Atomic Structure Chapter 4 4 1 Studying Atoms





- Slides: 9

Atomic Structure Chapter 4

4. 1 Studying Atoms n n n Democritus – believed all matter consisted of extremely small particles that could not be divided Called these particles Atoms = word means “indivisible”

Dalton – existence of Atoms n n Dalton proposed theory that all matter is made up of individual particles called atoms, which cannot be divided 1803 - Atomic Theory of Matter - All elements are composed of atoms - All atoms of the same element have the same mass, and atoms of different elements have different masses - Compounds contain atoms of more than one element - In a particular compound, atoms of different elements always combine in the same way

Oops! n n Many “holes” (errors) were found in his theories But other scientists adjusted/corrected what Dalton had done & went further

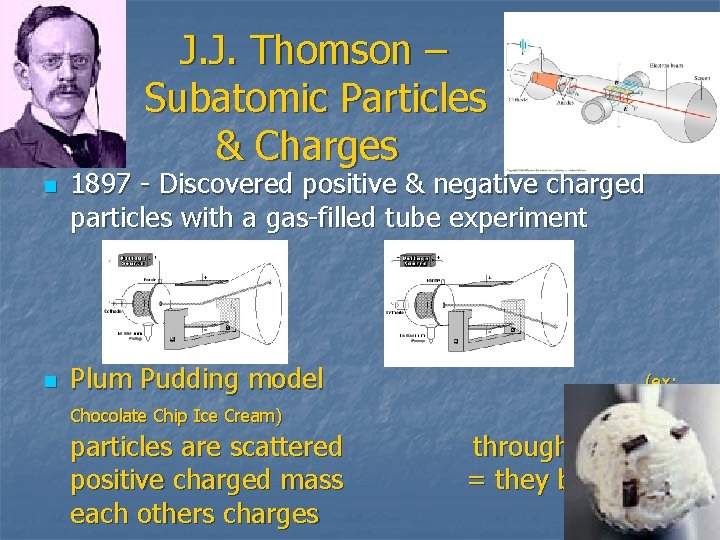
J. J. Thomson – Subatomic Particles & Charges n n 1897 - Discovered positive & negative charged particles with a gas-filled tube experiment Plum Pudding model Chocolate Chip Ice Cream) particles are scattered positive charged mass each others charges (ex: negative throughout the = they balance

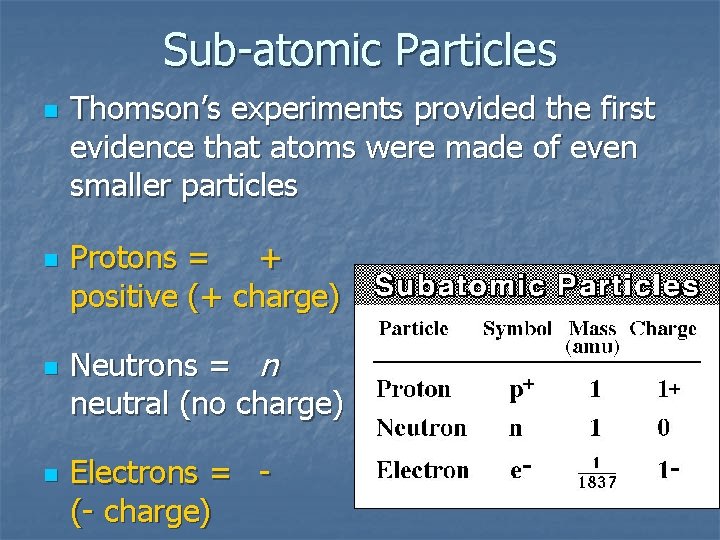
Sub-atomic Particles n n Thomson’s experiments provided the first evidence that atoms were made of even smaller particles Protons = + positive (+ charge) Neutrons = n neutral (no charge) Electrons = (- charge) negative

Rutherford n 1911 - Discovery of the Nucleus n Gold Foil experiment - positive charged of an atom is concentrated in a small central area (the nucleus) n According to Rutherford, all of an atom’s positive charge is concentrated in its nucleus n Model of a large stadium w/ a marble for the nucleus

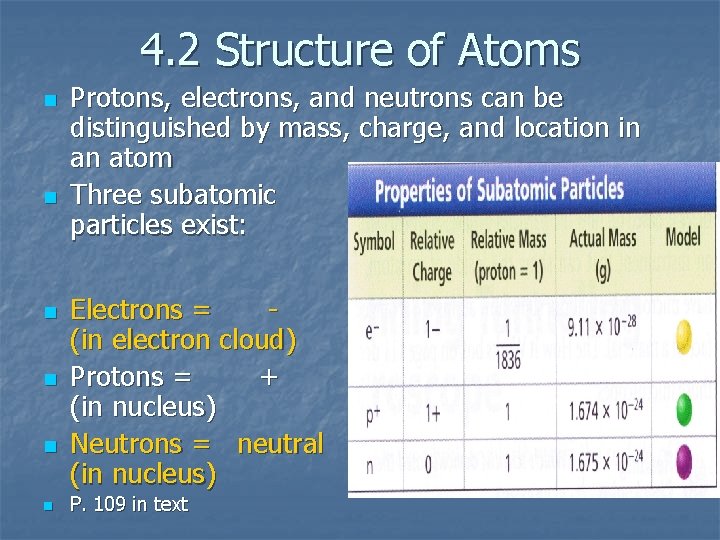
4. 2 Structure of Atoms n n n Protons, electrons, and neutrons can be distinguished by mass, charge, and location in an atom Three subatomic particles exist: Electrons = (in electron cloud) Protons = + (in nucleus) Neutrons = neutral (in nucleus) P. 109 in text

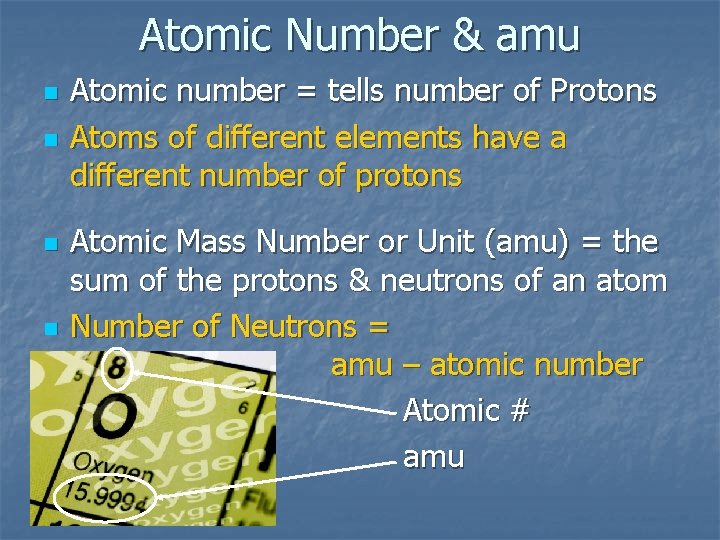
Atomic Number & amu n n n Atomic number = tells number of Protons Atoms of different elements have a different number of protons Atomic Mass Number or Unit (amu) = the sum of the protons & neutrons of an atom Number of Neutrons = amu – atomic number Atomic # amu