Adjunctive Devices for Patients With Acute Coronary Syndromes











- Slides: 39

Adjunctive Devices for Patients With Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention Prepared for: Agency for Healthcare Research and Quality (AHRQ) www. ahrq. gov

Outline of This CME Activity The comparative effectiveness review (CER) process Background Questions addressed in the CER for patients with acute coronary syndrome (ACS) who are undergoing percutaneous coronary intervention (PCI) of native vessels: The comparative effects of adjunctive devices on intermediate outcomes (e. g. , ST-segment resolution, myocardial blush grade 3 [MBG-3], thrombolysis in myocardial infarction flow grade 3 [TIMI-3], ejection fraction, distal embolization and no reflow) and terminal outcomes (mortality, myocardial infarction [MI], stroke, target revascularization, major adverse cardiovascular events [MACEs], and health-related quality of life). The rate and type of adverse events (e. g. , coronary dissection, coronary perforation, prolonged procedure time) and how they differ between device types when compared with PCI alone. Results Conclusions

Agency for Healthcare Research and Quality (AHRQ) Comparative Effectiveness Review Development Topics are nominated through a public process, which includes submissions from health care professionals, professional organizations, the private sector, policymakers, the public, and others. A systematic review of all relevant clinical studies is conducted by independent researchers, funded by AHRQ, to synthesize the evidence in a report summarizing what is known and not known about the select clinical issue. The research questions and the results of the report are subject to expert input, peer review, and public comment. The results of these reviews are summarized into Clinician Research Summaries and Consumer Research Summaries for use in decisionmaking and in discussions with patients. The Clinician Research Summary and full report are available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm. No Consumer Research Summary was prepared for the current topic. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

Rating the Strength of Evidence From the CER The strength of evidence was classified into four broad categories: High Further research is very unlikely to change the confidence in the estimate of effect. Moderate Further research may change the confidence in the estimate of effect and may change the estimate. Low Further research is likely to change the confidence in the estimate of effect and is likely to change the estimate. Insufficient Evidence either is unavailable or does not permit estimation of an effect. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

Background: Coronary Heart Disease Coronary heart disease (CHD) is a leading cause of morbidity and mortality in the United States. More than 650, 000 deaths were attributed to CHD in 2003. Treatment costs for CHD represent the largest health care expenditure for a single disease in the United States. Acute coronary syndromes (ACSs) account for more than 1. 5 million hospital admissions annually in the United States alone. ACSs include the clinical entities of: Unstable angina (UA) Non–ST-segment elevation MI (NSTEMI) ST-segment elevation MI (STEMI) Approximately 1 million of hospital admissions are classified as UA/NSTEMI, and approximately 500, 000 are STEMI. Smith SC Jr, Feldman TE, Hirshfeld JW, et al. J Am Coll Cardiol 2006; 47: 1 -121. PMID 16386656. Silber S, Albertsson P, Aviles FF, et al. Eur Heart J 2005; 26: 804 -47. PMID: 15769784.

Background: Percutaneous Coronary Intervention Percutaneous coronary intervention (PCI) has revolutionized the management of angina and MI. Often, coronary bypass surgery is not needed as a result. PCI permits a more rapid return to normal activities. In the United States, 664, 000 procedures were performed in 652, 000 patients in 2003. Coronary stents and adjunctive pharmacologic agents have improved the effect of PCI. Silber S, Albertsson P, Aviles FF, et al. Eur Heart J 2005; 26: 804 -47. PMID: 15769784.

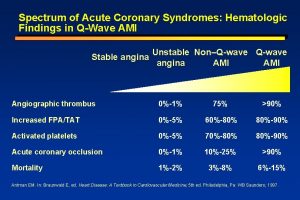
Background: The “No-Reflow Phenomenon” After PCI Dislodgement of atherothrombotic material from coronary lesions during PCI can result in distal embolization that leads to what is commonly referred to as the “no-reflow phenomenon. ” Characterized by inadequate flow at the cardiac tissue level despite patent coronary vessels is often defined as: A thrombolysis in MI (TIMI) flow grade ≤ 2 despite vessel patency and the absence of dissection, spasm, or distal macroembolus An MBG of 0 or 1 Or a contrast perfusion defect observed upon myocardial contrast echocardiography Patients with no-reflow experience a higher rate of adverse outcomes including: Larger infarcts More significant left ventricular systolic dysfunction Increased risk of MACEs Death Mehta RH, Harhai KJ, Cox D, et al. J Am Coll Cardiol 2003; 42: 1739. PMID: 14642681; Silber S, Albertsson P, Aviles FF, et al. Eur Heart J 2005; 26: 804 -47. PMID: 15769784; Smith SC Jr, Feldman TE, Hirshfeld JW, et al. J Am Coll Cardiol 2006; 47: e 1 -121. PMID: 16386656.

Thrombolysis in Myocardial Infarction Flow Grading System Defined Thrombolysis in Myocardial Infarction Flow Grading System Grade 0 Complete occlusion of the infarct-related artery Grade 1 Some penetration of contrast material beyond the point of obstruction but without perfusion of the distal coronary bed Grade 2 Perfusion of the entire infarct vessel into the distal bed but with delayed flow when compared with a normal artery Grade 3 Full perfusion of the infarct vessel with normal flow Chesebro JH, Knatterud G, Roberts R, et al. Circulation 1987; 76: 142 -54. PMID: 3109764.

Myocardial Blush Grades Defined Myocardial Blush Grades Grade 0 (MBG-0) Failure of dye to enter the microvasculature. Either minimal or no ground glass appearance (“blush”) or opacification of the myocardium in the distribution of the culprit artery indicating lack of tissue-level perfusion. Grade 1 (MBG-1) Dye slowly enters but fails to exit the microvasculature. There is the ground glass appearance (“blush”) or opacification of the myocardium in the distribution of the culprit lesion that fails to clear from the microvasculature, and dye staining is present on the next injection (approximately 30 seconds between injections). Grade 2 (MBG-2) Delayed entry and exit of dye from the microvasculature. There is the ground glass appearance (“blush”) or opacification of the myocardium in the distribution of the culprit lesion that is strongly persistent at the end of the washout phase (i. e. , dye is strongly persistent after three cardiac cycles of the washout phase and either does not or only minimally diminishes in intensity during washout). Grade 3 (MBG-3) Normal entry and exit of dye from the microvasculature. There is the ground glass appearance (“blush”) or opacification of the myocardium in the distribution of the culprit lesion that clears normally and is either gone or only mildly/moderately persistent at the end of the washout phase (i. e. , dye is gone or is mildly/moderately persistent after three cardiac cycles of the washout phase and noticeably diminishes in intensity during the washout phase), similar to that in an uninvolved artery. Blush that is of only mild intensity throughout the washout phase but fades minimally is also classified as grade 3. van 't Hof AW, Liem A, Suryapranata H, et al. Circulation 1998; 97: 2302 -6. PMID: 9639373.

Adjunctive Devices That Remove Thrombi and Protect Against Distal Embolization During PCI Adjunctive devices have been developed in an attempt to improve clinical outcomes by removing thrombi and to protect against distal embolization during PCI. Classes of devices include: Catheter aspiration thrombectomy devices Mechanical thrombectomy devices Embolic protection devices Chevalier B, et al. Euro. Intervention 2008; 4: 222 -8. PMID: 19110787; Ciszewski M, et al. Circulation 2008; 118: S 764. PMID: 21234920; Dudek D, De Feyter PJ. Polish-Italian-Hungarian Randomized Thrombectomy Trial (PHIRATE trial). Available at www. escardio. org/CONGRESSES/ESC 2008/CONGRESS-REPORTS/Pages/779 -980 -dudek-defeyter. aspx; Haeck JD, et al. JACC Cardiovasc Interv 2009; 2: 934 -43. PMID: 19850252; Ikari Y, et al. JACC Cardiovasc Interv 2008; 1: 424 -31. PMID: 19463340; Liistro F, et al. 2009; 2: 376 -83. PMID: 20031746; Lipiecki J, et al. Am Heart J 2009; 157: 583. e 1 -7. PMID: 19249433; Mamas MA, et al. Euro. Intervention 2008; 4: 115 -23. PMID: 19112733; Migliorini A, et al. J Am Coll Cardiol 2010; 56: 1298 -306. PMID: 20691553; Sardella G, et al. J Am Coll Cardiol 2009; 53: 309 -15. PMID: 19161878; Silber S, et al. Eur Heart J 2005; 26: 804 -47. PMID: 15769784; Smith SC Jr, et al. J Am Coll Cardiol 2006; 47: e 1 -121. PMID: 16386656; Tahk SJ, et al. Int J Cardiol 2008; 123: 162 -8. PMID: 17490759; U. S. Food and Drug Administration. Medical Devices. Available at www. fda. gov/Medical. Devices/default. htm.

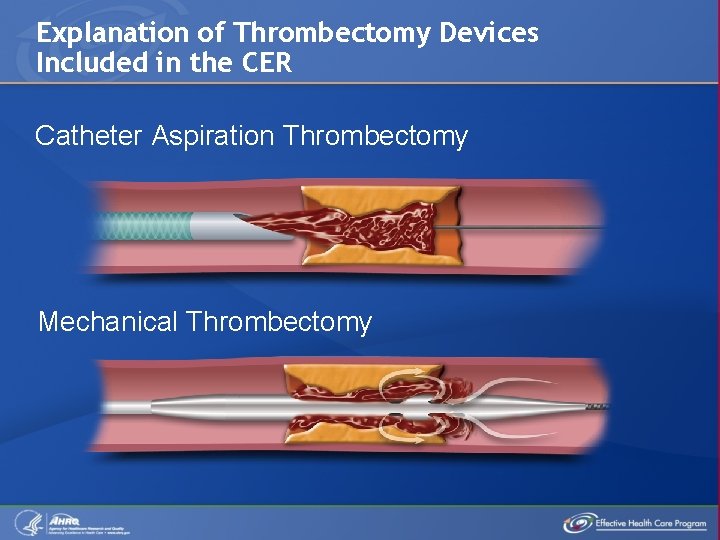
Explanation of Thrombectomy Devices Included in the CER Catheter Aspiration Thrombectomy Mechanical Thrombectomy

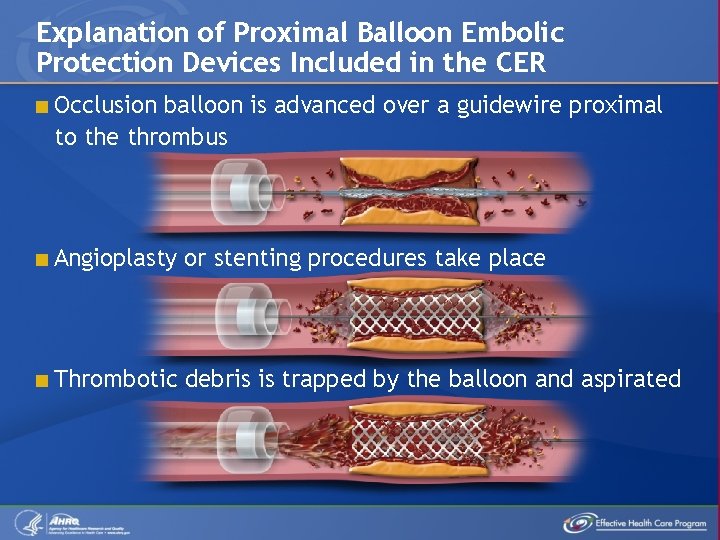
Explanation of Proximal Balloon Embolic Protection Devices Included in the CER < Occlusion balloon is advanced over a guidewire proximal to the thrombus < Angioplasty or stenting procedures take place < Thrombotic debris is trapped by the balloon and aspirated

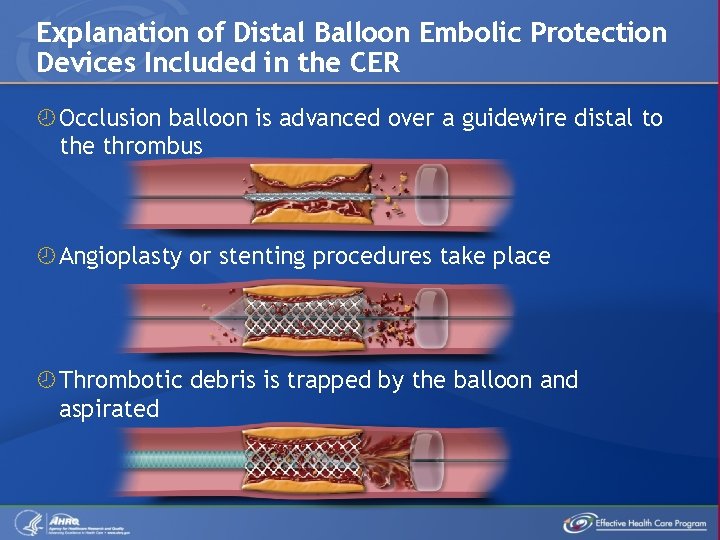
Explanation of Distal Balloon Embolic Protection Devices Included in the CER Occlusion balloon is advanced over a guidewire distal to the thrombus Angioplasty or stenting procedures take place Thrombotic debris is trapped by the balloon and aspirated

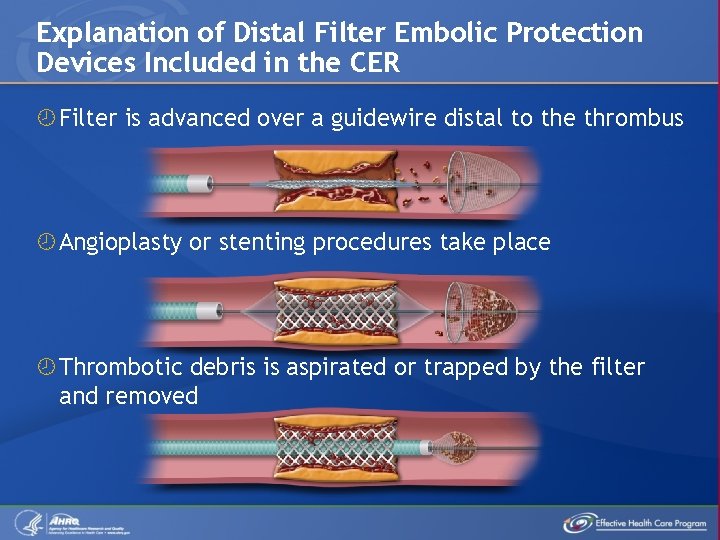
Explanation of Distal Filter Embolic Protection Devices Included in the CER Filter is advanced over a guidewire distal to the thrombus Angioplasty or stenting procedures take place Thrombotic debris is aspirated or trapped by the filter and removed

Current Practice for Patients Undergoing PCI Distal embolic protection devices are recommended for use in patients undergoing PCI of saphenous vein grafts due to previously demonstrated ability to reduce MACE. However, use of embolic protection devices in STEMI has been less well supported, mainly because of underpowered clinical trials that evaluated intermediate markers. Larger randomized controlled trials (RCTs) of patients with STEMI have evaluated MACE as an end point and followed patients beyond hospital discharge (typically 3 to 12 months) but have given conflicting results. The comparative effectiveness and the risk differences between these devices in patients with ACS undergoing PCI for native coronary arteries are not well established and need to be systematically evaluated. Antoniucci D, et al. Am J Cardiol 2004; 93: 1033 -5. PMID: 15081450; Chevalier B, et al. Euro. Intervention 2008; 4: 222 -8. PMID: 19110787; Ciszewski M, et al. Circulation 2008; 4: 222 -8. PMID: 21234920; Gartlehner G, et al. J Clin Epidemiol 2006; 59: 1040 -8. PMID: 16980143; Lefevre T, et al. J Am Coll Cardiol 2005; 46: 246 -52. PMID: 16022950; Liistro F, et al. 2009; 2: 376 -83. PMID: 20031746; Lipiecki J, et al. Am Heart J 2009; 157: 583. e 1 -7. PMID: 19249433; Mamas MA, et al. Euro. Intervention 2008; 4: 115 -23. PMID: 19112733; Migliorini A, et al. J Am Coll Cardiol 2010; 56: 1298 -306. PMID: 20691553; Napodano M, et al. J Am Coll Cardiol 2003; 42: 1395 -402. PMID: 14563581; Sardella G, et al. J Am Coll Cardiol 2009; 53: 309 -15. PMID: 19161878; Smith SC Jr, et al. J Am Coll Cardiol 2006; 47: e 1 -121. PMID: 16386656.

CER on Adjunctive Devices for Patients With ACS Undergoing PCI of Native Vessels A systematic review of 175 clinical studies published between January 1996 and March 2011 sought to determine the effectiveness, benefits, and adverse effects of adjunctive devices to remove thrombi or protect against embolization in patients with ACSs undergoing PCI of native vessels when compared with PCI alone. This CME activity is provided to assist in decisionmaking and should not be construed to represent clinical recommendations or guidelines. The full report is available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

CER Outcomes of Interest for Studies on Adjunctive Devices for Patients With ACS Undergoing PCI on Native Vessels Final health outcomes Mortality MI Stroke Target revascularization MACE (including reinfarction, target revascularization, and stroke) Health-related quality of life Intermediate health outcomes ST-segment resolution MBG-3 Thrombolysis in myocardial infarction 3 flow (TIMI-3) Ejection fraction Distal embolization Safety Coronary dissection Coronary perforation Prolonged procedure time Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

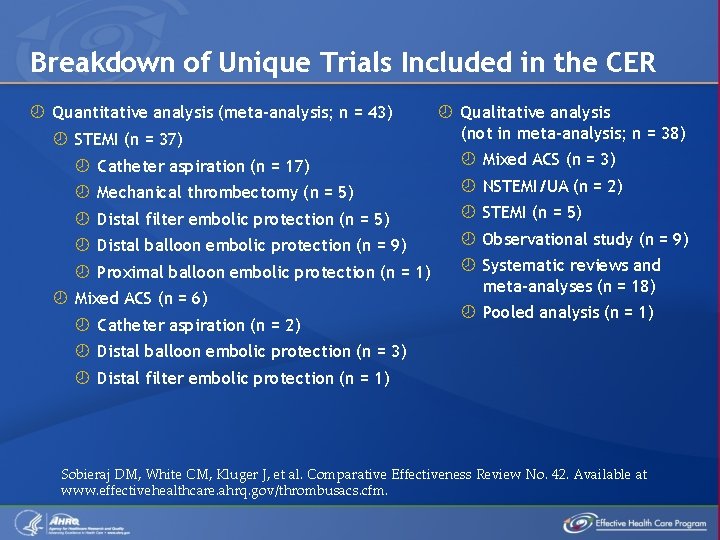
Breakdown of Unique Trials Included in the CER Quantitative analysis (meta-analysis; n = 43) STEMI (n = 37) Qualitative analysis (not in meta-analysis; n = 38) Catheter aspiration (n = 17) Mixed ACS (n = 3) Mechanical thrombectomy (n = 5) NSTEMI/UA (n = 2) Distal filter embolic protection (n = 5) STEMI (n = 5) Distal balloon embolic protection (n = 9) Observational study (n = 9) Proximal balloon embolic protection (n = 1) Systematic reviews and meta-analyses (n = 18) Mixed ACS (n = 6) Catheter aspiration (n = 2) Pooled analysis (n = 1) Distal balloon embolic protection (n = 3) Distal filter embolic protection (n = 1) Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

Comparative Effectiveness of Adjunctive Catheter Aspiration Thrombectomy Devices Versus PCI Alone in Patients With ACS Catheter Aspiration Devices in Patients With STEMI and Catheter Aspiration Devices in Other ACS Populations Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

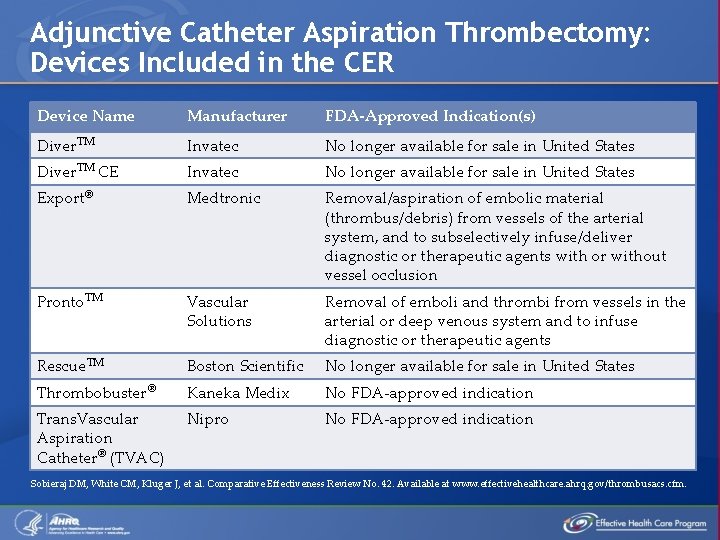
Adjunctive Catheter Aspiration Thrombectomy: Devices Included in the CER Device Name Manufacturer FDA-Approved Indication(s) Diver. TM Invatec No longer available for sale in United States Diver. TM CE Invatec No longer available for sale in United States Export® Medtronic Removal/aspiration of embolic material (thrombus/debris) from vessels of the arterial system, and to subselectively infuse/deliver diagnostic or therapeutic agents with or without vessel occlusion Pronto. TM Vascular Solutions Removal of emboli and thrombi from vessels in the arterial or deep venous system and to infuse diagnostic or therapeutic agents Rescue. TM Boston Scientific No longer available for sale in United States Thrombobuster® Kaneka Medix No FDA-approved indication Trans. Vascular Aspiration Catheter® (TVAC) Nipro No FDA-approved indication Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

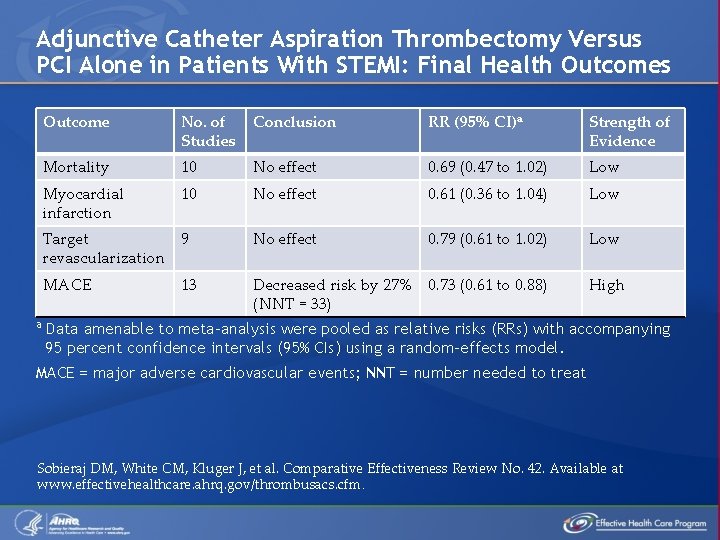
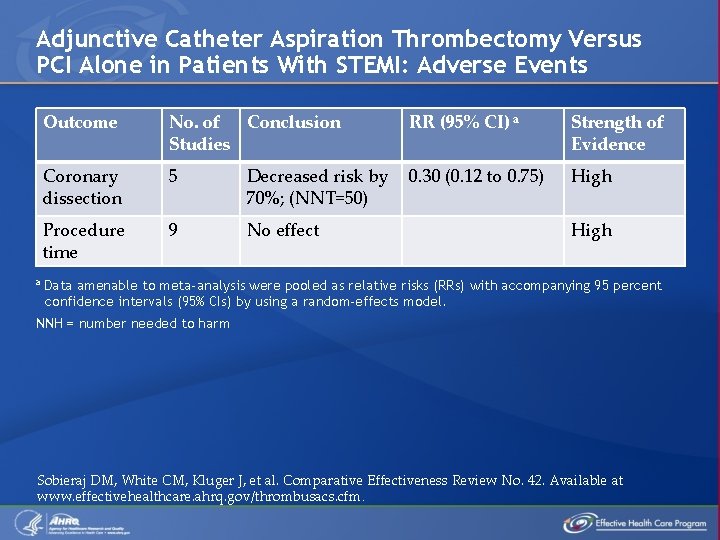
Adjunctive Catheter Aspiration Thrombectomy Versus PCI Alone in Patients With STEMI: Final Health Outcomes a Outcome No. of Studies Conclusion RR (95% CI)a Strength of Evidence Mortality 10 No effect 0. 69 (0. 47 to 1. 02) Low Myocardial infarction 10 No effect 0. 61 (0. 36 to 1. 04) Low Target 9 revascularization No effect 0. 79 (0. 61 to 1. 02) Low MACE Decreased risk by 27% 0. 73 (0. 61 to 0. 88) (NNT = 33) High 13 Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) using a random-effects model. MACE = major adverse cardiovascular events; NNT = number needed to treat Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

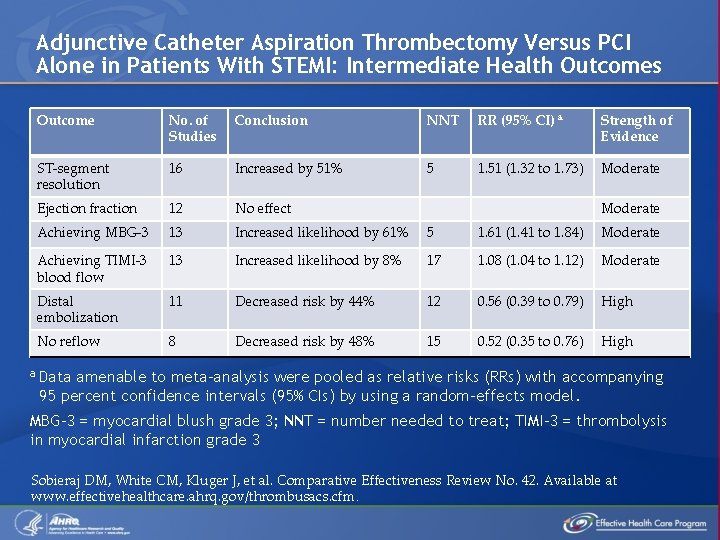
Adjunctive Catheter Aspiration Thrombectomy Versus PCI Alone in Patients With STEMI: Intermediate Health Outcomes Outcome No. of Studies Conclusion NNT RR (95% CI) a Strength of Evidence ST-segment resolution 16 Increased by 51% 5 1. 51 (1. 32 to 1. 73) Moderate Ejection fraction 12 No effect Achieving MBG-3 13 Increased likelihood by 61% 5 1. 61 (1. 41 to 1. 84) Moderate Achieving TIMI-3 blood flow 13 Increased likelihood by 8% 17 1. 08 (1. 04 to 1. 12) Moderate Distal embolization 11 Decreased risk by 44% 12 0. 56 (0. 39 to 0. 79) High No reflow 8 Decreased risk by 48% 15 0. 52 (0. 35 to 0. 76) High Moderate a Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) by using a random-effects model. MBG-3 = myocardial blush grade 3; NNT = number needed to treat; TIMI-3 = thrombolysis in myocardial infarction grade 3 Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

Adjunctive Catheter Aspiration Thrombectomy Versus PCI Alone in Patients With STEMI: Adverse Events a Outcome No. of Conclusion Studies RR (95% CI) a Strength of Evidence Coronary dissection 5 Decreased risk by 70%; (NNT=50) 0. 30 (0. 12 to 0. 75) High Procedure time 9 No effect High Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) by using a random-effects model. NNH = number needed to harm Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

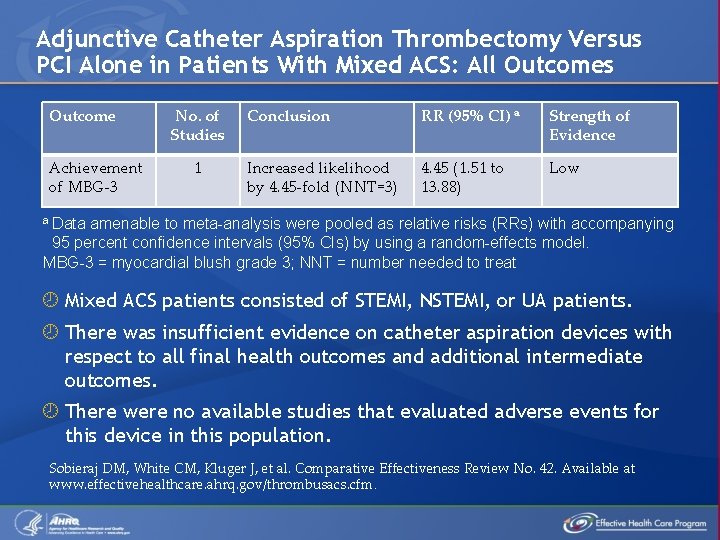
Adjunctive Catheter Aspiration Thrombectomy Versus PCI Alone in Patients With Mixed ACS: All Outcomes Outcome Achievement of MBG-3 No. of Studies 1 Conclusion RR (95% CI) a Strength of Evidence Increased likelihood by 4. 45 -fold (NNT=3) 4. 45 (1. 51 to 13. 88) Low a Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) by using a random-effects model. MBG-3 = myocardial blush grade 3; NNT = number needed to treat Mixed ACS patients consisted of STEMI, NSTEMI, or UA patients. There was insufficient evidence on catheter aspiration devices with respect to all final health outcomes and additional intermediate outcomes. There were no available studies that evaluated adverse events for this device in this population. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

Comparative Effectiveness of Adjunctive Mechanical Thrombectomy Devices Versus PCI Alone in Patients With ACS Mechanical Thrombectomy Devices in Patients With STEMI and Mechanical Thrombectomy Devices in Other ACS Populations Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

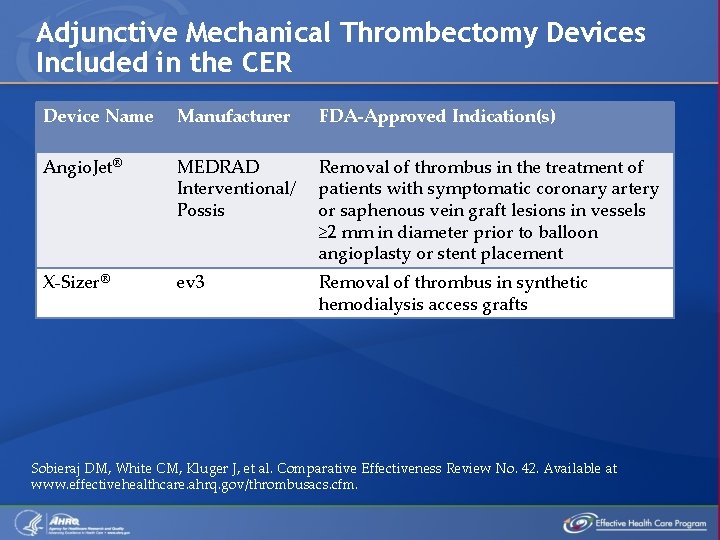
Adjunctive Mechanical Thrombectomy Devices Included in the CER Device Name Manufacturer FDA-Approved Indication(s) Angio. Jet® MEDRAD Interventional/ Possis Removal of thrombus in the treatment of patients with symptomatic coronary artery or saphenous vein graft lesions in vessels ≥ 2 mm in diameter prior to balloon angioplasty or stent placement X-Sizer® ev 3 Removal of thrombus in synthetic hemodialysis access grafts Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

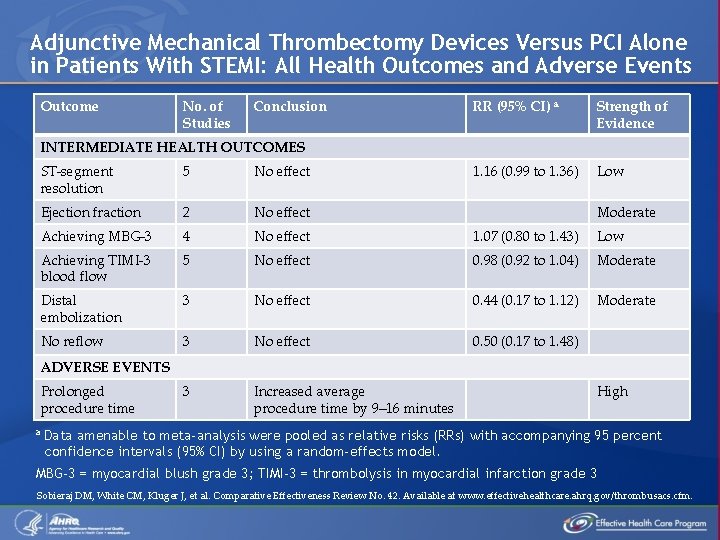
Adjunctive Mechanical Thrombectomy Devices Versus PCI Alone in Patients With STEMI: All Health Outcomes and Adverse Events Outcome No. of Studies Conclusion RR (95% CI) a Strength of Evidence 1. 16 (0. 99 to 1. 36) Low INTERMEDIATE HEALTH OUTCOMES ST-segment resolution 5 No effect Ejection fraction 2 No effect Achieving MBG-3 4 No effect 1. 07 (0. 80 to 1. 43) Low Achieving TIMI-3 blood flow 5 No effect 0. 98 (0. 92 to 1. 04) Moderate Distal embolization 3 No effect 0. 44 (0. 17 to 1. 12) Moderate No reflow 3 No effect 0. 50 (0. 17 to 1. 48) 3 Increased average procedure time by 9– 16 minutes Moderate ADVERSE EVENTS Prolonged procedure time a High Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CI) by using a random-effects model. MBG-3 = myocardial blush grade 3; TIMI-3 = thrombolysis in myocardial infarction grade 3 Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

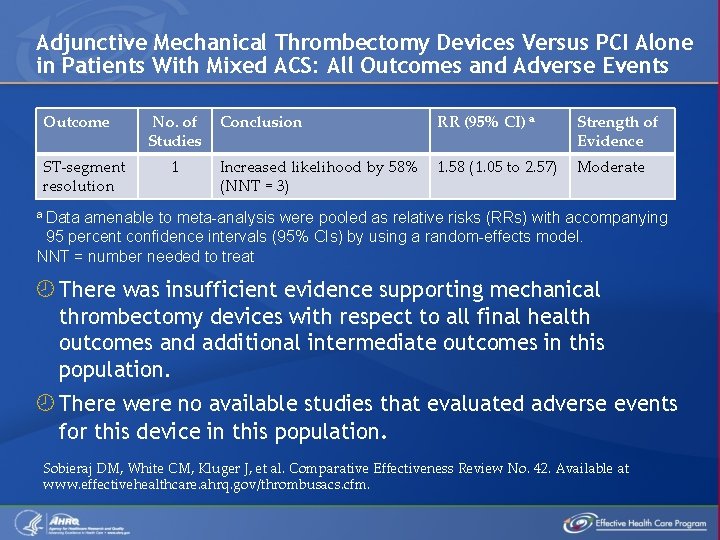
Adjunctive Mechanical Thrombectomy Devices Versus PCI Alone in Patients With Mixed ACS: All Outcomes and Adverse Events Outcome ST-segment resolution No. of Studies 1 Conclusion RR (95% CI) a Strength of Evidence Increased likelihood by 58% (NNT = 3) 1. 58 (1. 05 to 2. 57) Moderate a Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) by using a random-effects model. NNT = number needed to treat There was insufficient evidence supporting mechanical thrombectomy devices with respect to all final health outcomes and additional intermediate outcomes in this population. There were no available studies that evaluated adverse events for this device in this population. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

Comparative Effectiveness of Adjunctive Embolic Protection Devices Versus PCI Alone in Patients With ACS Distal Filter Embolic Protection Devices, Distal Balloon Embolic Protection Devices, and Proximal Balloon Embolic Protection Devices in Patients With ACS Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

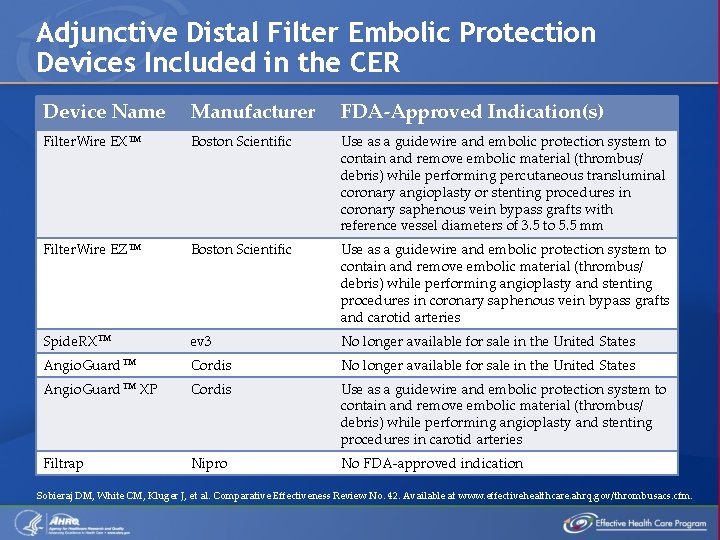
Adjunctive Distal Filter Embolic Protection Devices Included in the CER Device Name Manufacturer FDA-Approved Indication(s) Filter. Wire EXTM Boston Scientific Use as a guidewire and embolic protection system to contain and remove embolic material (thrombus/ debris) while performing percutaneous transluminal coronary angioplasty or stenting procedures in coronary saphenous vein bypass grafts with reference vessel diameters of 3. 5 to 5. 5 mm Filter. Wire EZTM Boston Scientific Use as a guidewire and embolic protection system to contain and remove embolic material (thrombus/ debris) while performing angioplasty and stenting procedures in coronary saphenous vein bypass grafts and carotid arteries Spide. RXTM ev 3 No longer available for sale in the United States Angio. Guard. TM Cordis No longer available for sale in the United States Angio. Guard. TM XP Cordis Use as a guidewire and embolic protection system to contain and remove embolic material (thrombus/ debris) while performing angioplasty and stenting procedures in carotid arteries Filtrap Nipro No FDA-approved indication Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

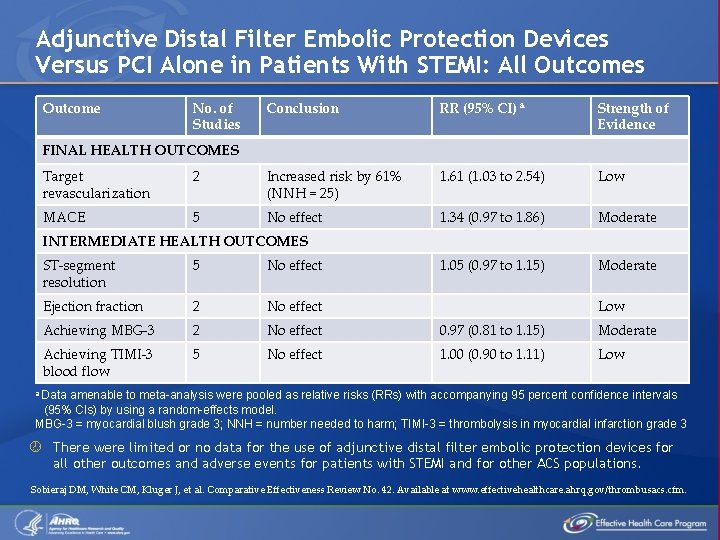
Adjunctive Distal Filter Embolic Protection Devices Versus PCI Alone in Patients With STEMI: All Outcomes Outcome No. of Studies Conclusion RR (95% CI) a Strength of Evidence FINAL HEALTH OUTCOMES Target revascularization 2 Increased risk by 61% (NNH = 25) 1. 61 (1. 03 to 2. 54) Low MACE 5 No effect 1. 34 (0. 97 to 1. 86) Moderate 1. 05 (0. 97 to 1. 15) Moderate INTERMEDIATE HEALTH OUTCOMES ST-segment resolution 5 No effect Ejection fraction 2 No effect Achieving MBG-3 2 No effect 0. 97 (0. 81 to 1. 15) Moderate Achieving TIMI-3 blood flow 5 No effect 1. 00 (0. 90 to 1. 11) Low a Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) by using a random-effects model. MBG-3 = myocardial blush grade 3; NNH = number needed to harm; TIMI-3 = thrombolysis in myocardial infarction grade 3 There were limited or no data for the use of adjunctive distal filter embolic protection devices for all other outcomes and adverse events for patients with STEMI and for other ACS populations. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

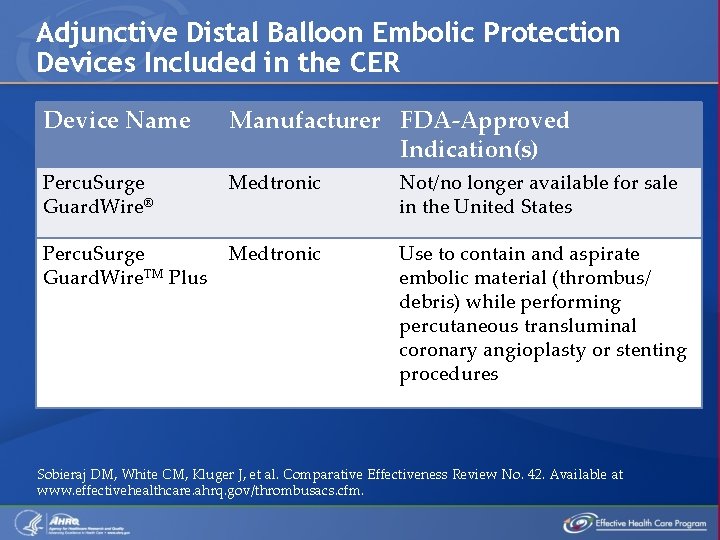
Adjunctive Distal Balloon Embolic Protection Devices Included in the CER Device Name Manufacturer FDA-Approved Indication(s) Percu. Surge Guard. Wire® Medtronic Not/no longer available for sale in the United States Percu. Surge Guard. Wire. TM Plus Medtronic Use to contain and aspirate embolic material (thrombus/ debris) while performing percutaneous transluminal coronary angioplasty or stenting procedures Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

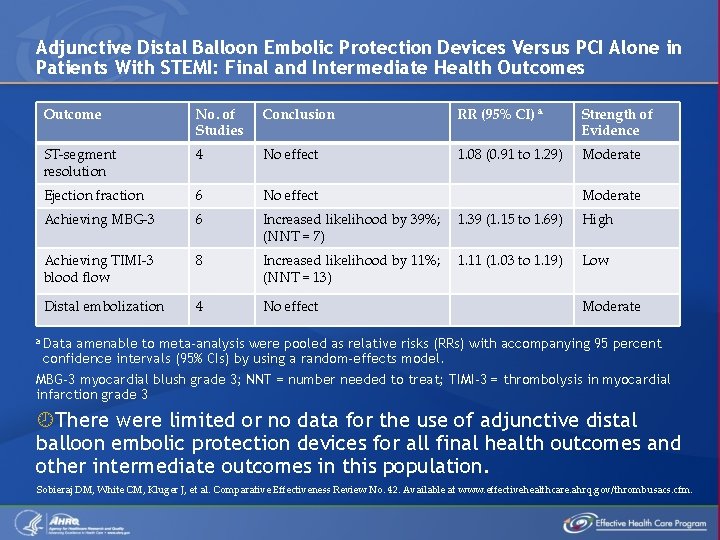
Adjunctive Distal Balloon Embolic Protection Devices Versus PCI Alone in Patients With STEMI: Final and Intermediate Health Outcomes Outcome No. of Studies Conclusion RR (95% CI) a Strength of Evidence ST-segment resolution 4 No effect 1. 08 (0. 91 to 1. 29) Moderate Ejection fraction 6 No effect Achieving MBG-3 6 Increased likelihood by 39%; (NNT = 7) 1. 39 (1. 15 to 1. 69) High Achieving TIMI-3 blood flow 8 Increased likelihood by 11%; (NNT = 13) 1. 11 (1. 03 to 1. 19) Low Distal embolization 4 No effect Moderate a Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) by using a random-effects model. MBG-3 myocardial blush grade 3; NNT = number needed to treat; TIMI-3 = thrombolysis in myocardial infarction grade 3 There were limited or no data for the use of adjunctive distal balloon embolic protection devices for all final health outcomes and other intermediate outcomes in this population. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

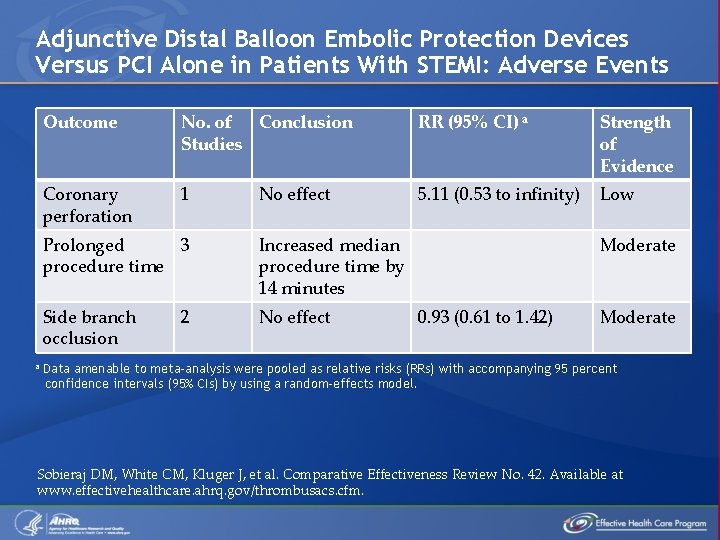
Adjunctive Distal Balloon Embolic Protection Devices Versus PCI Alone in Patients With STEMI: Adverse Events a Outcome No. of Conclusion Studies RR (95% CI) a Strength of Evidence Coronary perforation 1 5. 11 (0. 53 to infinity) Low No effect Prolonged 3 procedure time Increased median procedure time by 14 minutes Side branch occlusion No effect 2 Moderate 0. 93 (0. 61 to 1. 42) Moderate Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) by using a random-effects model. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

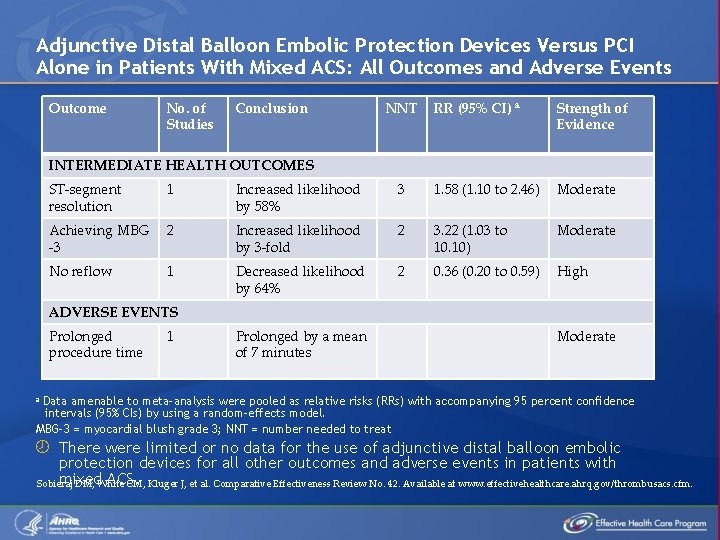
Adjunctive Distal Balloon Embolic Protection Devices Versus PCI Alone in Patients With Mixed ACS: All Outcomes and Adverse Events Outcome No. of Studies Conclusion NNT RR (95% CI) a Strength of Evidence INTERMEDIATE HEALTH OUTCOMES ST-segment resolution 1 Increased likelihood by 58% 3 1. 58 (1. 10 to 2. 46) Moderate Achieving MBG -3 2 Increased likelihood by 3 -fold 2 3. 22 (1. 03 to 10. 10) Moderate No reflow 1 Decreased likelihood by 64% 2 0. 36 (0. 20 to 0. 59) High ADVERSE EVENTS Prolonged procedure time 1 Prolonged by a mean of 7 minutes Moderate a Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) by using a random-effects model. MBG-3 = myocardial blush grade 3; NNT = number needed to treat There were limited or no data for the use of adjunctive distal balloon embolic protection devices for all other outcomes and adverse events in patients with mixed ACS. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

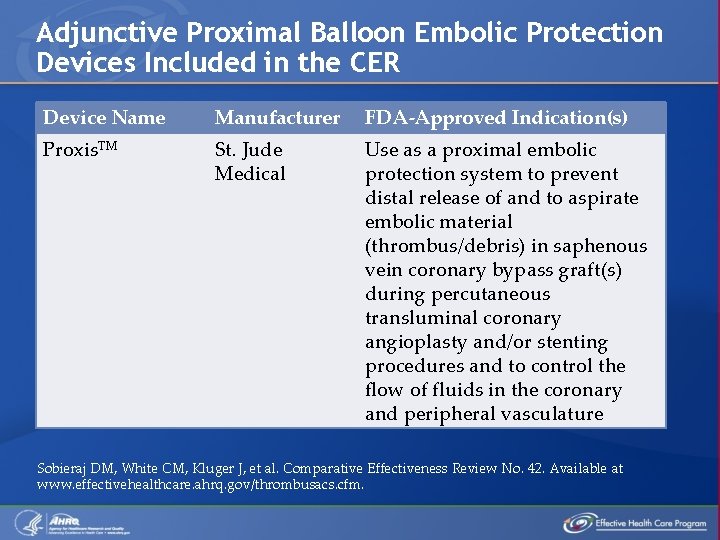
Adjunctive Proximal Balloon Embolic Protection Devices Included in the CER Device Name Manufacturer FDA-Approved Indication(s) Proxis. TM St. Jude Medical Use as a proximal embolic protection system to prevent distal release of and to aspirate embolic material (thrombus/debris) in saphenous vein coronary bypass graft(s) during percutaneous transluminal coronary angioplasty and/or stenting procedures and to control the flow of fluids in the coronary and peripheral vasculature Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

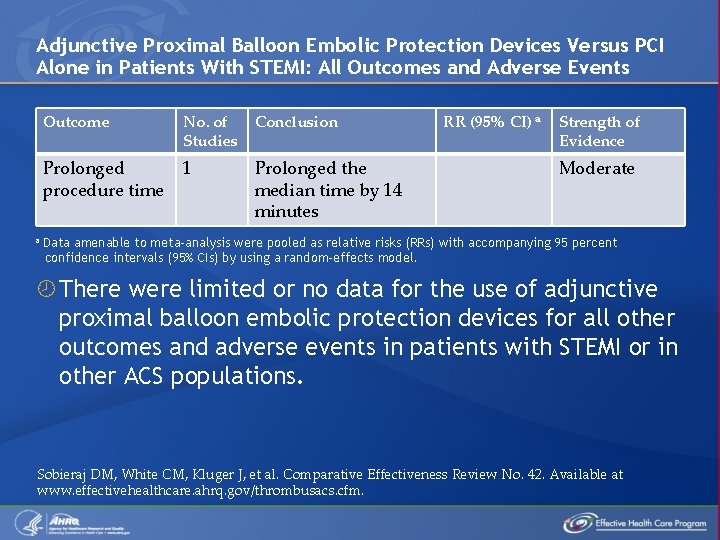
Adjunctive Proximal Balloon Embolic Protection Devices Versus PCI Alone in Patients With STEMI: All Outcomes and Adverse Events a Outcome No. of Studies Conclusion Prolonged procedure time 1 Prolonged the median time by 14 minutes RR (95% CI) a Strength of Evidence Moderate Data amenable to meta-analysis were pooled as relative risks (RRs) with accompanying 95 percent confidence intervals (95% CIs) by using a random-effects model. There were limited or no data for the use of adjunctive proximal balloon embolic protection devices for all other outcomes and adverse events in patients with STEMI or in other ACS populations. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

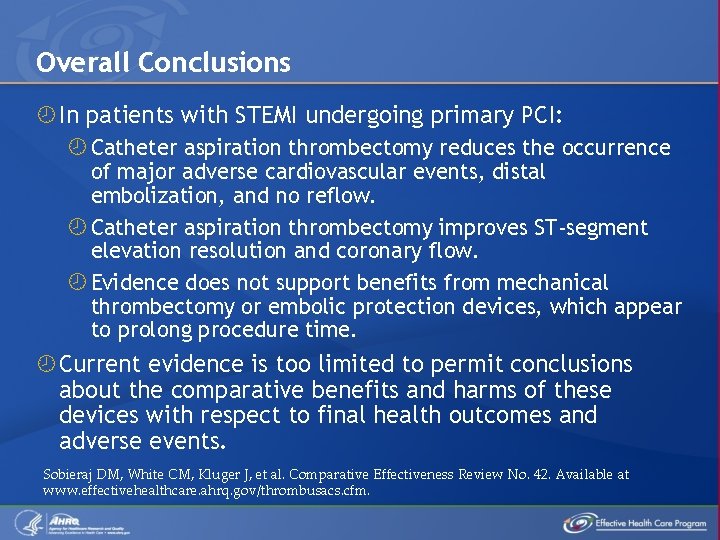
Overall Conclusions In patients with STEMI undergoing primary PCI: Catheter aspiration thrombectomy reduces the occurrence of major adverse cardiovascular events, distal embolization, and no reflow. Catheter aspiration thrombectomy improves ST-segment elevation resolution and coronary flow. Evidence does not support benefits from mechanical thrombectomy or embolic protection devices, which appear to prolong procedure time. Current evidence is too limited to permit conclusions about the comparative benefits and harms of these devices with respect to final health outcomes and adverse events. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.

Gaps in Knowledge Direct comparative RCTs are needed that compare one thrombectomy or embolic protection device with another and evaluate final health outcomes. Studies examining final health outcomes and using longer followup data are needed to fully determine the impact of adjunctive thrombectomy and embolic protection devices in patients with ACS who are undergoing PCI. Sobieraj DM, White CM, Kluger J, et al. Comparative Effectiveness Review No. 42. Available at www. effectivehealthcare. ahrq. gov/thrombusacs. cfm.
 Global registry of acute coronary events
Global registry of acute coronary events Acute coronary syndrome
Acute coronary syndrome Adjunctive adverbs
Adjunctive adverbs Adverbial vs adverb
Adverbial vs adverb Differential diagnosis of stroke
Differential diagnosis of stroke N
N Cerebellaris
Cerebellaris What is geriatric syndromes
What is geriatric syndromes Cerebellar syndromes
Cerebellar syndromes Neuroendocrine syndrome in gynecology
Neuroendocrine syndrome in gynecology Av
Av Dr eter
Dr eter Ali sepahdari
Ali sepahdari Coronary heart disease
Coronary heart disease Coronary blood flow
Coronary blood flow Dipyrimadole
Dipyrimadole Ligamentum arteriosum
Ligamentum arteriosum Trabeculae carinae
Trabeculae carinae Coronary circulation of heart
Coronary circulation of heart Pk papyrus covered coronary stent system
Pk papyrus covered coronary stent system Coronary artery disease
Coronary artery disease Shepherd crook rca
Shepherd crook rca Ischemic heart disease
Ischemic heart disease Anemia or hypoproteinemia will ______ blood viscosity.
Anemia or hypoproteinemia will ______ blood viscosity. Sircumflex
Sircumflex Pk papyrus stent
Pk papyrus stent Coronary personality
Coronary personality Where is the pulmonary semilunar valve located
Where is the pulmonary semilunar valve located Frog heart
Frog heart Coronary perfusion pressure
Coronary perfusion pressure Coronary calcium score guidelines
Coronary calcium score guidelines Structure of blood vessels
Structure of blood vessels Coronary artery disease pathophysiology
Coronary artery disease pathophysiology Anterior cardiac veins of the right ventricle
Anterior cardiac veins of the right ventricle Space of disse liver
Space of disse liver Left lung
Left lung Mesa coronary calcium score
Mesa coronary calcium score Intracapsular but extrasynovial
Intracapsular but extrasynovial Venae cordis minimi
Venae cordis minimi Qfr coronary
Qfr coronary