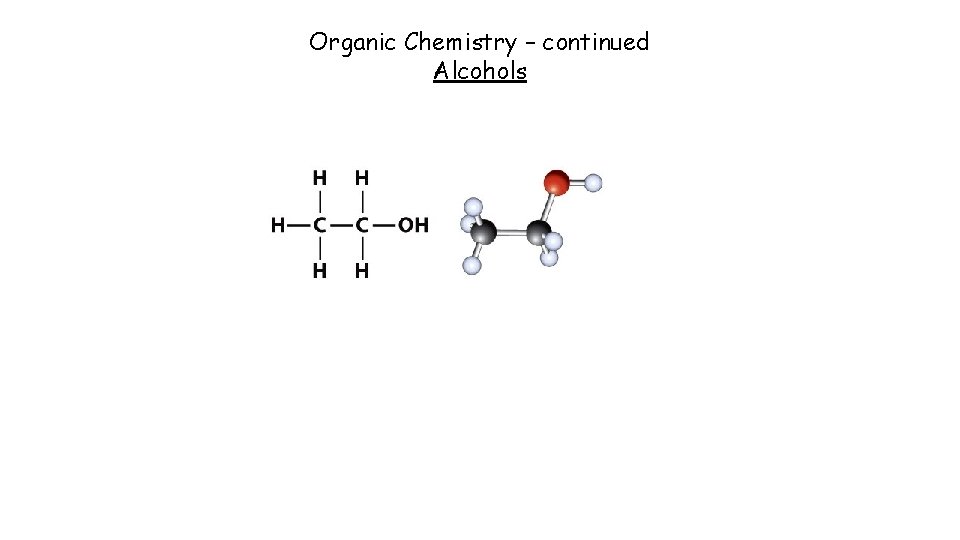
Organic Chemistry – continued Alcohols

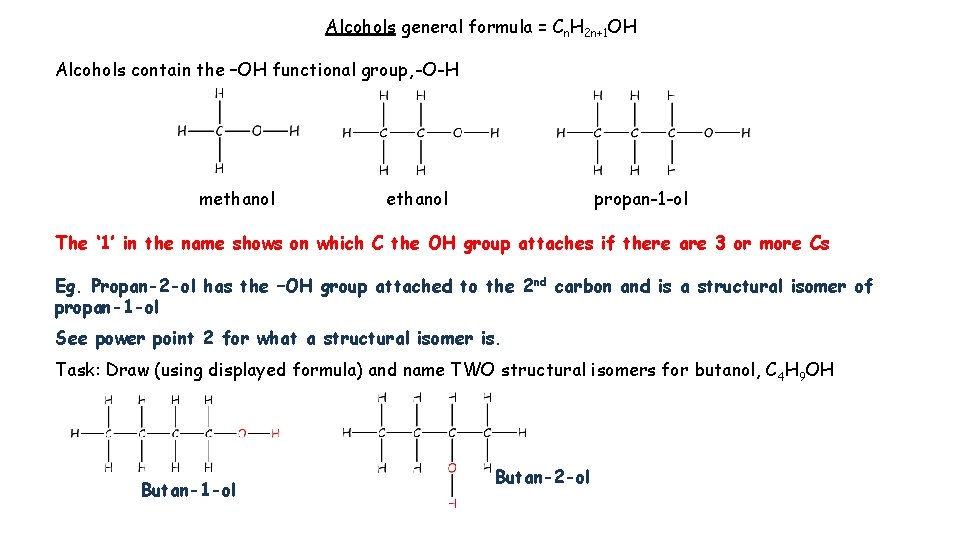
Alcohols general formula = Cn. H 2 n+1 OH Alcohols contain the –OH functional group, -O-H methanol propan-1 -ol The ‘ 1’ in the name shows on which C the OH group attaches if there are 3 or more Cs Eg. Propan-2 -ol has the –OH group attached to the 2 nd carbon and is a structural isomer of propan-1 -ol See power point 2 for what a structural isomer is. Task: Draw (using displayed formula) and name TWO structural isomers for butanol, C 4 H 9 OH Butan-1 -ol Butan-2 -ol

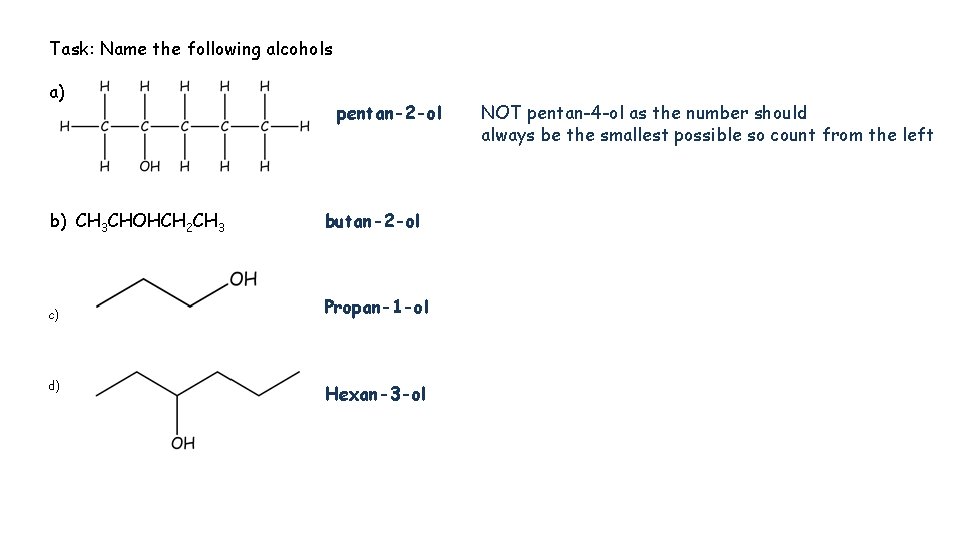
Task: Name the following alcohols a) pentan-2 -ol b) CH 3 CHOHCH 2 CH 3 butan-2 -ol c) Propan-1 -ol d) c Hexan-3 -ol NOT pentan-4 -ol as the number should always be the smallest possible so count from the left

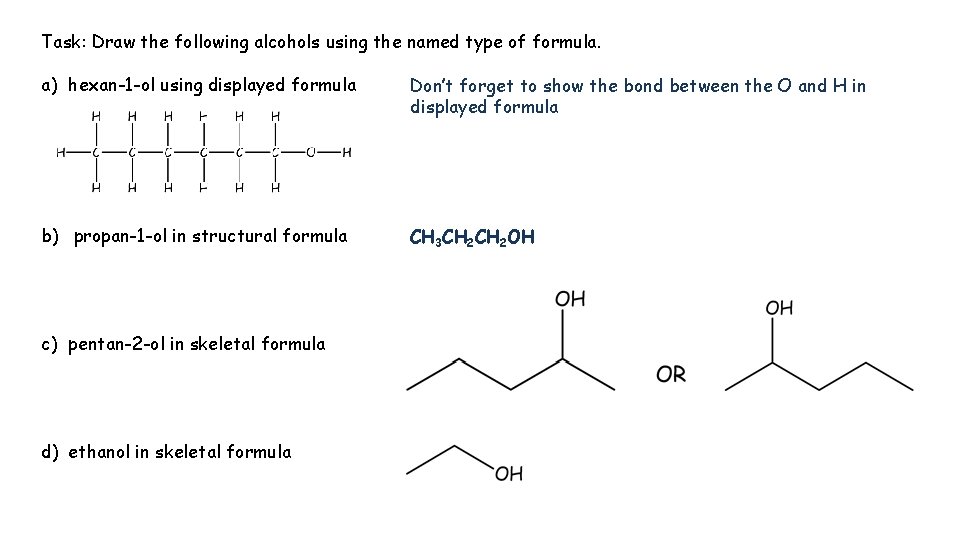
Task: Draw the following alcohols using the named type of formula. a) hexan-1 -ol using displayed formula Don’t forget to show the bond between the O and H in displayed formula b) propan-1 -ol in structural formula CH 3 CH 2 OH c) pentan-2 -ol in skeletal formula d) ethanol in skeletal formula

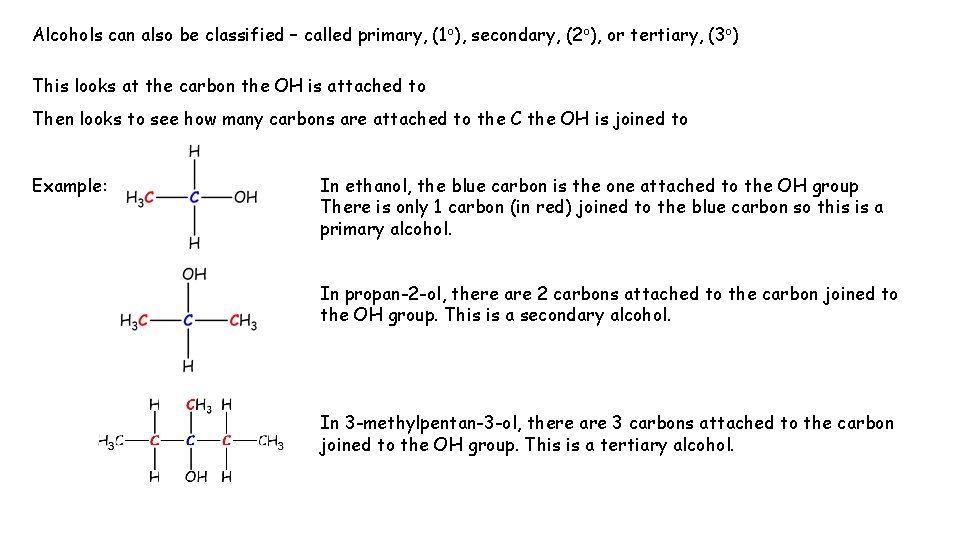
Alcohols can also be classified – called primary, (1 o), secondary, (2 o), or tertiary, (3 o) This looks at the carbon the OH is attached to Then looks to see how many carbons are attached to the C the OH is joined to Example: In ethanol, the blue carbon is the one attached to the OH group There is only 1 carbon (in red) joined to the blue carbon so this is a primary alcohol. In propan-2 -ol, there are 2 carbons attached to the carbon joined to the OH group. This is a secondary alcohol. In 3 -methylpentan-3 -ol, there are 3 carbons attached to the carbon joined to the OH group. This is a tertiary alcohol.

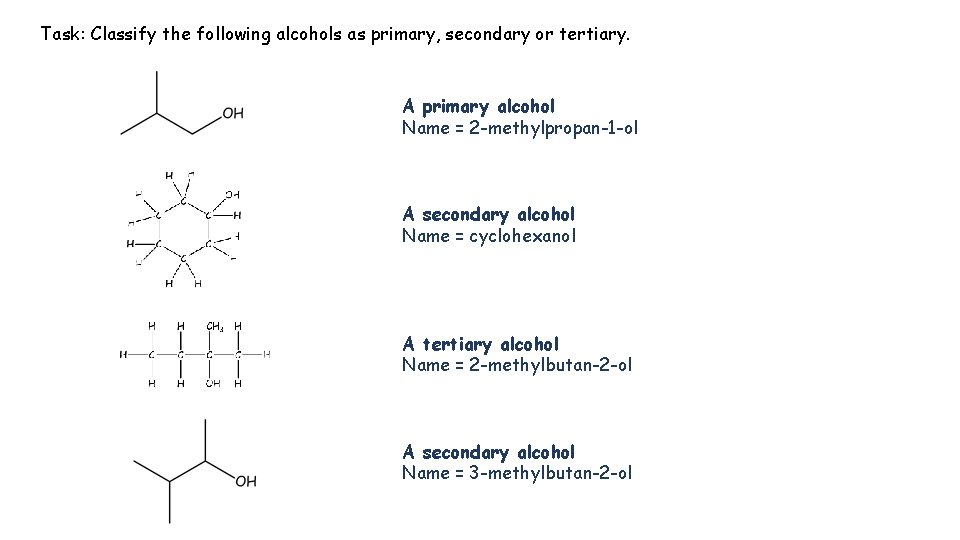
Task: Classify the following alcohols as primary, secondary or tertiary. A primary alcohol Name = 2 -methylpropan-1 -ol A secondary alcohol Name = cyclohexanol A tertiary alcohol Name = 2 -methylbutan-2 -ol A secondary alcohol Name = 3 -methylbutan-2 -ol

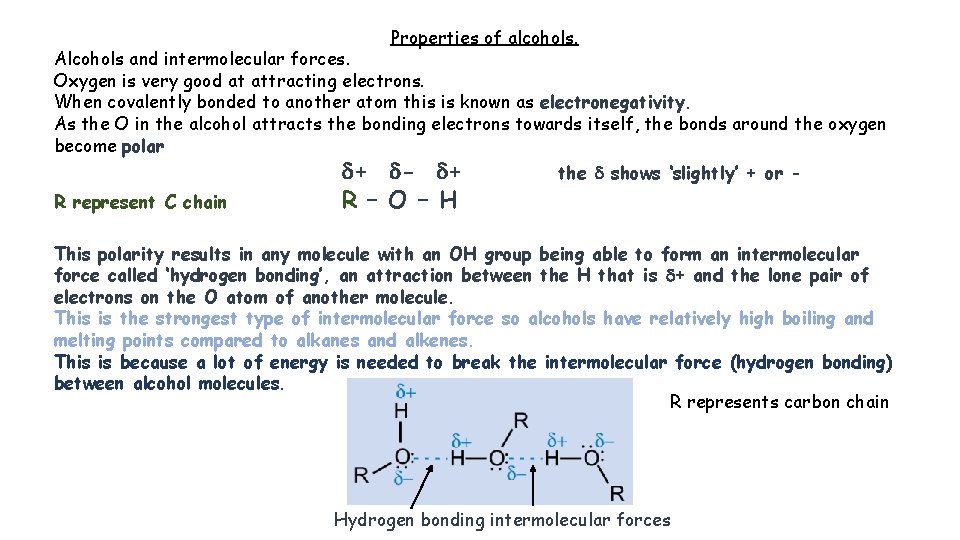
Properties of alcohols. Alcohols and intermolecular forces. Oxygen is very good at attracting electrons. When covalently bonded to another atom this is known as electronegativity. As the O in the alcohol attracts the bonding electrons towards itself, the bonds around the oxygen become polar R represent C chain d+ d- d+ R – O – H the d shows ‘slightly’ + or - This polarity results in any molecule with an OH group being able to form an intermolecular force called ‘hydrogen bonding’, an attraction between the H that is d+ and the lone pair of electrons on the O atom of another molecule. This is the strongest type of intermolecular force so alcohols have relatively high boiling and melting points compared to alkanes and alkenes. This is because a lot of energy is needed to break the intermolecular force (hydrogen bonding) between alcohol molecules. R represents carbon chain Hydrogen bonding intermolecular forces

Task: Put the following molecules in order of boiling point, highest to lowest ethene methane propan-1 -ol Propane Answer: Molecules with hydrogen bonding intermolecular forces have the highest boiling points as hydrogen bonding is the strongest intermolecular force. Alkanes and alkenes boiling points depend on the number of carbons and hydrogens in the molecule, (see power point 1). The bigger the molecule, the more electrons it has and the stronger the intermolecular forces are Propan-1 -ol Propane Ethene Methane highest bpt. lowest bpt.