PK Papyrus Covered Coronary Stent David E Kandzari

![PK Papyrus Covered Coronary Stent System PK Papyrus Graftmaster RX Stent Diameter [mm] 2. PK Papyrus Covered Coronary Stent System PK Papyrus Graftmaster RX Stent Diameter [mm] 2.](https://slidetodoc.com/presentation_image/4d1df1ab97c0df6155237e1a6411d79d/image-5.jpg)
- Slides: 11

PK Papyrus Covered Coronary Stent David E. Kandzari, MD, FACC, FSCAI Chief Scientific Officer Director, Interventional Cardiology Piedmont Heart Institute Atlanta, Georgia david. kandzari@piedmont. org

Disclosure Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below Affiliation/Financial Relationship Company Institutional Grant/Research Support Biotronik, Boston Scientific, Medtronic Cardio. Vascular, Medinol, Orbus Neich Consulting Fees/Honoraria Boston Scientific Corporation, Medtronic Cardio. Vascular, Biotronik, Cardinal Health Major Stock Shareholder/Equity None Royalty Income None Ownership/Founder None Intellectual Property Rights None Other Financial Benefit None

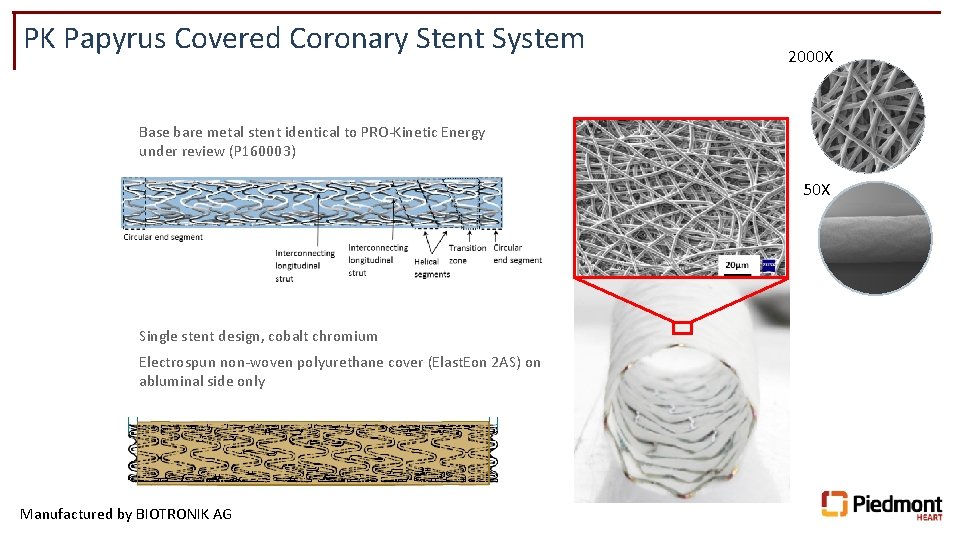
PK Papyrus Covered Coronary Stent System 2000 X Base bare metal stent identical to PRO-Kinetic Energy under review (P 160003) 50 X Single stent design, cobalt chromium Electrospun non-woven polyurethane cover (Elast. Eon 2 AS) on abluminal side only Manufactured by BIOTRONIK AG

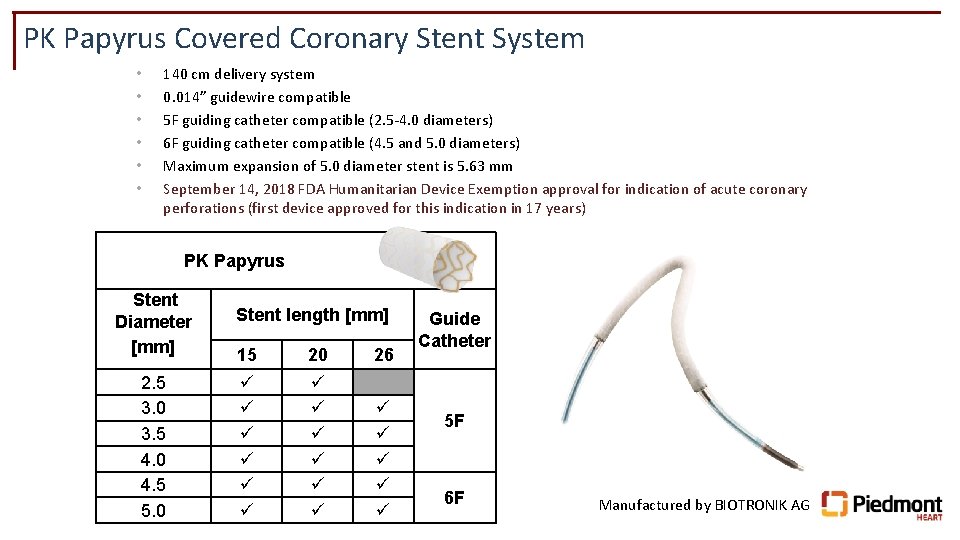
PK Papyrus Covered Coronary Stent System • • • 140 cm delivery system 0. 014” guidewire compatible 5 F guiding catheter compatible (2. 5 -4. 0 diameters) 6 F guiding catheter compatible (4. 5 and 5. 0 diameters) Maximum expansion of 5. 0 diameter stent is 5. 63 mm September 14, 2018 FDA Humanitarian Device Exemption approval for indication of acute coronary perforations (first device approved for this indication in 17 years) PK Papyrus Stent Diameter [mm] 2. 5 3. 0 3. 5 4. 0 4. 5 5. 0 Stent length [mm] 15 20 26 Guide Catheter 5 F 6 F Manufactured by BIOTRONIK AG
![PK Papyrus Covered Coronary Stent System PK Papyrus Graftmaster RX Stent Diameter mm 2 PK Papyrus Covered Coronary Stent System PK Papyrus Graftmaster RX Stent Diameter [mm] 2.](https://slidetodoc.com/presentation_image/4d1df1ab97c0df6155237e1a6411d79d/image-5.jpg)
PK Papyrus Covered Coronary Stent System PK Papyrus Graftmaster RX Stent Diameter [mm] 2. 8 3. 5 4. 0 4. 5 4. 8 Stent length [mm] 16 19 26 Stent Diameter [mm] Guide Cather 6 F 6 F 7 F 7 F − Smallest vessel which can be treated is 2. 75 mm − Smallest guide catheter which can be used is 6 F − Poor deliverability limits access to perforation sites in non-tortuous anatomy 1, 2 1. 2. 3. 2. 5 3. 0 3. 5 4. 0 4. 5 5. 0 Stent length [mm] 15 20 26 Guide Cather 5 F 5 F 6 F 6 F + 5 F guide catheter can be utilized with up to 4. 0 mm stent diameter + Guide catheter extension compatibility: 6 Fr: 2. 5 to 4. 0 mm 7/8 Fr: 4. 5 and 5. 0 mm + Improved deliverability allows access to tortuous anatomy and distal segments C. Hendry, D. Fraser, et al. Coronary perforation in the drug-eluting stent era: incidence, risk factors, management and outcome: the UK experience. Euro. Intervention 2012; 7: 79 -86. S. Chen, C. Lotan, et al. Pericardial covered stent for coronary perforations. Catheter Cardiovascular Intervention. 2015 Sep; 86(3): 400 -4. Survey data includes 2. 0 mm

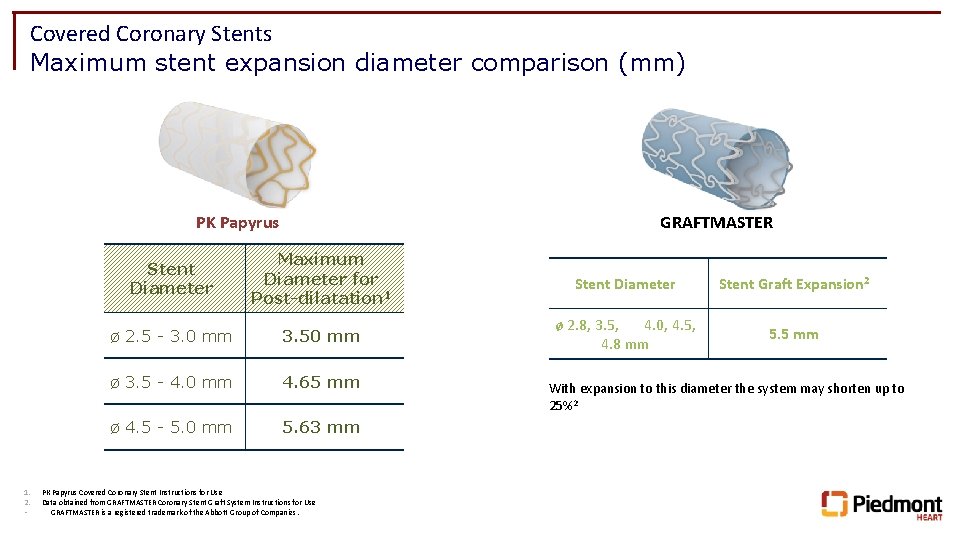
Covered Coronary Stents Maximum stent expansion diameter comparison (mm) GRAFTMASTER PK Papyrus 1. 2. • Stent Diameter Maximum Diameter for Post-dilatation 1 Stent Diameter Stent Graft Expansion 2 ø 2. 5 - 3. 0 mm 3. 50 mm ø 2. 8, 3. 5, 4. 0, 4. 5, 4. 8 mm 5. 5 mm ø 3. 5 - 4. 0 mm 4. 65 mm ø 4. 5 - 5. 0 mm 5. 63 mm PK Papyrus Covered Coronary Stent Instructions for Use Data obtained from GRAFTMASTER Coronary Stent Graft System Instructions for Use GRAFTMASTER is a registered trademark of the Abbott Group of Companies. With expansion to this diameter the system may shorten up to 25%2

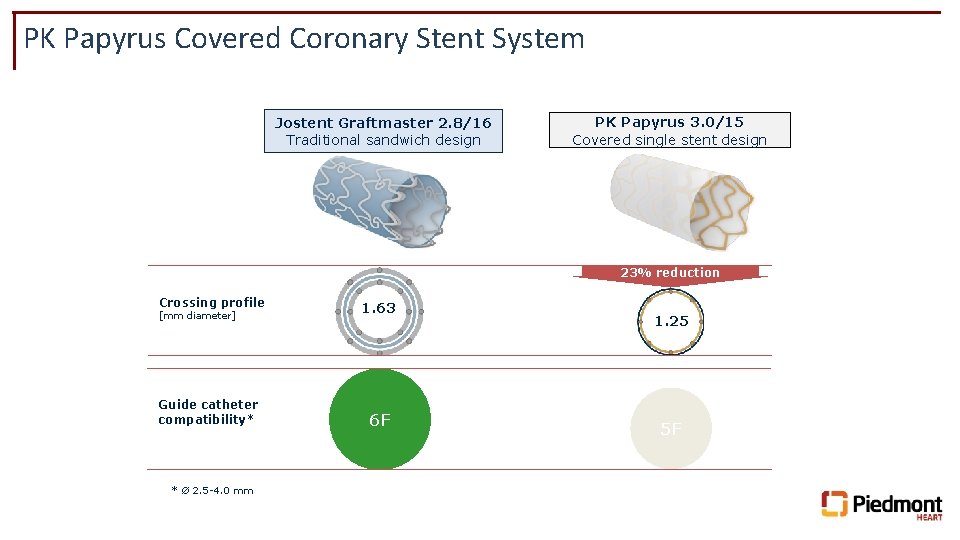
PK Papyrus Covered Coronary Stent System Jostent Graftmaster 2. 8/16 Traditional sandwich design PK Papyrus 3. 0/15 Covered single stent design 23% reduction Crossing profile [mm diameter] Guide catheter compatibility* * Ø 2. 5 -4. 0 mm 1. 63 6 F 1. 25 5 F

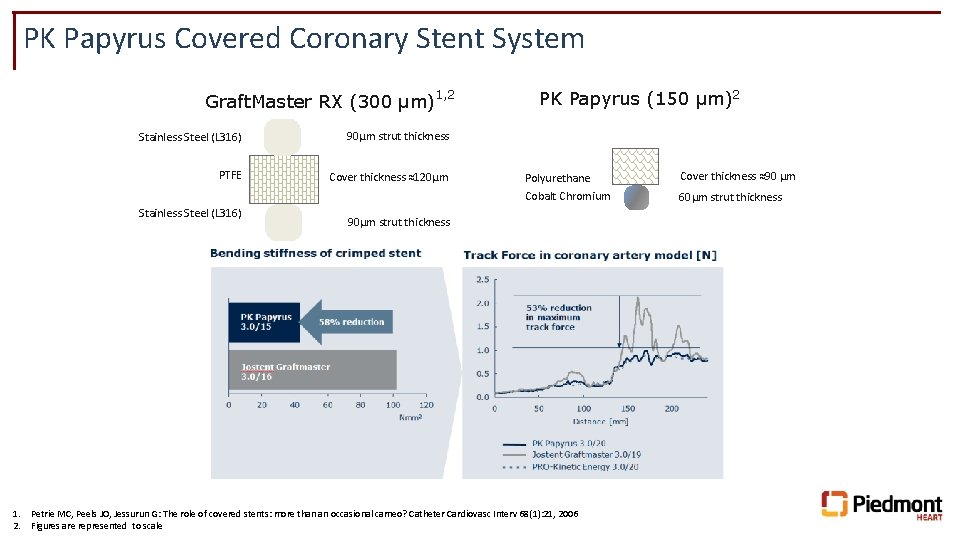
PK Papyrus Covered Coronary Stent System 1, 2 Graft. Master RX (300 µm) Stainless Steel (L 316) 90µm strut thickness PTFE Cover thickness ≈120µm Stainless Steel (L 316) PK Papyrus (150 µm)2 Polyurethane Cover thickness ≈90 µm Cobalt Chromium 60µm strut thickness 90µm strut thickness 1. Petrie MC, Peels JO, Jessurun G: The role of covered stents: more than an occasional cameo? Catheter Cardiovasc Interv 68(1): 21, 2006 2. Figures are represented to scale

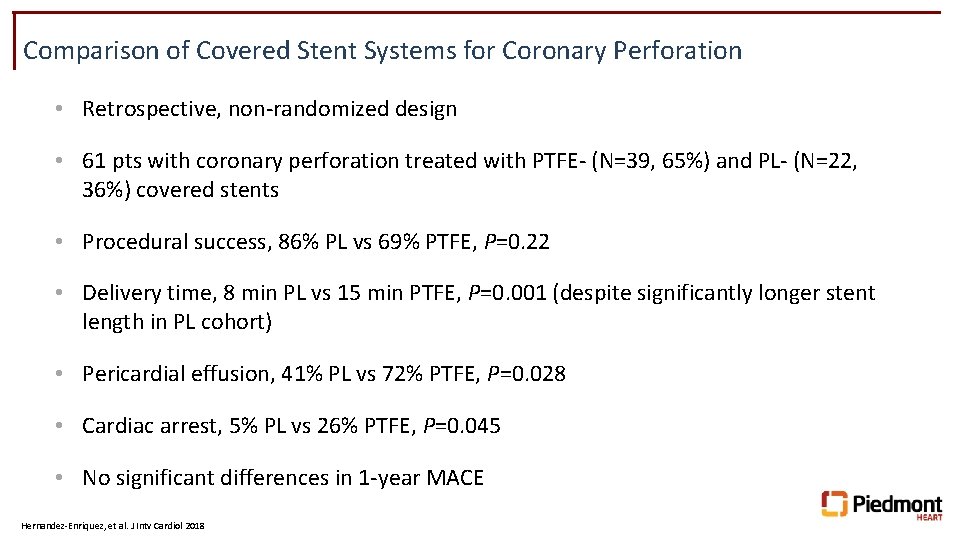
Comparison of Covered Stent Systems for Coronary Perforation • Retrospective, non-randomized design • 61 pts with coronary perforation treated with PTFE- (N=39, 65%) and PL- (N=22, 36%) covered stents • Procedural success, 86% PL vs 69% PTFE, P=0. 22 • Delivery time, 8 min PL vs 15 min PTFE, P=0. 001 (despite significantly longer stent length in PL cohort) • Pericardial effusion, 41% PL vs 72% PTFE, P=0. 028 • Cardiac arrest, 5% PL vs 26% PTFE, P=0. 045 • No significant differences in 1 -year MACE Hernandez-Enriquez, et al. J Intv Cardiol 2018

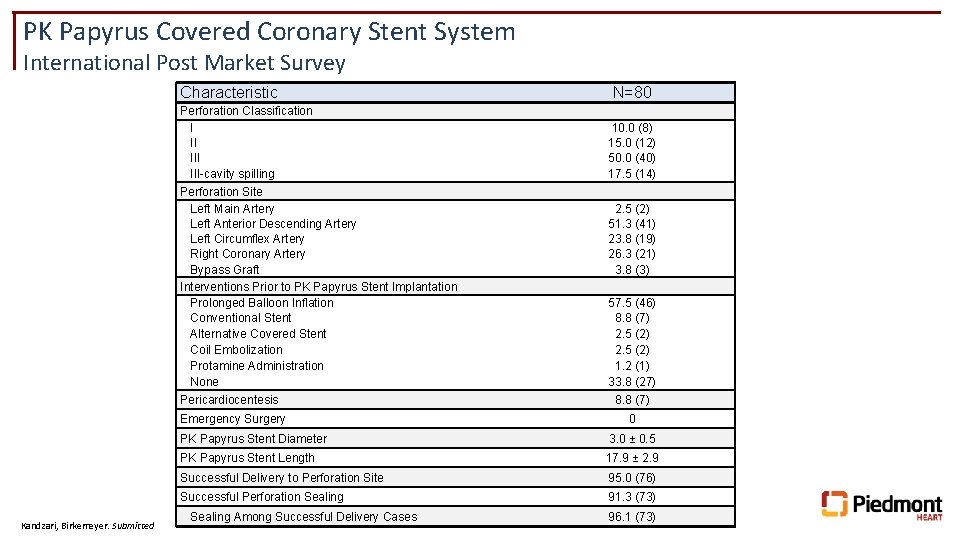
PK Papyrus Covered Coronary Stent System International Post Market Survey Characteristic Perforation Classification I III-cavity spilling Perforation Site Left Main Artery Left Anterior Descending Artery Left Circumflex Artery Right Coronary Artery Bypass Graft Interventions Prior to PK Papyrus Stent Implantation Prolonged Balloon Inflation Conventional Stent Alternative Covered Stent Coil Embolization Protamine Administration None Pericardiocentesis Emergency Surgery Kandzari, Birkemeyer. Submitted N=80 10. 0 (8) 15. 0 (12) 50. 0 (40) 17. 5 (14) 2. 5 (2) 51. 3 (41) 23. 8 (19) 26. 3 (21) 3. 8 (3) 57. 5 (46) 8. 8 (7) 2. 5 (2) 1. 2 (1) 33. 8 (27) 8. 8 (7) 0 PK Papyrus Stent Diameter 3. 0 ± 0. 5 PK Papyrus Stent Length 17. 9 ± 2. 9 Successful Delivery to Perforation Site 95. 0 (76) Successful Perforation Sealing 91. 3 (73) Sealing Among Successful Delivery Cases 96. 1 (73)

PK Papyrus Covered Coronary Stent System Summary • PK Papyrus is a novel covered stent design intended to overcome limitations of existing therapies to facilitate device delivery and effectively treat coronary artery perforations – Ultrathin strut stent combined with polyurethane matrix cover results in halving of device profile and 24% reduction in overall delivery system crossing profile – Permits wider range of vessel treatment and with 5 Fr and 6 Fr guiding catheters • Recent (September 2018) FDA approval for coronary perforations under Humanitarian Device Exemption pathway • In an international survey among unselected patients with coronary perforation, use of PK Papyrus is associated with a high rate of delivery to the perforation site (95. 0%) and successful perforation sealing with device delivery (96. 0%)