A SEMISYNTHETIC STRATEGY TO FUCOSYLATED CHONDROITIN SULFATE POLYSACCHARIDES

![BIOLOGICAL ACTIVITIES f. CS may exhibit activities related to: • Coagulation and thrombosis[1] Pomin, BIOLOGICAL ACTIVITIES f. CS may exhibit activities related to: • Coagulation and thrombosis[1] Pomin,](https://slidetodoc.com/presentation_image_h2/208e6b3c521a4d818417d58bff01db24/image-3.jpg)




- Slides: 20

A SEMI-SYNTHETIC STRATEGY TO FUCOSYLATED CHONDROITIN SULFATE POLYSACCHARIDES FROM MICROBIAL-SOURCED CHONDROITIN Laezza Antonio 1, Iadonisi Alfonso 1, De Castro Cristina 2, De Rosa Mario 3, Schiraldi Chiara 3, Parrilli Michelangelo 4, Bedini Emiliano 1 1 Dipartimento di Scienze Chimiche, Università di Napoli Federico II, Complesso Universitario Monte S. Angelo, via Cintia 4, I-80126 Napoli, Italy 2 Dipartimento di Agraria, Università di Napoli Federico II, via Università 100, I-80055 Portici, Italy 3 Dipartimento di Medicina Sperimentale, Seconda Università di Napoli, via De Crecchio 7, I-80138 Napoli, Italy 4 Dipartimento di Biologia, Università di Napoli Federico II, Complesso Universitario Monte S. Angelo, via Cintia 4, I-80126 Napoli, Italy 4 th EPNOE International Polysaccharide Conference, Warsaw, 19 -22 Oct 2015

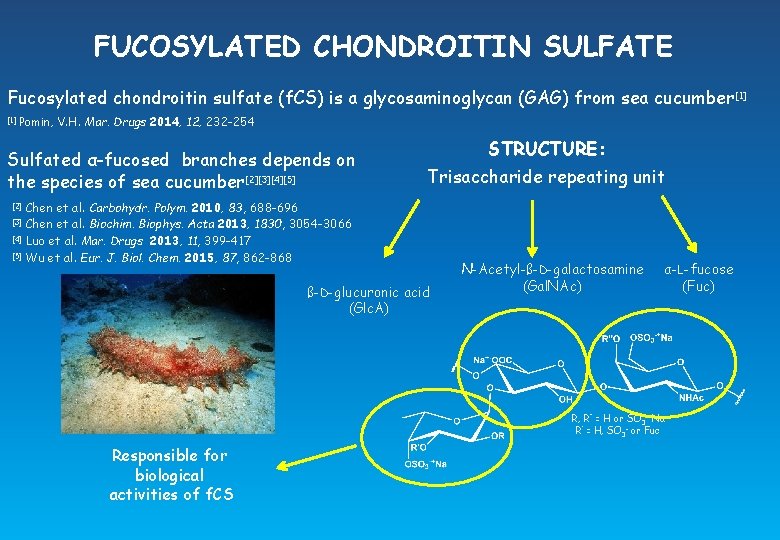
FUCOSYLATED CHONDROITIN SULFATE Fucosylated chondroitin sulfate (f. CS) is a glycosaminoglycan (GAG) from sea cucumber [1] Pomin, V. H. Mar. Drugs 2014, 12, 232 -254 Sulfated α-fucosed branches depends on the species of sea cucumber[2][3][4][5] [2] [3] [4] [5] STRUCTURE: Trisaccharide repeating unit Chen et al. Carbohydr. Polym. 2010, 83, 688 -696 Chen et al. Biochim. Biophys. Acta 2013, 1830, 3054 -3066 Luo et al. Mar. Drugs 2013, 11, 399 -417 Wu et al. Eur. J. Biol. Chem. 2015, 87, 862 -868 ß-D-glucuronic acid (Glc. A) N-Acetyl-ß-D-galactosamine (Gal. NAc) α-L-fucose (Fuc) R, R’’ = H or SO 3 -+Na R’ = H, SO 3 - or Fuc Responsible for biological activities of f. CS
![BIOLOGICAL ACTIVITIES f CS may exhibit activities related to Coagulation and thrombosis1 Pomin BIOLOGICAL ACTIVITIES f. CS may exhibit activities related to: • Coagulation and thrombosis[1] Pomin,](https://slidetodoc.com/presentation_image_h2/208e6b3c521a4d818417d58bff01db24/image-3.jpg)
BIOLOGICAL ACTIVITIES f. CS may exhibit activities related to: • Coagulation and thrombosis[1] Pomin, V. H. Mar. Drugs 2014, 12, 232 -254 • Atherosclerosis[6] Tovar et al. Altherosclerosis 1996, 26, 185 -195 • Cancer metastasis and inflammation[7] Borsig et al. J. Biol. Chem. 2007, 282, 14984 -14991 • Viral infection[8][9] [8] [9] Lian et al. Biochim. Biophys. Acta 2013, 1830, 4681 -4691 Huang et al. Carbohydr. Res. 2013, 380, 64 -69

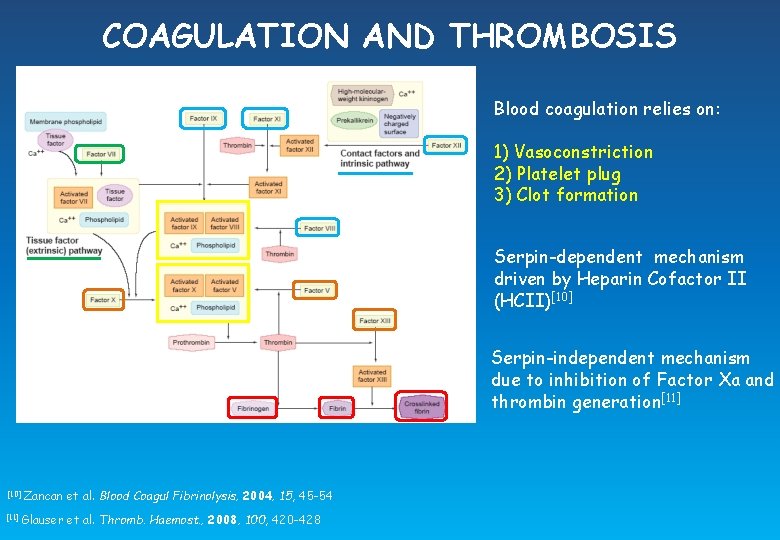
COAGULATION AND THROMBOSIS Blood coagulation relies on: 1) Vasoconstriction 2) Platelet plug 3) Clot formation Serpin-dependent mechanism driven by Heparin Cofactor II (HCII)[10] Serpin-independent mechanism due to inhibition of Factor Xa and thrombin generation[11] [10] [11] Zancan et al. Blood Coagul Fibrinolysis, 2004, 15, 45 -54 Glauser et al. Thromb. Haemost. , 2008, 100, 420 -428

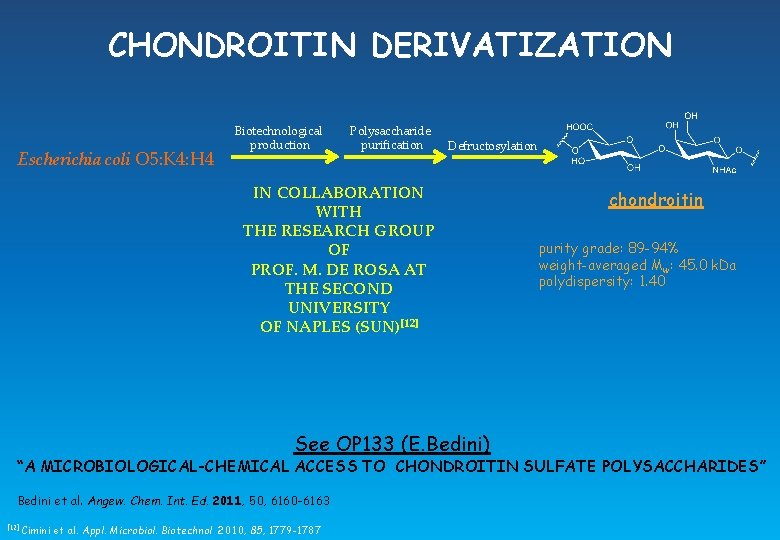
CHONDROITIN DERIVATIZATION Escherichia coli O 5: K 4: H 4 Biotechnological production Polysaccharide purification Defructosylation IN COLLABORATION WITH THE RESEARCH GROUP OF PROF. M. DE ROSA AT THE SECOND UNIVERSITY OF NAPLES (SUN)[12] See OP 133 (E. Bedini) chondroitin purity grade: 89 -94% weight-averaged Mw: 45. 0 k. Da polydispersity: 1. 40 “A MICROBIOLOGICAL-CHEMICAL ACCESS TO CHONDROITIN SULFATE POLYSACCHARIDES” Bedini et al. Angew. Chem. Int. Ed. 2011, 50, 6160 -6163 [12] Cimini et al. Appl. Microbiol. Biotechnol 2010, 85, 1779 -1787

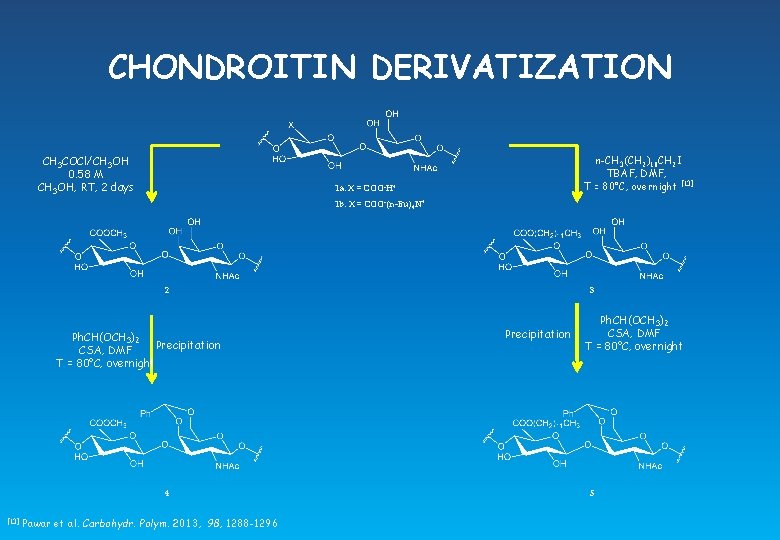
CHONDROITIN DERIVATIZATION CH 3 COCl/CH 3 OH 0. 58 M CH 3 OH, RT, 2 days n-CH 3(CH 2)10 CH 2 I TBAF, DMF, T = 80°C, overnight [13] 1 a: X = COO-H+ 1 b: X = COO-(n-Bu)4 N+ 2 Ph. CH(OCH 3)2 Precipitation CSA, DMF T = 80°C, overnight 4 [13] Pawar et al. Carbohydr. Polym. 2013, 98, 1288 -1296 3 Precipitation Ph. CH(OCH 3)2 CSA, DMF T = 80°C, overnight 5

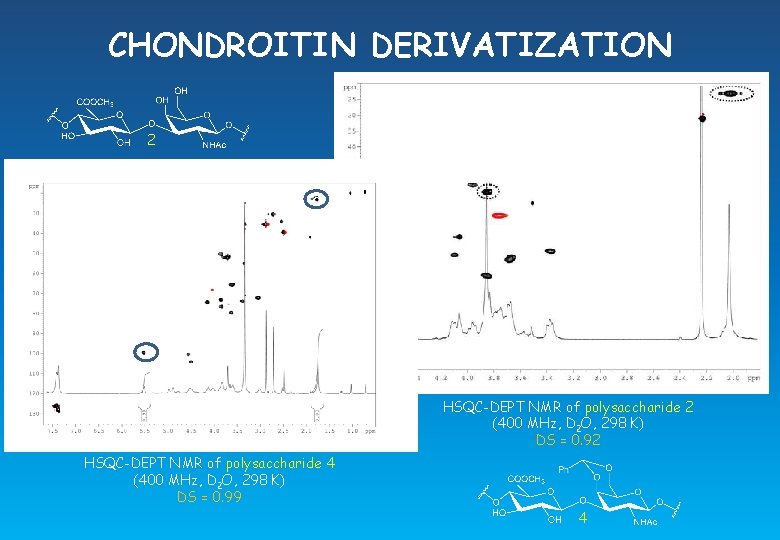
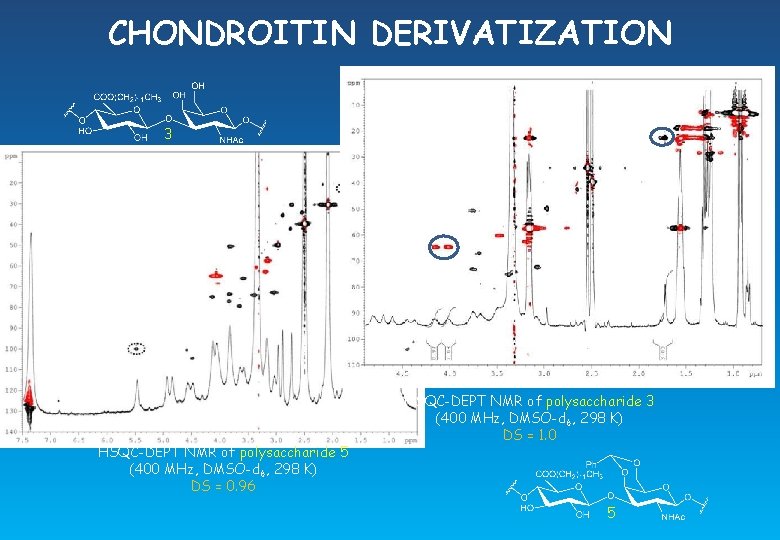
CHONDROITIN DERIVATIZATION 2 c HSQC-DEPT NMR of polysaccharide 2 (400 MHz, D 2 O, 298 K) DS = 0. 92 HSQC-DEPT NMR of polysaccharide 4 (400 MHz, D 2 O, 298 K) DS = 0. 99 4

CHONDROITIN DERIVATIZATION 3 HSQC-DEPT NMR of polysaccharide 5 (400 MHz, DMSO-d 6, 298 K) DS = 0. 96 HSQC-DEPT NMR of polysaccharide 3 (400 MHz, DMSO-d 6, 298 K) DS = 1. 0 5

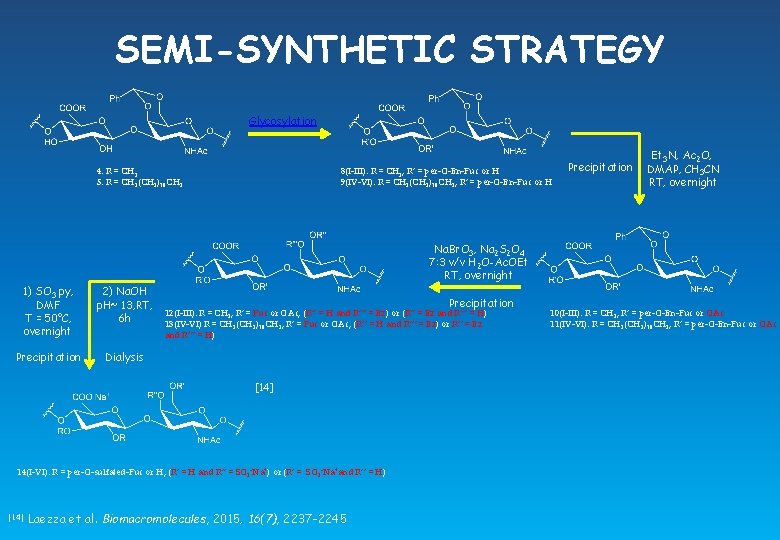
SEMI-SYNTHETIC STRATEGY Glycosylation 4: R = CH 3 5: R = CH 2(CH 2)10 CH 3 8(I-III): R = CH 3, R’ = per-O-Bn-Fuc or H 9(IV-VI): R = CH 2(CH 2)10 CH 3, R’ = per-O-Bn-Fuc or H Precipitation Et 3 N, Ac 2 O, DMAP, CH 3 CN RT, overnight Na. Br. O 3, Na 2 S 2 O 4 7: 3 v/v H 2 O-Ac. OEt RT, overnight 1) SO 3. py, DMF T = 50°C, overnight 2) Na. OH p. H~ 13, RT, 6 h Precipitation Dialysis Precipitation 12(I-III): R = CH 3, R’ = Fuc or OAc, (R’’ = H and R’’’ = Bz) or (R’’ = Bz and R’’’ = H) 13(IV-VI) R = CH 2(CH 2)10 CH 3, R’ = Fuc or OAc, (R’’ = H and R’’’ = Bz) or R’’ = Bz and R’’’ = H) [14] 14(I-VI): R = per-O-sulfated-Fuc or H, (R’ = H and R’’ = SO 3 -Na+) or (R’ = SO 3 -Na+and R’’ = H) [14] Laezza et al. Biomacromolecules, 2015, 16(7), 2237 -2245 10(I-III): R = CH 3, R’ = per-O-Bn-Fuc or OAc 11(IV-VI): R = CH 2(CH 2)10 CH 3, R’ = per-O-Bn-Fuc or OAc

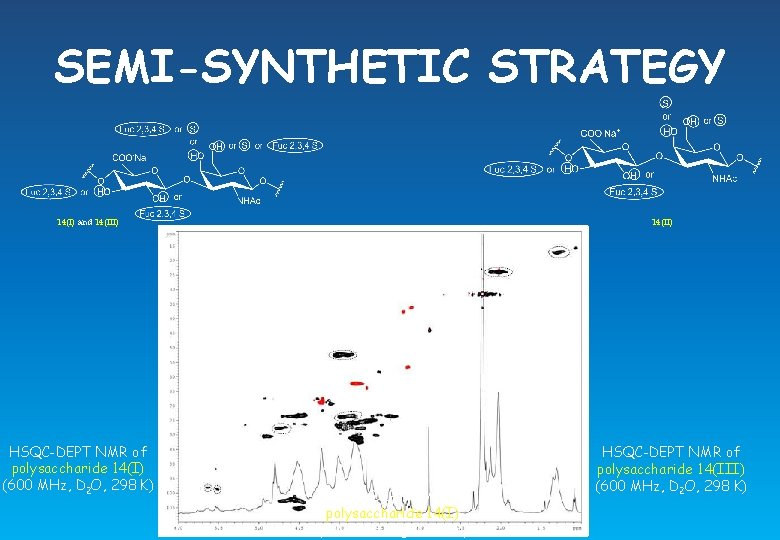
SEMI-SYNTHETIC STRATEGY 14(II) 14(I) and 14(III) HSQC-DEPT NMR of polysaccharide 14(I) (600 MHz, D 2 O, 298 K) HSQC-DEPT NMR of polysaccharide 14(III) (600 MHz, D 2 O, 298 K)

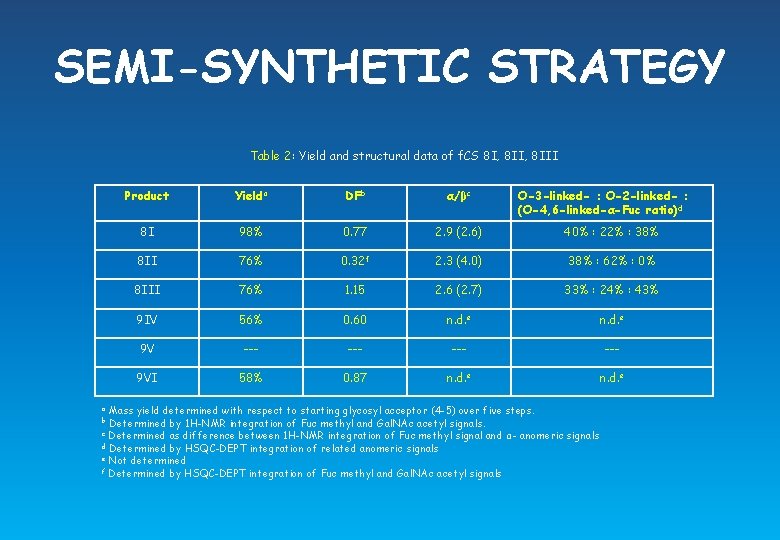
SEMI-SYNTHETIC STRATEGY Table 2: Yield and structural data of f. CS 8 I, 8 III Product Yielda DFb α/βc O-3 -linked- : O-2 -linked- : (O-4, 6 -linked-α-Fuc ratio)d 8 I 98% 0. 77 2. 9 (2. 6) 40% : 22% : 38% 8 II 76% 0. 32 f 2. 3 (4. 0) 38% : 62% : 0% 8 III 76% 1. 15 2. 6 (2. 7) 33% : 24% : 43% 9 IV 56% 0. 60 n. d. e 9 V --- --- 9 VI 58% 0. 87 n. d. e Mass yield determined with respect to starting glycosyl acceptor (4 -5) over five steps. Determined by 1 H-NMR integration of Fuc methyl and Gal. NAc acetyl signals. c Determined as difference between 1 H-NMR integration of Fuc methyl signal and α- anomeric signals d Determined by HSQC-DEPT integration of related anomeric signals e Not determined f Determined by HSQC-DEPT integration of Fuc methyl and Gal. NAc acetyl signals a b

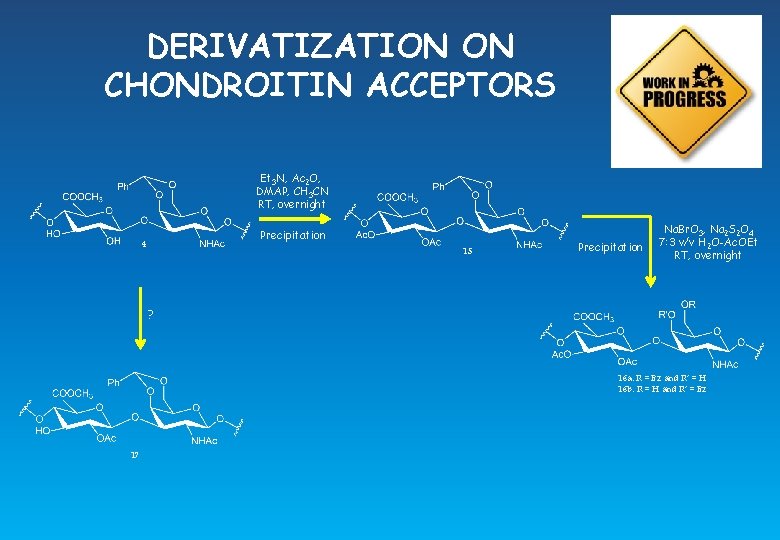
DERIVATIZATION ON CHONDROITIN ACCEPTORS Et 3 N, Ac 2 O, DMAP, CH 3 CN RT, overnight Precipitation 4 15 Precipitation Na. Br. O 3, Na 2 S 2 O 4 7: 3 v/v H 2 O-Ac. OEt RT, overnight ? 16 a: R = Bz and R’ = H 16 b: R = H and R’ = Bz 17

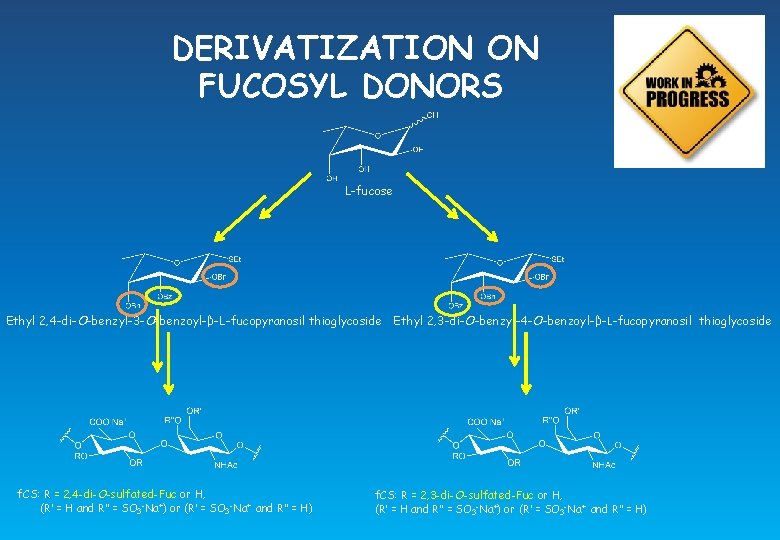
DERIVATIZATION ON FUCOSYL DONORS L-fucose Ethyl 2, 4 -di-O-benzyl-3 -O-benzoyl-β-L-fucopyranosil thioglycoside Ethyl 2, 3 -di-O-benzyl-4 -O-benzoyl-β-L-fucopyranosil thioglycoside f. CS: R = 2, 4 -di-O-sulfated-Fuc or H, (R’ = H and R’’ = SO 3 -Na+) or (R’ = SO 3 -Na+ and R’’ = H) f. CS: R = 2, 3 -di-O-sulfated-Fuc or H, (R’ = H and R’’ = SO 3 -Na+) or (R’ = SO 3 -Na+ and R’’ = H)

CONCLUSIONS First seven-step semi-synthetic strategy from microbial source chondroitin to fucosylated chondroitin sulfate First O-glycosylation of secondary hydroxyls of polysaccharides • HIGH GLOBAL YIELD • CHEAPNESS OF THE USED REAGENTS • RESEMBLANCE WITH NATURAL f. CS

FUTURE PERSPECTIVES Different protection patterns on Fuc donors Different protection patterns on chondroitin intermediates Synthesis of a library of f. CS polysaccharides Anticoagulant tests on f. CS polysaccharides

ACKNOWLEDGMENT University of Naples “Federico II” - Prof. M. Parrilli - Dr. E. Bedini -Prof. A. Iadonisi -Prof. C. De Castro Second University of Naples (SUN) - Prof. M. De Rosa - Prof. C. Schiraldi MIUR L. 297 project “Produzione biotecnologica di condroitina” Bio. Tek. Net


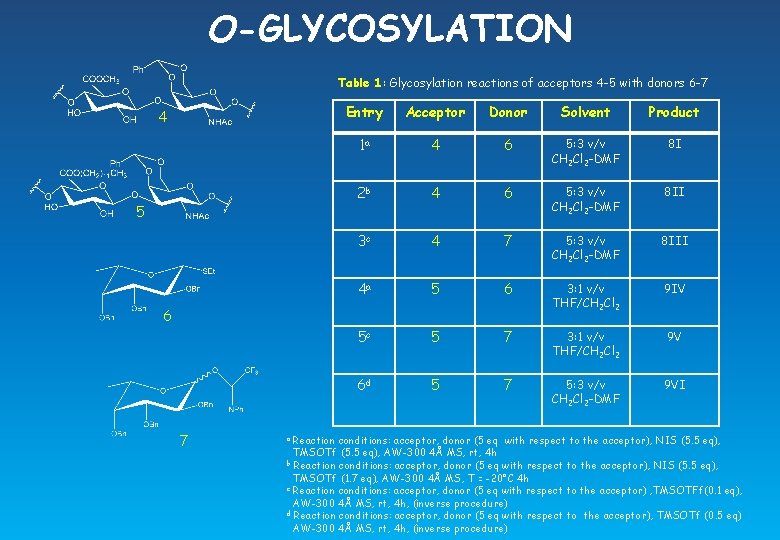
O-GLYCOSYLATION Table 1: Glycosylation reactions of acceptors 4 -5 with donors 6 -7 4 5 Entry Acceptor Donor Solvent Product 1 a 4 6 5: 3 v/v CH 2 Cl 2 -DMF 8 I 2 b 4 6 5: 3 v/v CH 2 Cl 2 -DMF 8 II 3 c 4 7 5: 3 v/v CH 2 Cl 2 -DMF 8 III 4 a 5 6 3: 1 v/v THF/CH 2 Cl 2 9 IV 5 c 5 7 3: 1 v/v THF/CH 2 Cl 2 9 V 6 d 5 7 5: 3 v/v CH 2 Cl 2 -DMF 9 VI 6 7 Reaction conditions: acceptor, donor (5 eq with respect to the acceptor), NIS (5. 5 eq), TMSOTf (5. 5 eq), AW-300 4Å MS, rt, 4 h b Reaction conditions: acceptor, donor (5 eq with respect to the acceptor), NIS (5. 5 eq), TMSOTf (1. 7 eq), AW-300 4Å MS, T = -20°C 4 h c Reaction conditions: acceptor, donor (5 eq with respect to the acceptor) , TMSOTFf(0. 1 eq), AW-300 4Å MS, rt, 4 h, (inverse procedure) d Reaction conditions: acceptor, donor (5 eq with respect to the acceptor), TMSOTf (0. 5 eq) AW-300 4Å MS, rt, 4 h, (inverse procedure) a

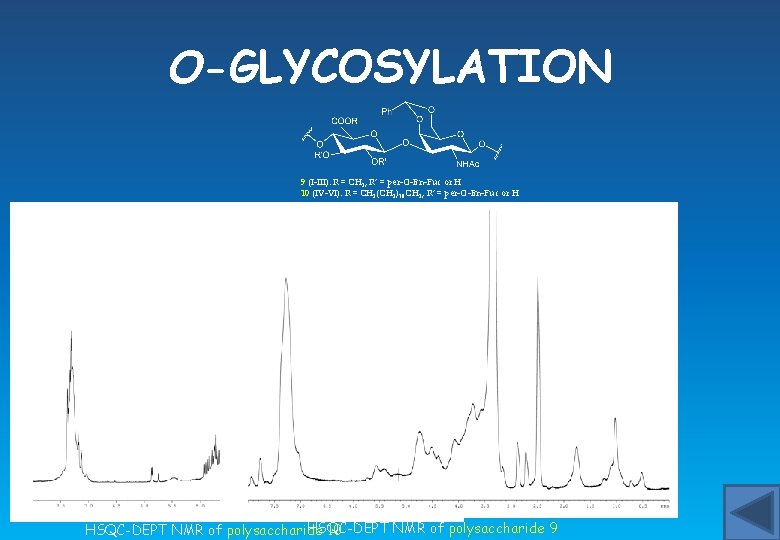
O-GLYCOSYLATION 9 (I-III): R = CH 3, R’ = per-O-Bn-Fuc or H 10 (IV-VI): R = CH 2(CH 2)10 CH 3, R’ = per-O-Bn-Fuc or H HSQC-DEPT NMR of polysaccharide 9 HSQC-DEPT NMR of polysaccharide 10

 Polysaccharides
Polysaccharides Structure of sucrose maltose and lactose
Structure of sucrose maltose and lactose Hexose examples
Hexose examples Monosaccharides disaccharides and polysaccharides
Monosaccharides disaccharides and polysaccharides Storage polysaccharide
Storage polysaccharide Monosaccharides, disaccharides, polysaccharides
Monosaccharides, disaccharides, polysaccharides Polysaccharides
Polysaccharides Oligosaccharides vs polysaccharides
Oligosaccharides vs polysaccharides Barium nitrate and sodium sulfate
Barium nitrate and sodium sulfate Cristal de sulfate de cuivre
Cristal de sulfate de cuivre Sulfate anion test
Sulfate anion test Baruim sulfate
Baruim sulfate Aluminum sulfate and calcium hydroxide
Aluminum sulfate and calcium hydroxide Side effects of magnesium sulfate in pregnancy
Side effects of magnesium sulfate in pregnancy Norisodrine sulfate aerohaler
Norisodrine sulfate aerohaler Dic medical abbreviation
Dic medical abbreviation Ag2scompound name
Ag2scompound name Magnesium sulfate and urine output
Magnesium sulfate and urine output Al+h2so4
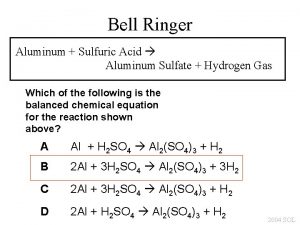
Al+h2so4 Ammonium sulfate precipitation
Ammonium sulfate precipitation Calcium carbonate nitric acid reaction
Calcium carbonate nitric acid reaction