33 1 Electric Fields The force field that















































- Slides: 52

33. 1 Electric Fields

The force field that surrounds a mass is a gravitational field. If you throw a ball into the air, it follows a curved path. Earlier chapters showed that it curves because there is an interaction between the ball and Earth— between their centers of gravity, to be exact. Their centers of gravity are quite far apart, so this is "action at a distance. "

The idea that things not in contact could exert forces on one another bothered Isaac Newton and many others. The concept of a force field explains how Earth can exert a force on things without touching them, like a tossed ball. The ball is in contact with the field all the time. The ball curves because it interacts with Earth's gravitational field. You can think of distant space probes as interacting with gravitational fields rather than with the masses of Earth and other astronomical bodies that are responsible for the fields.

Just as the space around Earth and every other mass is filled with a gravitational field, the space around every electric charge is filled with an electric field. An electric field is a force field that surrounds an electric charge or group of charges.

A gravitational force holds a satellite in orbit about a planet, and an electrical force holds an electron in orbit about a proton. In both cases there is no contact between the objects, and the forces are "acting at a distance. "

In terms of the field concept, the satellite and electron interact with the force fields of the planet and the proton and are everywhere in contact with these fields. Just as in the gravitational case, the force that one electric charge exerts on another can be described as the interaction between one charge and the electric field set up by the other.

An electric field has both magnitude and direction. The magnitude (strength) of an electric field can be measured by its effect on charges located in the field. Imagine a small positive "test charge" that is placed in an electric field. Where the force is greatest on the test charge, the field is strongest. Where the force on the test charge is weak, the field is small.

The direction of an electric field at any point, by convention, is the direction of the electrical force on a small positive test charge placed at that point. Thus, if the charge that sets up the field is positive, the field points away from that charge. If the charge that sets up the field is negative, the field points toward that charge. (Be sure to distinguish between the hypothetical small test charge and the charge that sets up the CONCEPTHow are the magnitude and direction of an electric field. ) determined?

33. 2 Electric Field Lines Since an electric field has both magnitude and direction, it is a vector quantity and can be represented by vectors. The negatively charged particle in Figure 17 -15 b is surrounded by vectors that point toward the particle. (If the particle were positively charged, the, vectors would point away from the particle. The vectors always point in the direction of the force that would act on a positive test charge. )

The magnitude of the field is indicated by the length of the vectors. The electric field is greater where the vectors are long than it is where the vectors are short. To represent a complete electric field by vectors, you would have to show a vector at every point in the space around the charge. Such a diagram would be totally unreadable!

You can use electric field lines (also called lines of force) to represent an electric field. Where the lines are farther apart, the field is weaker. For an isolated charge, the lines extend to infinity, while for two or more opposite charges, the lines emanate from a positive charge and terminate on a negative charge. Some electric field configurations are shown in Figure 17 -16 through 17 -18.

The photographs in Figure 17 -16 through 17 -18. show bits of thread that are suspended in an oil bath surrounding charged conductors. The ends of the bits of thread line up end-to-end with the electric field lines. In Figures 17 -16 and 17 -17, we see the field lines are characteristic of a single pair of point charges.

Refer to Figure 17 -18 In this case, only half the lines originating from the positive charge terminate on the negative charge because the positive chare is twice as great as the negative charge. CONCEPT; How can you represent an electric field?

33. 3 Electric Shielding When a car being struck by lightning. The occupant inside the car is completely safe. This is because the electrons that shower down upon the car are mutually repelled and spread over the outer metal surface, finally discharging when additional sparks jump from the car's body to the ground.

The configuration of electrons on the car's surface at any moment is such that the electric fields inside the car practically cancel to zero. This is true of any charged conductor. If the charge on a conductor is not moving, the electric field inside the conductor is exactly zero.

Charged Conductors The absence of electric field within a conductor holding static charge does not arise from the inability of an electric field to penetrate metals. It comes about because free electrons within the conductor can "settle down" and stop moving only when the electric field is zero. So the charges arrange themselves to ensure a zero field with the material. .

Consider the charged metal sphere shown in Figure 17. 21. Because of mutual repulsion, the electrons spread as far apart from one another as possible. They distribute themselves uniformly over the surface of the sphere. A positive test charge located exactly in the middle of the sphere would feel no force.

The electrons on the left side of the sphere would tend to pull the test charge to the left, but the electrons on the right side of the sphere would tend to pull the test charge to the right equally hard. The net force on the test charge would be zero. Thus, the electric field is also zero. Interestingly enough, complete cancellation will occur anywhere inside the sphere

What is the formula for the compound formed by aluminum(III) and the sulfate ion, SO 42–? a. Al. SO 4 b. Al 2 SO 4 c. Al 2(SO 4)3 d. Al(SO 4)3 c

If the conductor is not spherical, then the charge distribution will not be uniform. The remarkable thing is this: The exact charge distribution over the surface is such that the electric field everywhere inside the conductor is zero. Look at it this way: If there were an electric field inside a conductor, then free electrons inside the conductor would be set in motion. How far would they move? Until equilibrium is established, which is to say, when the positions of all the electrons produce a zero field inside the conductor.

How to Shield an Electric Field There is no way to shield gravity, because gravity only attracts. There are no repelling parts of gravity to offset attracting parts. Shielding electric fields, however, is quite simple. Surround yourself or whatever you wish to shield with a conducting surface. Put this surface in an electric field of whatever field strength. field within a conductor holding static charge?

The free charges in the conducting surface will arrange themselves on the surface of the conductor in a way such that all field contributions inside cancel one another. That's why certain electronic components are encased in metal boxes, and why certain cables have a metal covering—to shield them from all outside electrical activity. CONCEPT- How can you describe the electric

33. 4 Electrical Potential Energy Recall the relationship between work and potential energy. Work is done when a force moves something in the direction of the force. An object has potential energy by virtue of its location, say in a force field. For example, if you lift an object, you apply a force equal to its weight. When you raise it through some distance, you are doing work on the object.

You are also increasing its gravitational potential energy. The greater the distance it is raised, the greater is the increase in its gravitational potential energy. Doing work increases its gravitational potential energy.

What is the metallic ion in the compound Cu. Cl 2? a. Cu 2– b. Cu– c. Cu+ d. Cu 2+ d

The indium(II) ion and indium(III) ion a. have the same charge. b. are polyatomic ions. c. have charges of 1+ and 2+, respectively. d. have charges of 2+ and 3+, respectively. d

What is the ratio of cations to anions in a compound composed of sodium ions, Na+, and carbonate ions, ? a. 1 to 1 b. 1 to 2 c. 2 to 1 d. 3 to 1 c

A compound that has the same number of positive and negative charges is said to be a. cationic. b. anionic. c. electroneutral. d. isoelectronic. c

An ion and its parent atom have the same a. electron configuration. b. number of charges c. atomic number. d. chemical reactivity. c

Once an atom has full s and p orbitals in its outermost energy level, a. it is highly reactive only with alkali metals. b. it is highly reactive only with halogens. c. it can be combined with most elements. d. it has a stable octet and is unreactive. d

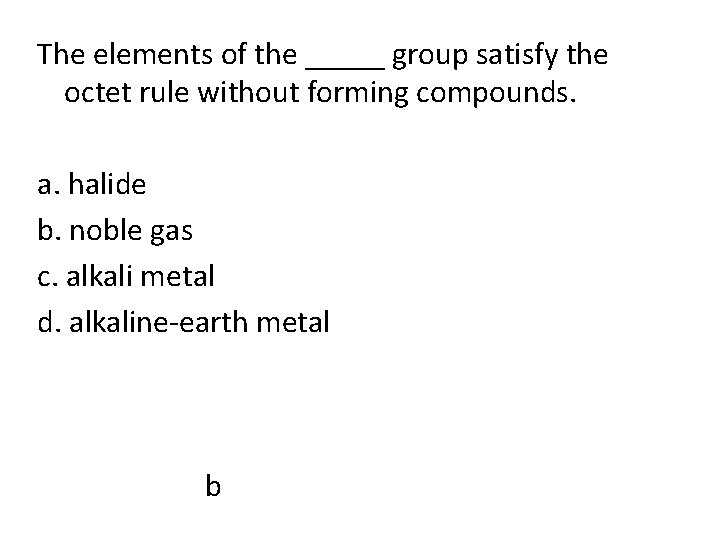
The elements of the _____ group satisfy the octet rule without forming compounds. a. halide b. noble gas c. alkali metal d. alkaline-earth metal b

An anion a. is an ion with a negative charge. b. attracts ions with negative charges. c. results when an alkaline-earth metal loses one of its two outermost electrons. d. has more protons than electrons. a

Atoms of copper and iron a. generally form stable bonds with transition elements. b. have stable electron configurations. c. tend to form cations. d. tend to form anions. c

The electron configuration of nitrogen is 1 s 22 p 3. How many more electrons does nitrogen need to have an electron configuration similar to neon? a. 1 b. 3 c. 5 d. 8 b

In the compound sodium fluoride, Na. F, the sodium atom loses one electron and the fluorine atom gains one electron to form ions that have electron configurations similar to a. helium. b. oxygen. c. neon. d. calcium. c

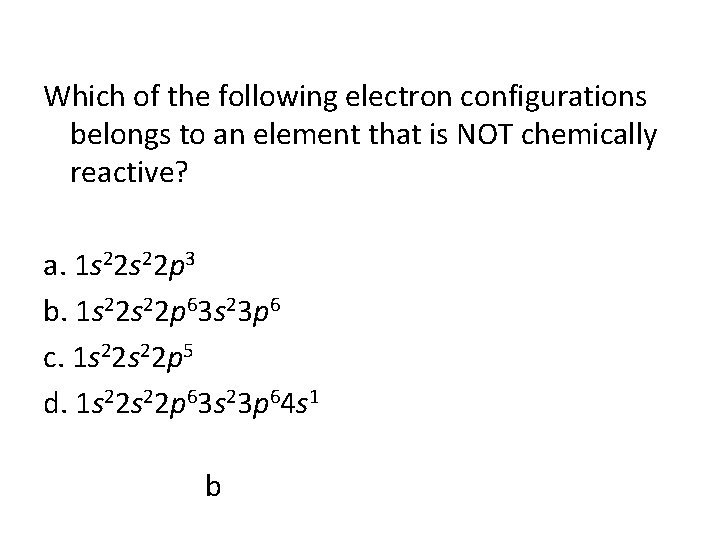
Which of the following electron configurations belongs to an element that is NOT chemically reactive? a. 1 s 22 p 3 b. 1 s 22 p 63 s 23 p 6 c. 1 s 22 p 5 d. 1 s 22 p 63 s 23 p 64 s 1 b

Manganese has the electron configuration, 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 5. Which of the following forms is most likely that of the ion? a. Mn 5_ b. Mn 2_ c. Mn 2+ d. Mn 6_ b

According to the octet rule, a calcium atom has a tendency to a. lose one electron. b. lose two electrons. c. gain one electron. d. gain two electrons. b

The correct name for the salt with the formula Ca. I 2 is a. calcium diodide. b. calcium iodine. c. calcium iodide. d. dicalcium iodine. c

The pair of ions listed below with similar chemical properties is a. F- and Cl-. b. F- and Li+. c. Na+ and Cl-. d. Na+ and F-. a

How many electrons does Barium have in its outermost shell? a. 1 b. 2 c. 3 d. 4 b

Which of the following elements is a transition metal? a. calcium b. iron c. sodium d. sulfur b

The alkali metal elements are found in of the periodic table. a. Group 1 b. Group 2 c. Period 1 d. Period 2 a

How many electrons does carbon have in its outermost shell? a. 1 b. 2 c. 3 d. 4 d

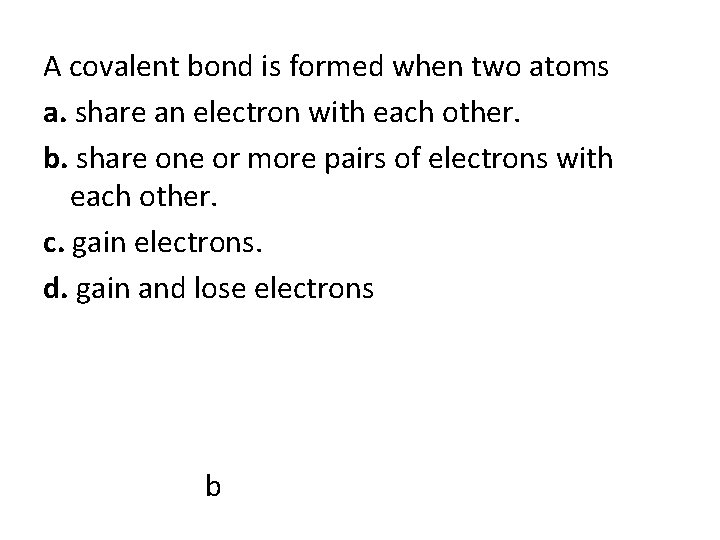
A covalent bond is formed when two atoms a. share an electron with each other. b. share one or more pairs of electrons with each other. c. gain electrons. d. gain and lose electrons b

A polyatomic anion consists of a. a single atom with a negative charge. b. a single atom with a positive charge. c. multiple atoms with an overall negative charge. d. multiple atoms with an overall positive charge c

In the word equation sodium chloride → chlorine gas + sodium a. sodium is a product. b. chlorine gas is a reactant. c. sodium chloride is a product. d. None of the above a

The formation of a solid product in a chemical reaction is represented by the symbol a. (aq). b. (g). c. (l). d. (s). d

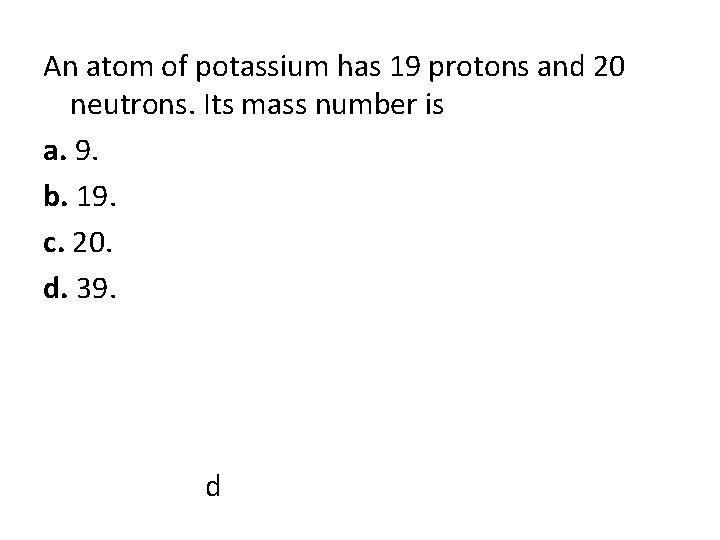
An atom of potassium has 19 protons and 20 neutrons. Its mass number is a. 9. b. 19. c. 20. d. 39. d

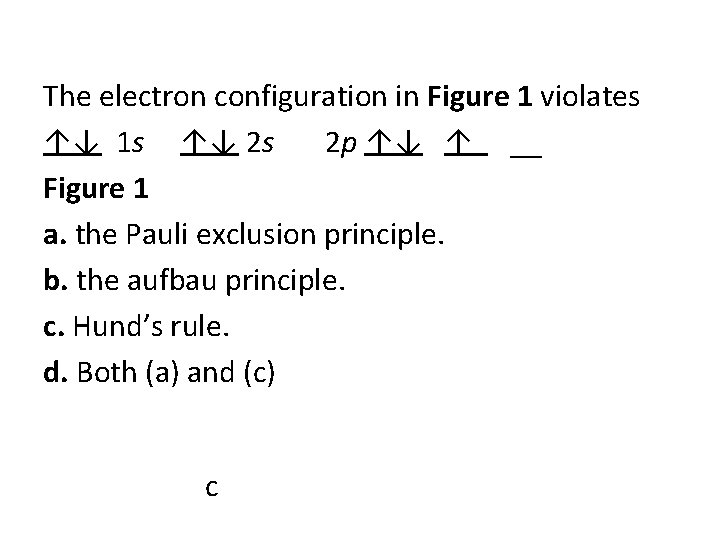
The electron configuration in Figure 1 violates ↑↓ 1 s ↑↓ 2 s 2 p ↑↓ ↑ __ Figure 1 a. the Pauli exclusion principle. b. the aufbau principle. c. Hund’s rule. d. Both (a) and (c) c

c

c