4 1 Studying Atoms Chapter 4 Atomic Structure











- Slides: 23

4. 1 Studying Atoms Chapter 4 Atomic Structure Details of the atom are too small to be seen, but highly magnified images can be produced with a scanning electron microscope

4. 1 Studying Atoms Key Concepts • What was Dalton’s theory of the structure of matter? • What contributions did Thomson and Rutherford make to the development of atomic theory?

4. 1 Studying Atoms Early Ideas About Matter • People have always be interested in the study of matter – Ancient Greek philosophers thought that matter was made up of four fundamental elements… air • ______ fire • ____________ earth • ______ water

4. 1 Studying Atoms • Democritus, who lived from 460 to _______ 370 _____ B. C. was the first person to propose the atoms concept of _____ • “Atomos” – Geek word uncut meaning _____ or indivisible ________ tested • However, he never _____ his ideas

4. 1 Studying Atoms Dalton’s Atomic Theory Dalton • John _______ (1766 -1844), an English schoolteacher and chemist, used experimental ________ evidence to support the existence of atoms

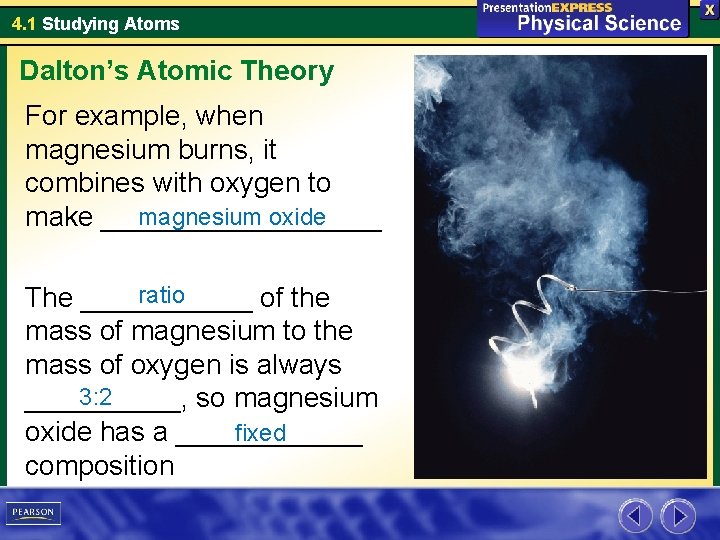
4. 1 Studying Atoms Dalton’s Atomic Theory For example, when magnesium burns, it combines with oxygen to magnesium oxide make _________ ratio The ______ of the mass of magnesium to the mass of oxygen is always 3: 2 _____, so magnesium oxide has a ______ fixed composition

4. 1 Studying Atoms atomic • Dalton proposed his ____________ of matter in 1803. theory • Although his theory has been ______ changed _______ to accommodate new slightly discoveries, Dalton’s theory was so insightful that it has remained essentially intact up to the present time.

4. 1 Studying Atoms • The following statements are the main points of Dalton’s atomic theory. atoms 1. All elements are composed of _______. 2. All atoms of the same element have the ______ mass, and atoms of different same elements have ________ masses different more 3. Compounds contain atoms of ____ than one element Dalton’s Atom 4. In a particular compound, atoms of different elements always combine the ________ same way

4. 1 Studying Atoms The Electron • Because of Dalton’s atomic theory, most scientists in the 1800 s believed that the atom was like a tiny ______ indestructible ball that could not be broken up into parts. • In 1897, a British physicist, J. J. _______, discovered that this solid. Thomson NOT ball model was ____ accurate.

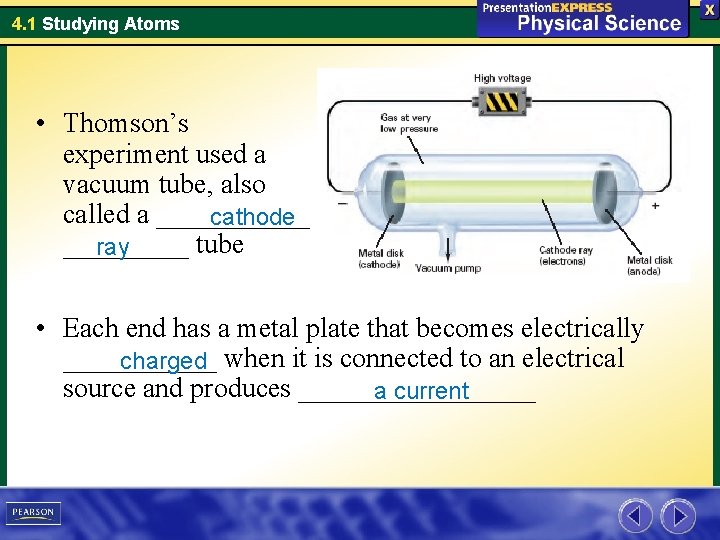
4. 1 Studying Atoms • Thomson’s experiment used a vacuum tube, also called a ______ cathode _____ tube ray • Each end has a metal plate that becomes electrically ______ charged when it is connected to an electrical source and produces _________ a current

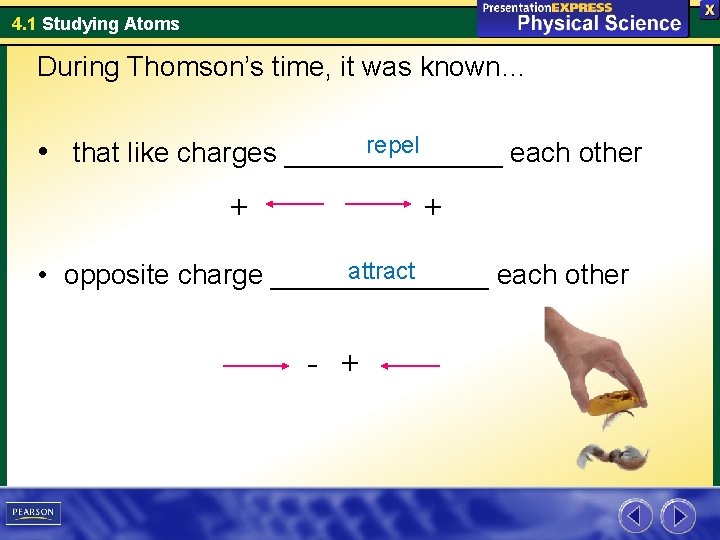
4. 1 Studying Atoms During Thomson’s time, it was known… repel • that like charges _______ each other + + attract • opposite charge _______ each other - +

4. 1 Studying Atoms • When Thomson place the positive end of magnet near the rays, they bent towards ______ the magnet • Are the particles in the rays positive or negative? negative

4. 1 Studying Atoms Cathode-Ray Tube • So Thomson concluded that cathode rays contain negative particles referred to as _________ electrons • Cathode ray tubes were also used to demonstrate the positive existence of _______ particles called protons ________ • Thomson’s new model of the atom included ________ positive and negative charges both

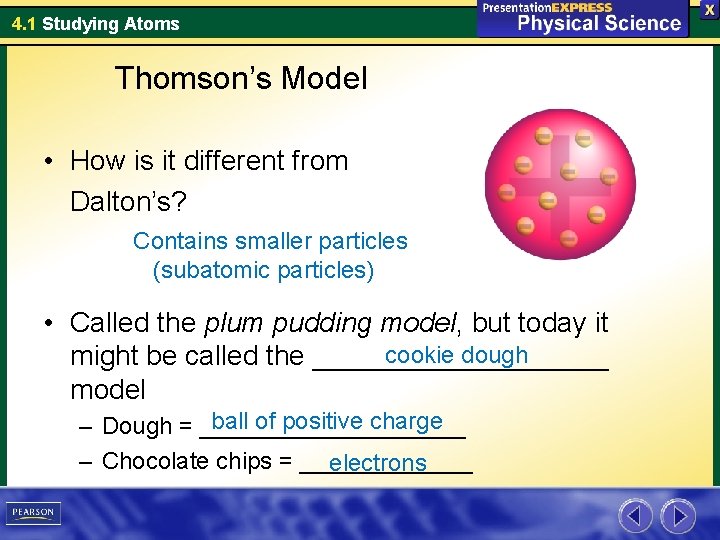
4. 1 Studying Atoms Thomson’s Model • How is it different from Dalton’s? Contains smaller particles (subatomic particles) • Called the plum pudding model, but today it cookie dough might be called the __________ model ball of positive charge – Dough = __________ – Chocolate chips = _______ electrons

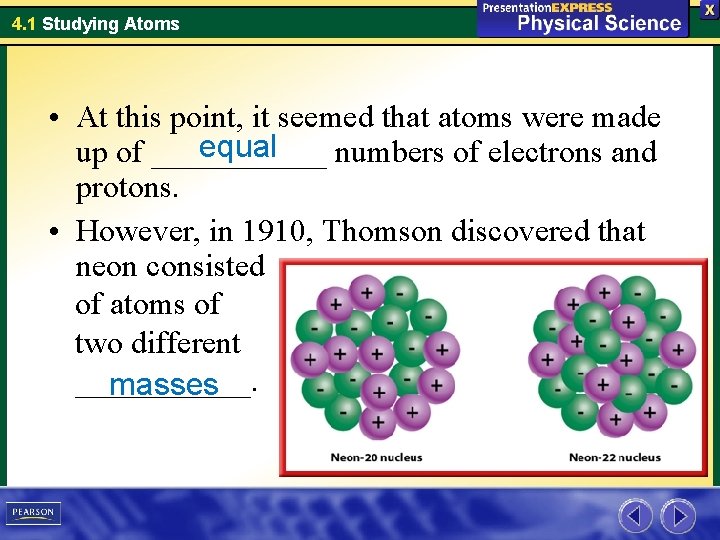
4. 1 Studying Atoms • At this point, it seemed that atoms were made equal up of ______ numbers of electrons and protons. • However, in 1910, Thomson discovered that neon consisted of atoms of two different ______. masses

4. 1 Studying Atoms Isotopes and Neutrons • Atoms of an element that are chemically mass are _______ but differ in ____ alike called _______ isotopes of the element. • This difference in mass was attributed to a third, ________, subatomic particle neutral called the ________ neutron

4. 1 Studying Atoms Rutherford’s Gold Foil Experiment • In 1909, a team of scientists led by Ernest ______ Rutherford set out to determine the arrangement of ________ the atom

4. 1 Studying Atoms • In Rutherford’s ____ gold ____ foil experiment, they used a radioactive polonium element called ______ • Polonium releases radiation in the form of alpha _____ particles, which are ________. positive

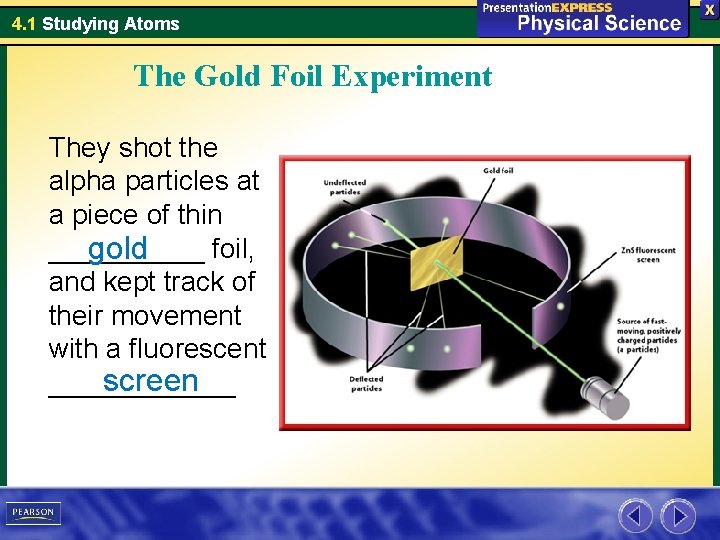
4. 1 Studying Atoms The Gold Foil Experiment They shot the alpha particles at a piece of thin _____ foil, gold and kept track of their movement with a fluorescent screen ______

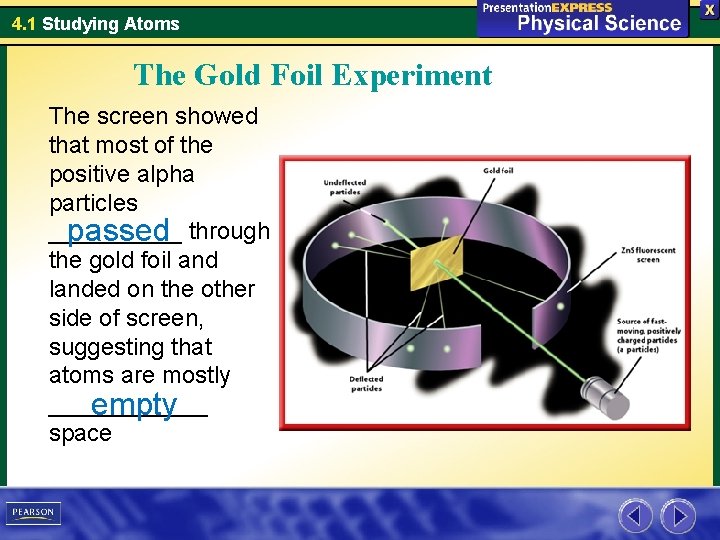
4. 1 Studying Atoms The Gold Foil Experiment The screen showed that most of the positive alpha particles _____ passed through the gold foil and landed on the other side of screen, suggesting that atoms are mostly ______ empty space

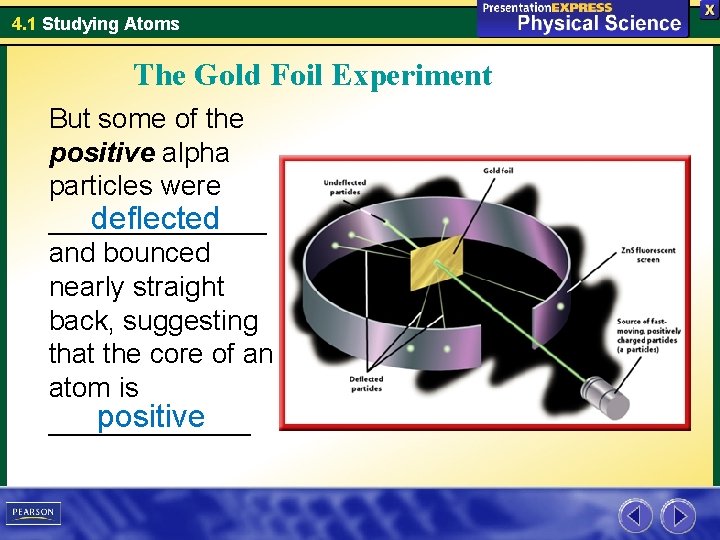
4. 1 Studying Atoms The Gold Foil Experiment But some of the positive alpha particles were deflected _______ and bounced nearly straight back, suggesting that the core of an atom is positive _______

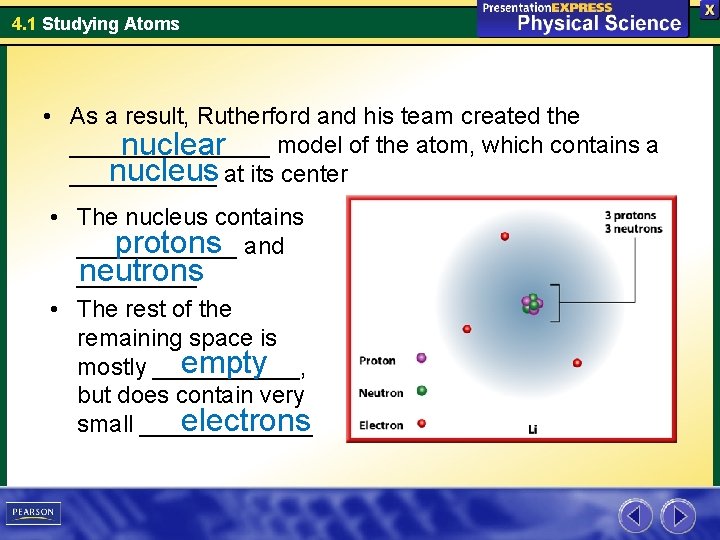
4. 1 Studying Atoms • As a result, Rutherford and his team created the ________ model of the atom, which contains a nuclear nucleus at its center ______ • The nucleus contains protons and ______ neutrons _____ • The rest of the remaining space is empty mostly ______, but does contain very electrons small _______

4. 1 Studying Atoms Review Write down the name of the philosopher or scientist who correctly matches the following descriptions First person to propose the idea of atoms Democritus Thought that the atom was a solid, indivisible, and indestructible sphere Dalton Used a cathode ray tube to demonstrate the existence of electrons Thomson Conducted the gold foil experiment Rutherford