A semisynthetic strategy to fucosylated chondroitin sulfate polysaccharides

![BIOLOGICAL ACTIVITIES f. CS may exhibit activities related to: • Coagulation and thrombosis[1] Pomin, BIOLOGICAL ACTIVITIES f. CS may exhibit activities related to: • Coagulation and thrombosis[1] Pomin,](https://slidetodoc.com/presentation_image_h2/491d6ab0b00aac7b044e90e60eabd8c1/image-4.jpg)

![ANTICOAGULANT ACTIVITY Activity [IU/mg] f. CII f. CSVIII f. CSIX f. CS-X Heparin 0. ANTICOAGULANT ACTIVITY Activity [IU/mg] f. CII f. CSVIII f. CSIX f. CS-X Heparin 0.](https://slidetodoc.com/presentation_image_h2/491d6ab0b00aac7b044e90e60eabd8c1/image-19.jpg)




- Slides: 25

A semi-synthetic strategy to fucosylated chondroitin sulfate polysaccharides from microbial sourced chondroitin Laezza Antonio 1, Iadonisi Alfonso 1, De Castro Cristina 2, De Rosa Mario 3, Schiraldi Chiara 3, Parrilli Michelangelo 4, Bedini Emiliano 1 1 Dipartimento di Scienze Chimiche, Università di Napoli Federico II, Complesso Universitario Monte S. Angelo, via Cintia 4, I-80126 Napoli, Italy 2 Dipartimento di Agraria, Università di Napoli Federico II, Via Università 100, I-80055, Portici, Italy 3 Dipartimento di Medicina Sperimentale, Seconda Università di Napoli, via De Crecchio 7, I-80138 Napoli, Italy 4 Dipartimento di Biologia, Università di Napoli Federico II, Complesso Universitario Monte S. Angelo, via Cintia 4, I 80126 Napoli, Italy 7 th Baltic Meeting on Microbial Carbohydrates, Gϋstrow, 25 -29 September

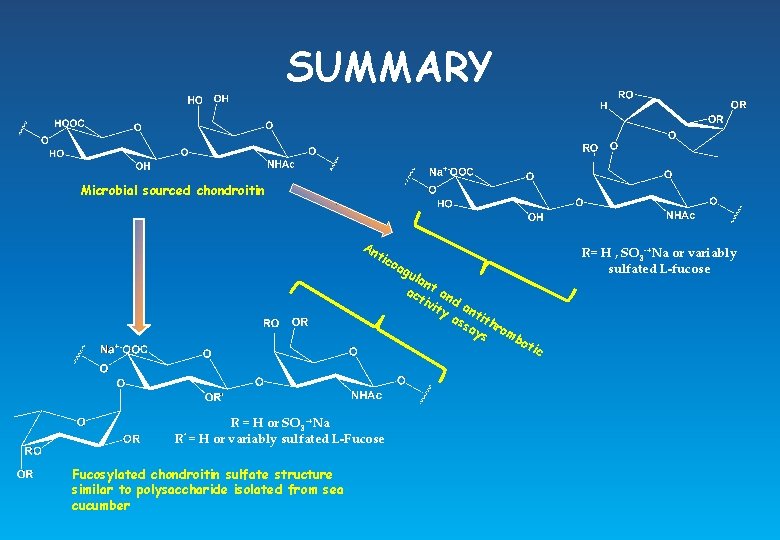
SUMMARY Microbial sourced chondroitin An tic R= H , SO 3 -+Na or variably sulfated L-fucose oa g ula n ac t an tiv d ity an as tith sa ys romb o tic R = H or SO 3 -+Na R’ = H or variably sulfated L-Fucose Fucosylated chondroitin sulfate structure similar to polysaccharide isolated from sea cucumber

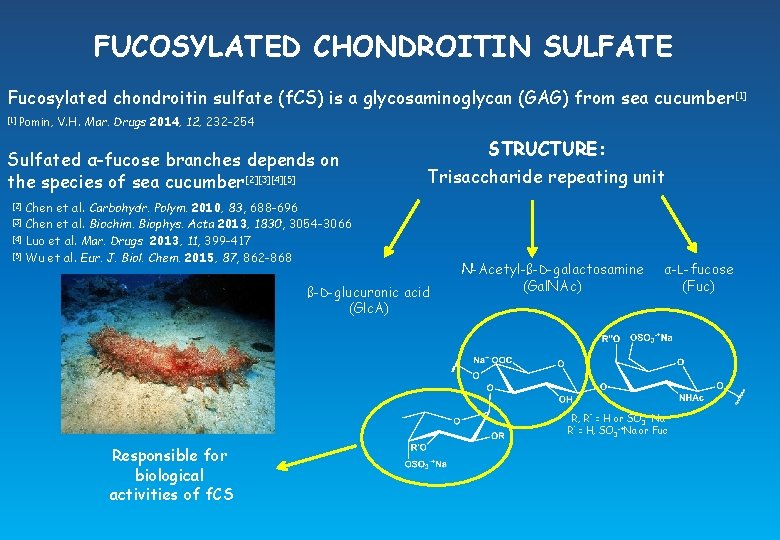
FUCOSYLATED CHONDROITIN SULFATE Fucosylated chondroitin sulfate (f. CS) is a glycosaminoglycan (GAG) from sea cucumber [1] Pomin, V. H. Mar. Drugs 2014, 12, 232 -254 Sulfated α-fucose branches depends on the species of sea cucumber[2][3][4][5] [2] [3] [4] [5] STRUCTURE: Trisaccharide repeating unit Chen et al. Carbohydr. Polym. 2010, 83, 688 -696 Chen et al. Biochim. Biophys. Acta 2013, 1830, 3054 -3066 Luo et al. Mar. Drugs 2013, 11, 399 -417 Wu et al. Eur. J. Biol. Chem. 2015, 87, 862 -868 ß-D-glucuronic acid (Glc. A) N-Acetyl-ß-D-galactosamine (Gal. NAc) α-L-fucose (Fuc) R, R’’ = H or SO 3 -+Na R’ = H, SO 3 -+Na or Fuc Responsible for biological activities of f. CS
![BIOLOGICAL ACTIVITIES f CS may exhibit activities related to Coagulation and thrombosis1 Pomin BIOLOGICAL ACTIVITIES f. CS may exhibit activities related to: • Coagulation and thrombosis[1] Pomin,](https://slidetodoc.com/presentation_image_h2/491d6ab0b00aac7b044e90e60eabd8c1/image-4.jpg)
BIOLOGICAL ACTIVITIES f. CS may exhibit activities related to: • Coagulation and thrombosis[1] Pomin, V. H. Mar. Drugs 2014, 12, 232 -254 • Atherosclerosis[6] Tovar et al. Altherosclerosis 1996, 26, 185 -195 • Cancer metastasis and inflammation[7] Borsig et al. J. Biol. Chem. 2007, 282, 14984 -14991 • Viral infection[8][9] [8] [9] Lian et al. Biochim. Biophys. Acta 2013, 1830, 4681 -4691 Huang et al. Carbohydr. Res. 2013, 380, 64 -69

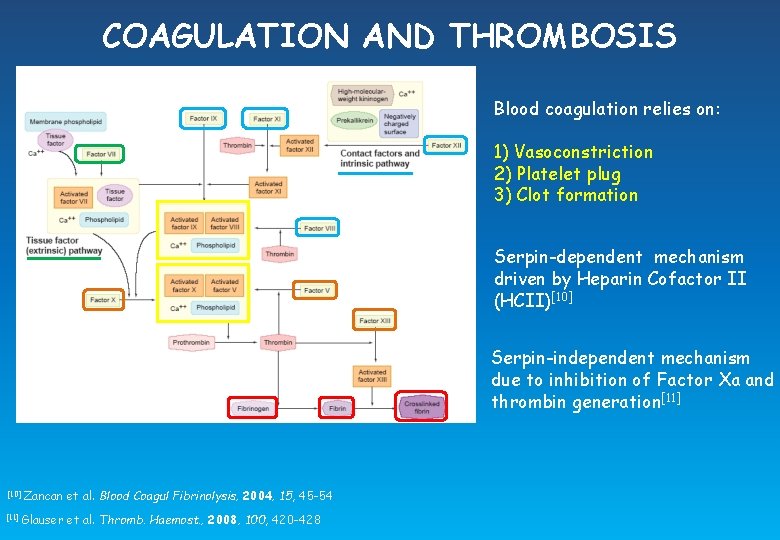
COAGULATION AND THROMBOSIS Blood coagulation relies on: 1) Vasoconstriction 2) Platelet plug 3) Clot formation Serpin-dependent mechanism driven by Heparin Cofactor II (HCII)[10] Serpin-independent mechanism due to inhibition of Factor Xa and thrombin generation[11] [10] [11] Zancan et al. Blood Coagul Fibrinolysis, 2004, 15, 45 -54 Glauser et al. Thromb. Haemost. , 2008, 100, 420 -428

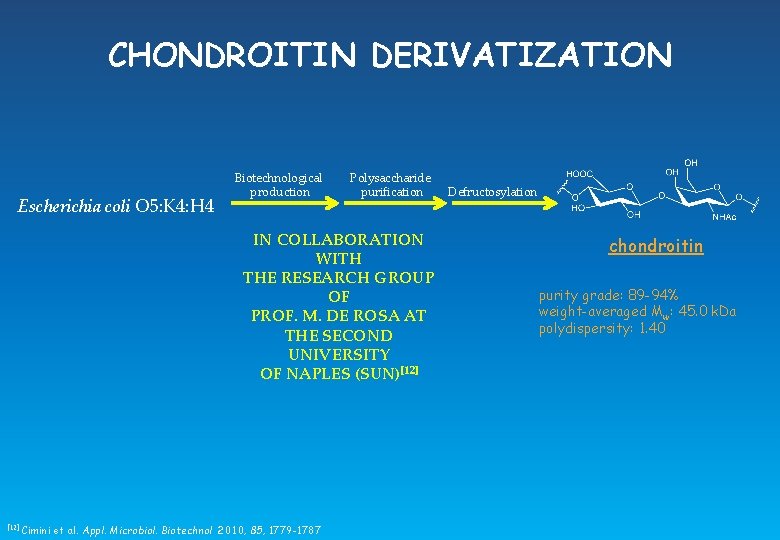
CHONDROITIN DERIVATIZATION Escherichia coli O 5: K 4: H 4 Biotechnological production Polysaccharide purification IN COLLABORATION WITH THE RESEARCH GROUP OF PROF. M. DE ROSA AT THE SECOND UNIVERSITY OF NAPLES (SUN)[12] Cimini et al. Appl. Microbiol. Biotechnol 2010, 85, 1779 -1787 Defructosylation chondroitin purity grade: 89 -94% weight-averaged Mw: 45. 0 k. Da polydispersity: 1. 40

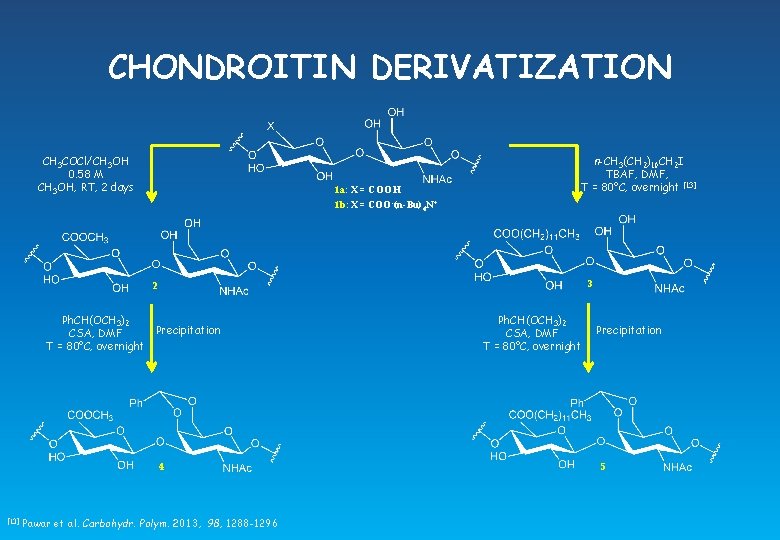
CHONDROITIN DERIVATIZATION CH 3 COCl/CH 3 OH 0. 58 M CH 3 OH, RT, 2 days 1 a: X = COOH 1 b: X = COO-(n-Bu)4 N+ n-CH 3(CH 2)10 CH 2 I TBAF, DMF, T = 80°C, overnight [13] 3 2 Ph. CH(OCH 3)2 Precipitation CSA, DMF T = 80°C, overnight 4 [13] Pawar et al. Carbohydr. Polym. 2013, 98, 1288 -1296 Ph. CH(OCH 3)2 CSA, DMF T = 80°C, overnight Precipitation 5

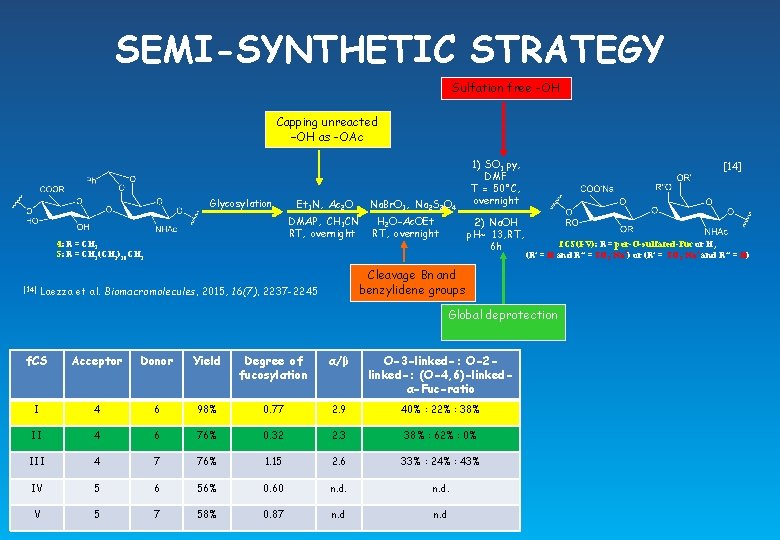
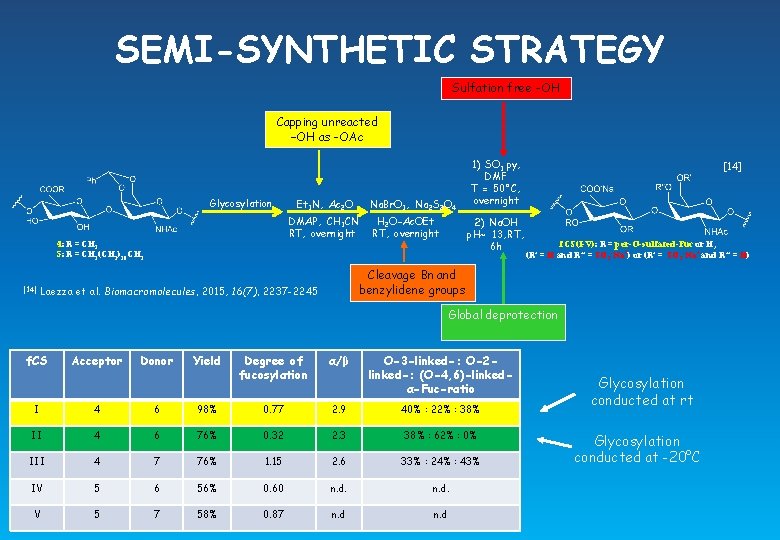
SEMI-SYNTHETIC STRATEGY Sulfation free -OH Capping unreacted –OH as -OAc Glycosylation DMAP, CH 3 CN RT, overnight 4: R = CH 3 5: R = CH 2(CH 2)10 CH 3 [14] Et 3 N, Ac 2 O Na. Br. O 3, Na 2 S 2 O 4 H 2 O-Ac. OEt RT, overnight 1) SO 3. py, DMF T = 50°C, overnight 2) Na. OH p. H~ 13, RT, 6 h [14] f. CS(I-V): R = per-O-sulfated-Fuc or H, (R’ = H and R’’ = SO 3 -Na+) or (R’ = SO 3 -Na+ and R’’ = H) Cleavage Bn and benzylidene groups Laezza et al. Biomacromolecules, 2015, 16(7), 2237 -2245 Global deprotection f. CS Acceptor Donor Yield Degree of fucosylation α/β O-3 -linked-: O-2 linked-: (O-4, 6)-linkedα-Fuc-ratio I 4 6 98% 0. 77 2. 9 40% : 22% : 38% II 4 6 76% 0. 32 2. 3 38% : 62% : 0% III 4 7 76% 1. 15 2. 6 33% : 24% : 43% IV 5 6 56% 0. 60 n. d. V 5 7 58% 0. 87 n. d

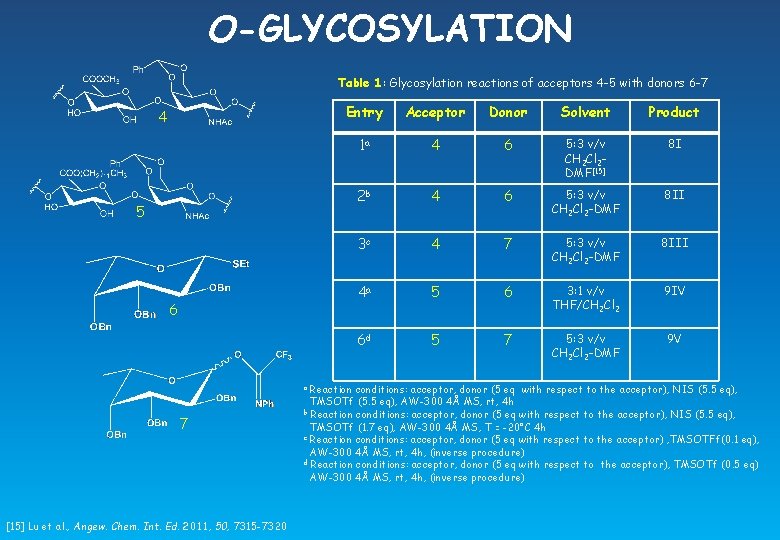
O-GLYCOSYLATION Table 1: Glycosylation reactions of acceptors 4 -5 with donors 6 -7 4 5 6 [15] Lu et al. , Angew. Chem. Int. Ed. 2011, 50, 7315 -7320 Acceptor Donor Solvent Product 1 a 4 6 5: 3 v/v CH 2 Cl 2 DMF[15] 8 I 2 b 4 6 5: 3 v/v CH 2 Cl 2 -DMF 8 II 3 c 4 7 5: 3 v/v CH 2 Cl 2 -DMF 8 III 4 a 5 6 3: 1 v/v THF/CH 2 Cl 2 9 IV 6 d 5 7 5: 3 v/v CH 2 Cl 2 -DMF 9 V Reaction conditions: acceptor, donor (5 eq with respect to the acceptor), NIS (5. 5 eq), TMSOTf (5. 5 eq), AW-300 4Å MS, rt, 4 h b Reaction conditions: acceptor, donor (5 eq with respect to the acceptor), NIS (5. 5 eq), TMSOTf (1. 7 eq), AW-300 4Å MS, T = -20°C 4 h c Reaction conditions: acceptor, donor (5 eq with respect to the acceptor) , TMSOTFf(0. 1 eq), AW-300 4Å MS, rt, 4 h, (inverse procedure) d Reaction conditions: acceptor, donor (5 eq with respect to the acceptor), TMSOTf (0. 5 eq) AW-300 4Å MS, rt, 4 h, (inverse procedure) a 7 Entry

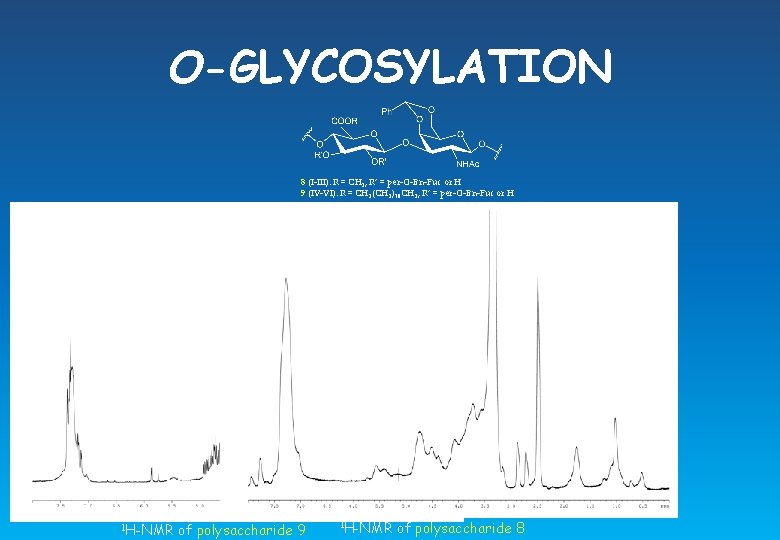
O-GLYCOSYLATION 8 (I-III): R = CH 3, R’ = per-O-Bn-Fuc or H 9 (IV-VI): R = CH 2(CH 2)10 CH 3, R’ = per-O-Bn-Fuc or H 1 H-NMR of polysaccharide 9 1 H-NMR of polysaccharide 8

SEMI-SYNTHETIC STRATEGY Sulfation free -OH Capping unreacted –OH as -OAc Glycosylation DMAP, CH 3 CN RT, overnight 4: R = CH 3 5: R = CH 2(CH 2)10 CH 3 [14] Et 3 N, Ac 2 O Na. Br. O 3, Na 2 S 2 O 4 H 2 O-Ac. OEt RT, overnight 1) SO 3. py, DMF T = 50°C, overnight 2) Na. OH p. H~ 13, RT, 6 h [14] f. CS(I-V): R = per-O-sulfated-Fuc or H, (R’ = H and R’’ = SO 3 -Na+) or (R’ = SO 3 -Na+ and R’’ = H) Cleavage Bn and benzylidene groups Laezza et al. Biomacromolecules, 2015, 16(7), 2237 -2245 Global deprotection f. CS Acceptor Donor Yield Degree of fucosylation α/β O-3 -linked-: O-2 linked-: (O-4, 6)-linkedα-Fuc-ratio I 4 6 98% 0. 77 2. 9 40% : 22% : 38% II 4 6 76% 0. 32 2. 3 38% : 62% : 0% III 4 7 76% 1. 15 2. 6 33% : 24% : 43% IV 5 6 56% 0. 60 n. d. V 5 7 58% 0. 87 n. d Glycosylation conducted at rt Glycosylation conducted at -20°C

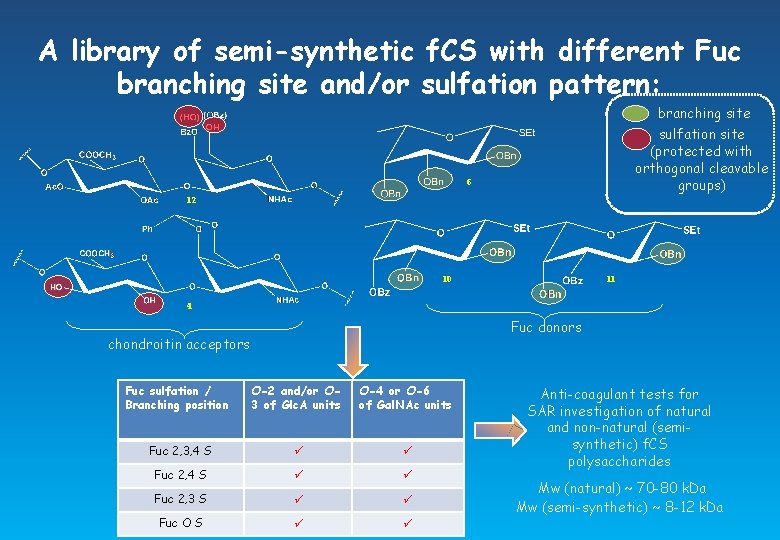
A library of semi-synthetic f. CS with different Fuc branching site and/or sulfation pattern: branching site sulfation site (protected with orthogonal cleavable groups) 6 12 10 11 4 Fuc donors chondroitin acceptors Fuc sulfation / Branching position O-2 and/or O 3 of Glc. A units O-4 or O-6 of Gal. NAc units Fuc 2, 3, 4 S Fuc 2, 3 S Fuc O S Anti-coagulant tests for SAR investigation of natural and non-natural (semisynthetic) f. CS polysaccharides Mw (natural) ~ 70 -80 k. Da Mw (semi-synthetic) ~ 8 -12 k. Da

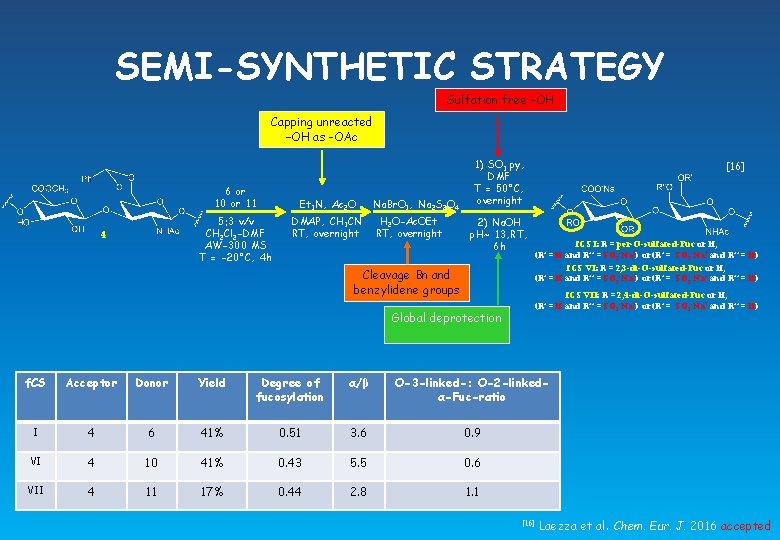
SEMI-SYNTHETIC STRATEGY Sulfation free -OH Capping unreacted –OH as -OAc 6 or 10 or 11 5: 3 v/v CH 2 Cl 2 -DMF AW-300 MS T = -20°C, 4 h 4 Et 3 N, Ac 2 O DMAP, CH 3 CN RT, overnight Na. Br. O 3, Na 2 S 2 O 4 H 2 O-Ac. OEt RT, overnight 1) SO 3. py, DMF T = 50°C, overnight 2) Na. OH p. H~ 13, RT, 6 h Cleavage Bn and benzylidene groups Global deprotection [16] f. CS I: R = per-O-sulfated-Fuc or H, (R’ = H and R’’ = SO 3 -Na+) or (R’ = SO 3 -Na+ and R’’ = H) f. CS VI: R = 2, 3 -di-O-sulfated-Fuc or H, (R’ = H and R’’ = SO 3 -Na+) or (R’ = SO 3 -Na+ and R’’ = H) f. CS VII: R = 2, 4 -di-O-sulfated-Fuc or H, (R’ = H and R’’ = SO 3 -Na+) or (R’ = SO 3 -Na+ and R’’ = H) f. CS Acceptor Donor Yield Degree of fucosylation α/β O-3 -linked-: O-2 -linkedα-Fuc-ratio I 4 6 41% 0. 51 3. 6 0. 9 VI 4 10 41% 0. 43 5. 5 0. 6 VII 4 11 17% 0. 44 2. 8 1. 1 [16] Laezza et al. Chem. Eur. J. 2016 accepted

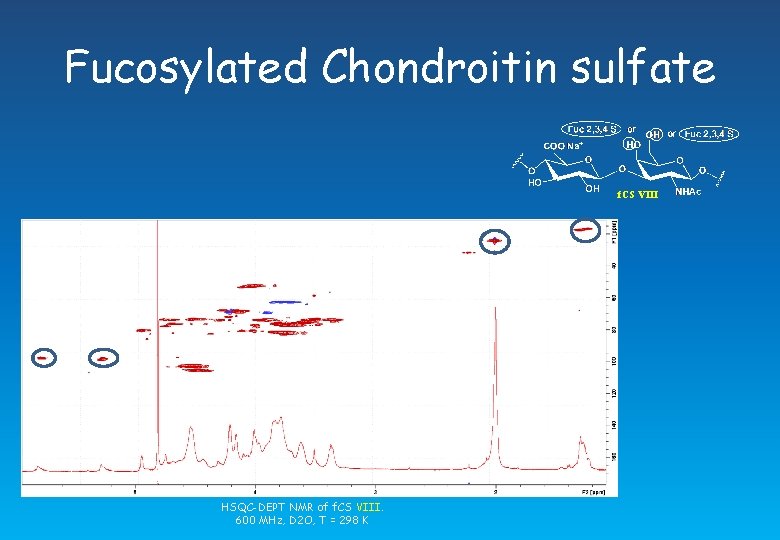
Fucosylated chondroitin sulfate f. CS VII HSQC-DEPT NMR of f. CS VII. 600 MHz, D 2 O, T = 298 K

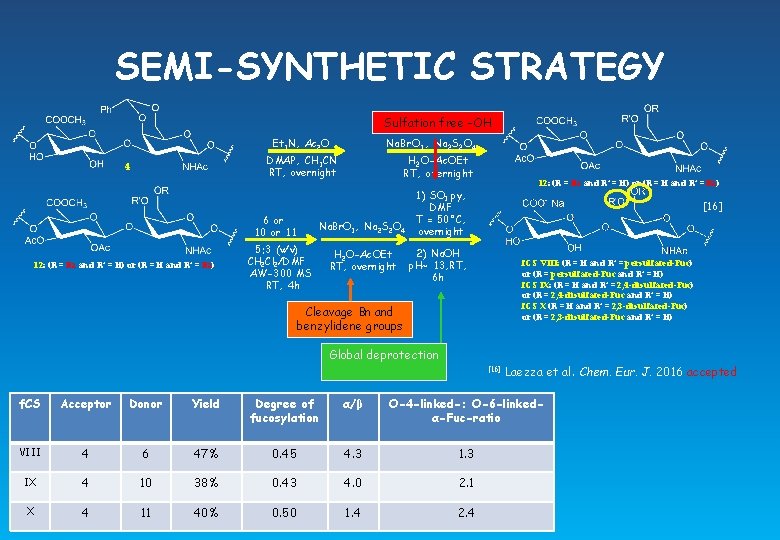
SEMI-SYNTHETIC STRATEGY Sulfation free -OH Na. Br. O 3, Na 2 S 2 O 4 Et 3 N, Ac 2 O H 2 O-Ac. OEt RT, overnight DMAP, CH 3 CN RT, overnight 4 6 or 10 or 11 12: (R = Bz and R’ = H) or (R = H and R’ = Bz) Na. Br. O 3, Na 2 S 2 O 4 5: 3 (v/v) CH 2 Cl 2/DMF AW-300 MS RT, 4 h H 2 O-Ac. OEt RT, overnight 12: (R = Bz and R’ = H) or (R = H and R’ = Bz) 1) SO 3. py, DMF T = 50°C, overnight [16] 2) Na. OH p. H~ 13, RT, 6 h f. CS VIII: (R = H and R’ = persulfated-Fuc) or (R = persulfated-Fuc and R’ = H) f. CS IX: (R = H and R’ = 2, 4 -disulfated-Fuc) or (R = 2, 4 -disulfated-Fuc and R’ = H) f. CS X (R = H and R’ = 2, 3 -disulfated-Fuc) or (R = 2, 3 -disulfated-Fuc and R’ = H) Cleavage Bn and benzylidene groups Global deprotection [16] Laezza et al. Chem. Eur. J. 2016 accepted f. CS Acceptor Donor Yield Degree of fucosylation α/β O-4 -linked-: O-6 -linkedα-Fuc-ratio VIII 4 6 47% 0. 45 4. 3 1. 3 IX 4 10 38% 0. 43 4. 0 2. 1 X 4 11 40% 0. 50 1. 4 2. 4

Fucosylated Chondroitin sulfate f. CS VIII HSQC-DEPT NMR of f. CS VIII. 600 MHz, D 2 O, T = 298 K

SEMI-SYNTHETIC STRATEGY 4 5: 3 (v/v) CH 2 Cl 2/DMF AW-300 MS RT, 4 h DMAP, CH 3 CN RT, overnight H 2 O-Ac. OEt RT, overnight 12: (R = Bz and R’ = H) or (R = H and R’ = Bz) 5: 3 (v/v) CH 2 Cl 2/DMF AW-300 MS RT, 4 h 6 H 2 O-Ac. OEt RT, overnight Et 3 N, Ac 2 O 6 Na. Br. O 3, Na 2 S 2 O 4 Na. OH p. H~ 13, RT, 6 h Na. Br. O 3, Na 2 S 2 O 4 [16] Na. OH p. H~ 13, RT, 6 h [16] f. C-II: (R = H and R’ = Fuc) or (R = Fuc and R’ = H) f. C-I: R = Fuc or H, (R’ = R’’ = H) [16] Laezza et al. Chem. Eur. J. 2016 accepted

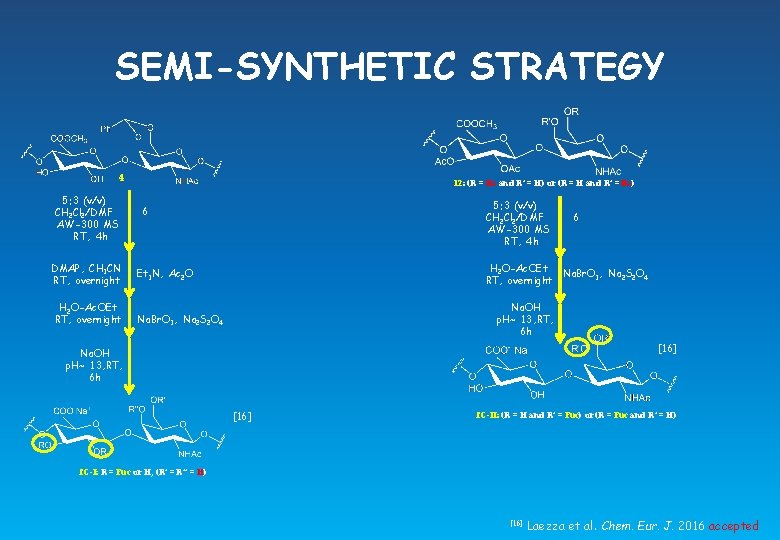
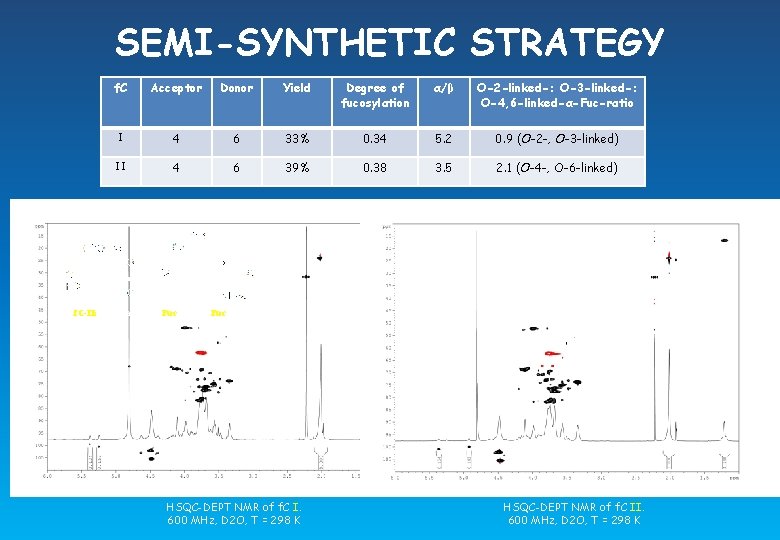
SEMI-SYNTHETIC STRATEGY f. C Acceptor Donor Yield Degree of fucosylation α/β O-2 -linked-: O-3 -linked-: O-4, 6 -linked-α-Fuc-ratio I 4 6 33% 0. 34 5. 2 0. 9 (O-2 -, O-3 -linked) II 4 6 39% 0. 38 3. 5 2. 1 (O-4 -, O-6 -linked) f. C-I: R = Fuc or H, (R’ = R’’ = H) f. C-II: (R = H and R’ = Fuc) or (R = Fuc and R’ = H) HSQC-DEPT NMR of f. C I. 600 MHz, D 2 O, T = 298 K HSQC-DEPT NMR of f. C II. 600 MHz, D 2 O, T = 298 K
![ANTICOAGULANT ACTIVITY Activity IUmg f CII f CSVIII f CSIX f CSX Heparin 0 ANTICOAGULANT ACTIVITY Activity [IU/mg] f. CII f. CSVIII f. CSIX f. CS-X Heparin 0.](https://slidetodoc.com/presentation_image_h2/491d6ab0b00aac7b044e90e60eabd8c1/image-19.jpg)
ANTICOAGULANT ACTIVITY Activity [IU/mg] f. CII f. CSVIII f. CSIX f. CS-X Heparin 0. 20 0. 25 0. 18 0. 27 n. d. 0. 26 0. 33 0. 23 198 Table 2: AT-dependent activity against factor Xa AT-dependent activity is very similar to that reported for low molecular mass f. CS [17] a) Wu et al. Eur. J. Med. Chem. 2015, 92, 257 -269 b) Panagos et al. J. Biol. Chem. 2014, 289, 28284 -28298 c) Mourão et al. J. Biol. Chem. 1996, 271, 23973 -23984 HC-II concentration [ng/m. L] 160 140 120 100 80 60 40 20 0 f. C-II f. CS-VIII f. CS-IX f. CS-X heparin HC-II-mediated anti-factor IIa activity

FUTURE PERSPECTIVES • Different protection patterns on chondroitin intermediates • Different glycosyl activators • Glycomar LTD 1) Anticoagulant assays • University of Edinburgh (Prof. Dusan Uhrin) 2) df. CS-selectins interactions

CONCLUSIONS Semi-synthetic strategies from microbial sourced chondroitin to fucosylated chondroitin sulfate First O-glycosylation of secondary hydroxyls of polysaccharides Library of different O-glycosylated chondroitin polysaccharides

Thanks to University of Naples Federico II Second University of Naples (SUN) University of Edinburgh - Prof. M. Parrilli - Dr. E. Bedini -Prof. A. Iadonisi -Prof. C. De Castro - Prof. M. De Rosa - Prof. C. Schiraldi - Dr. Dusan Uhrin L. 297 project “Produzione biotecnologica di condroitina”


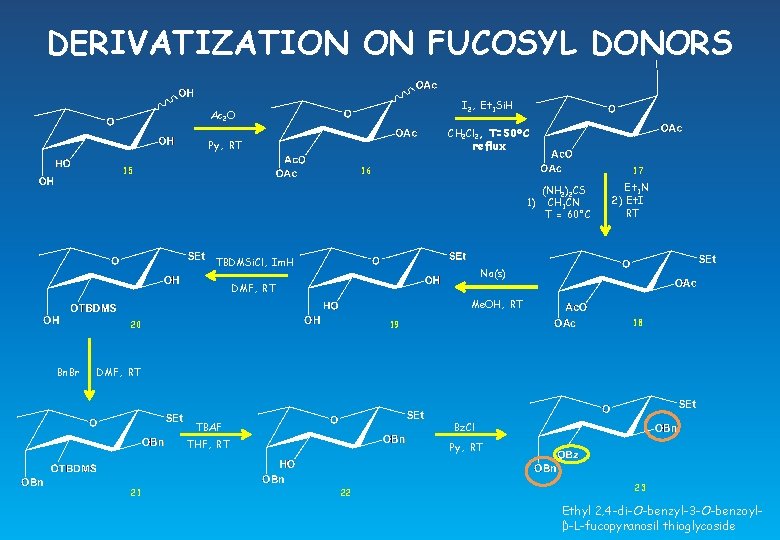
DERIVATIZATION ON FUCOSYL DONORS I 2, Et 3 Si. H Ac 2 O CH 2 Cl 2, T=50°C reflux Py, RT 15 16 17 (NH 2)2 CS 1) CH 3 CN T = 60°C TBDMSi. Cl, Im. H Et 3 N 2) Et. I RT Na(s) DMF, RT Me. OH, RT 20 Bn. Br 18 19 DMF, RT 21 TBAF Bz. Cl THF, RT Py, RT 22 23 Ethyl 2, 4 -di-O-benzyl-3 -O-benzoylβ-L-fucopyranosil thioglycoside

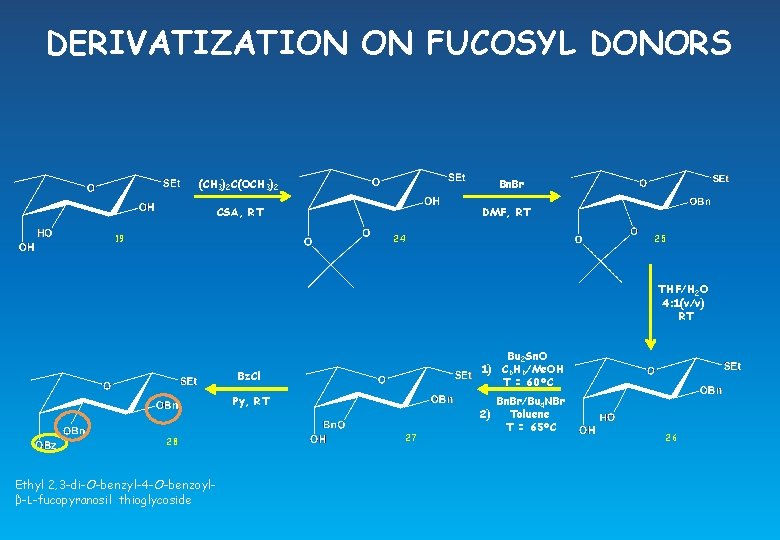
DERIVATIZATION ON FUCOSYL DONORS (CH 3)2 C(OCH 3)2 Bn. Br CSA, RT 19 DMF, RT 24 25 THF/H 2 O 4: 1(v/v) RT Bu 2 Sn. O 1) C 6 H 6/Me. OH T = 60°C Bz. Cl Py, RT 28 Ethyl 2, 3 -di-O-benzyl-4 -O-benzoylβ-L-fucopyranosil thioglycoside 2) 27 Bn. Br/Bu 4 NBr Toluene T = 65°C 26
 Difference between monosaccharide and polysaccharide
Difference between monosaccharide and polysaccharide Biological molecules
Biological molecules L-ketopentose
L-ketopentose Polysaccharides
Polysaccharides Structure of sucrose maltose and lactose
Structure of sucrose maltose and lactose Hexoses
Hexoses Monosaccharides disaccharides and polysaccharides
Monosaccharides disaccharides and polysaccharides Storage polysaccharide
Storage polysaccharide Identify the correct example of surgical dusting powder
Identify the correct example of surgical dusting powder Ammonium sulfate precipitation
Ammonium sulfate precipitation Dic.n
Dic.n Magnesium sulfate toxicity level
Magnesium sulfate toxicity level Magnesium sulfate and urine output
Magnesium sulfate and urine output Sulphuric acid and aluminium
Sulphuric acid and aluminium Rtp273
Rtp273 Preterm labor definition
Preterm labor definition Copper sulfate and potassium iodide
Copper sulfate and potassium iodide Copper oxide and hydrogen reaction
Copper oxide and hydrogen reaction Ammonium sulfate cation and anion
Ammonium sulfate cation and anion Berotec nebs
Berotec nebs Anion test for sulfate
Anion test for sulfate Sulfate reducing bacteria
Sulfate reducing bacteria Lithium oxide dot and cross
Lithium oxide dot and cross Mgso4 side effects
Mgso4 side effects Spectator ions
Spectator ions Cristal de sulfate de cuivre
Cristal de sulfate de cuivre