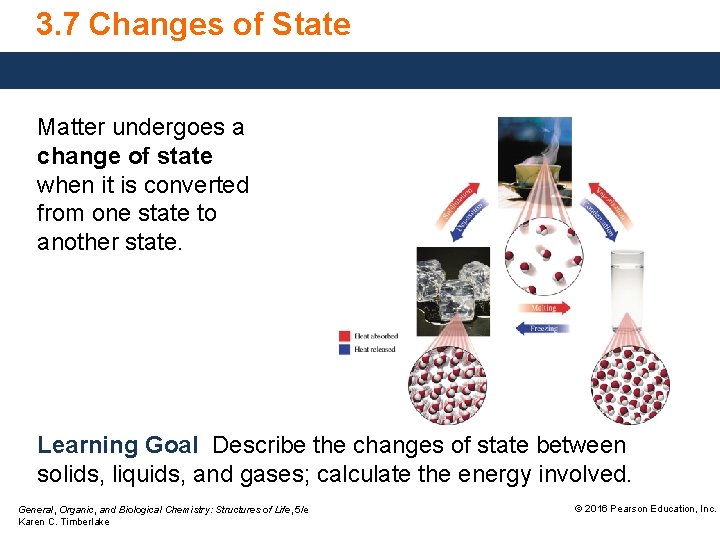
3 7 Changes of State Matter undergoes a
- Slides: 37

3. 7 Changes of State Matter undergoes a change of state when it is converted from one state to another state. Learning Goal Describe the changes of state between solids, liquids, and gases; calculate the energy involved. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

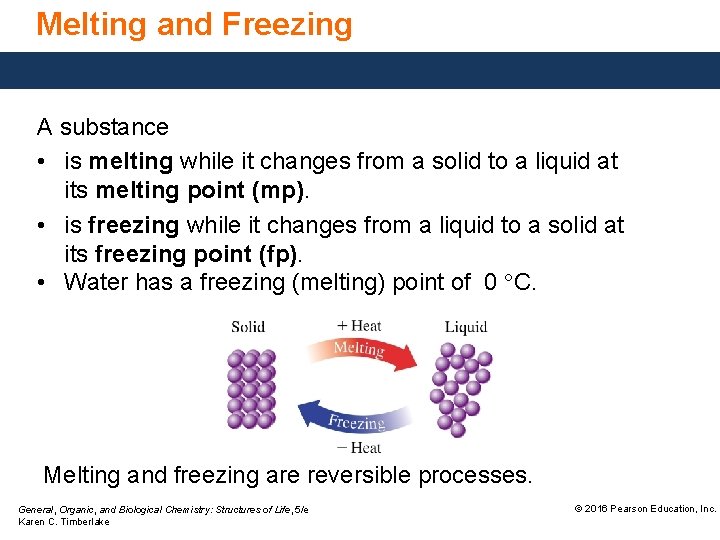
Melting and Freezing A substance • is melting while it changes from a solid to a liquid at its melting point (mp). • is freezing while it changes from a liquid to a solid at its freezing point (fp). • Water has a freezing (melting) point of 0 °C. Melting and freezing are reversible processes. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Heat of Fusion The heat of fusion • is the amount of heat released when 1 g of liquid freezes (at its freezing point). • is the amount of heat needed to melt 1 g of solid (at its melting point). For a given amount of substance, heat released during freezing = heat needed during melting General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

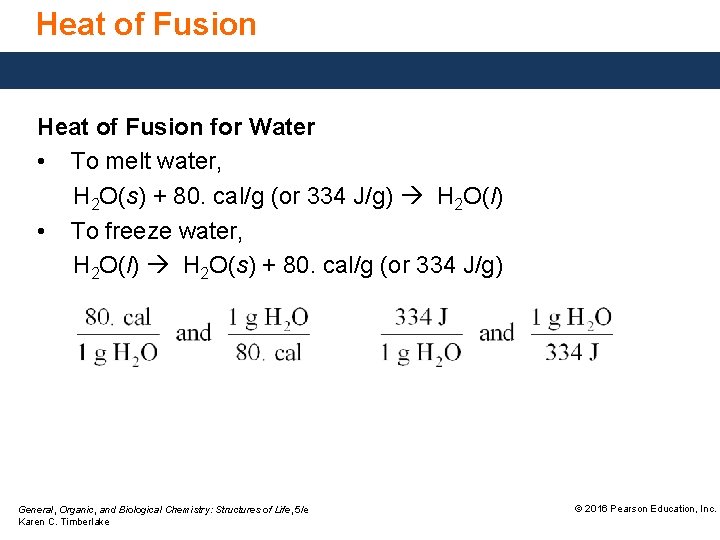
Heat of Fusion for Water • To melt water, H 2 O(s) + 80. cal/g (or 334 J/g) H 2 O(l) • To freeze water, H 2 O(l) H 2 O(s) + 80. cal/g (or 334 J/g) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

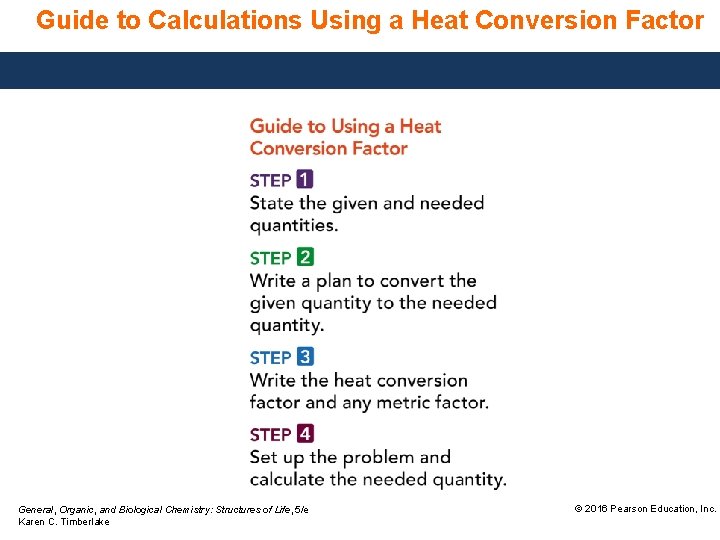
Guide to Calculations Using a Heat Conversion Factor General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

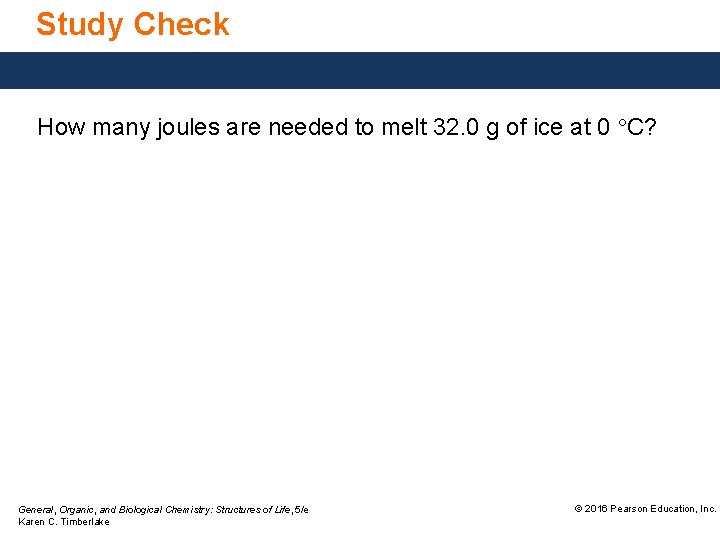
Study Check How many joules are needed to melt 32. 0 g of ice at 0 °C? General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

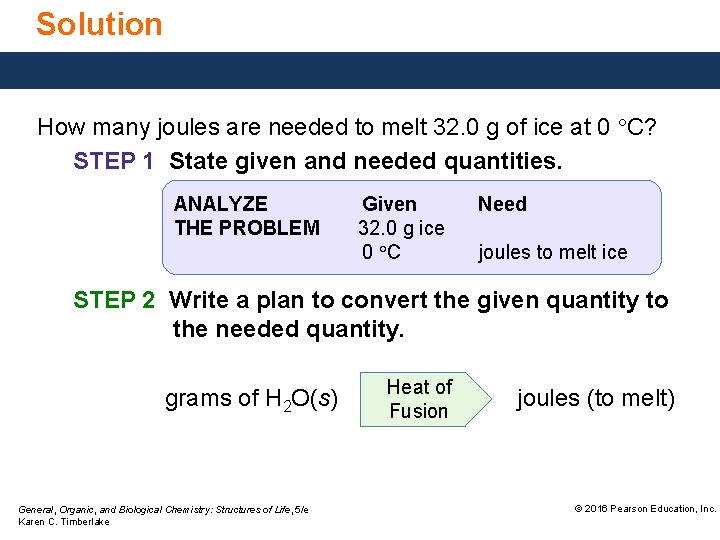
Solution How many joules are needed to melt 32. 0 g of ice at 0 °C? STEP 1 State given and needed quantities. ANALYZE THE PROBLEM Given 32. 0 g ice 0 °C Need joules to melt ice STEP 2 Write a plan to convert the given quantity to the needed quantity. grams of H 2 O(s) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Heat of Fusion joules (to melt) © 2016 Pearson Education, Inc.

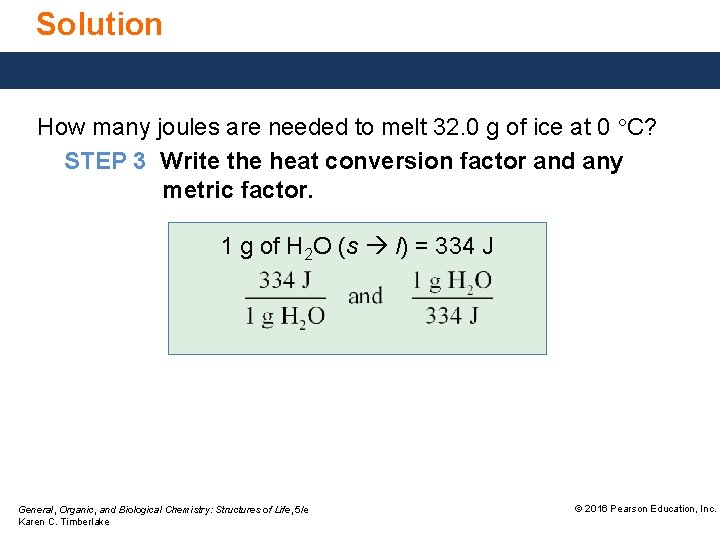
Solution How many joules are needed to melt 32. 0 g of ice at 0 °C? STEP 3 Write the heat conversion factor and any metric factor. 1 g of H 2 O (s l) = 334 J General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

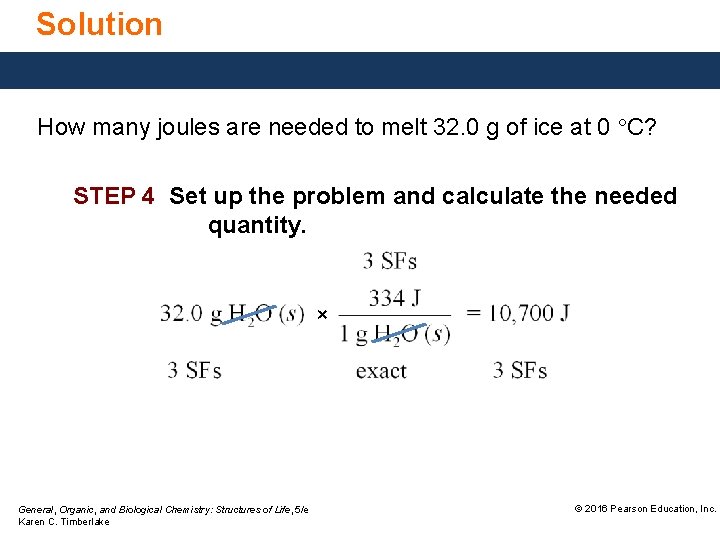
Solution How many joules are needed to melt 32. 0 g of ice at 0 °C? STEP 4 Set up the problem and calculate the needed quantity. × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

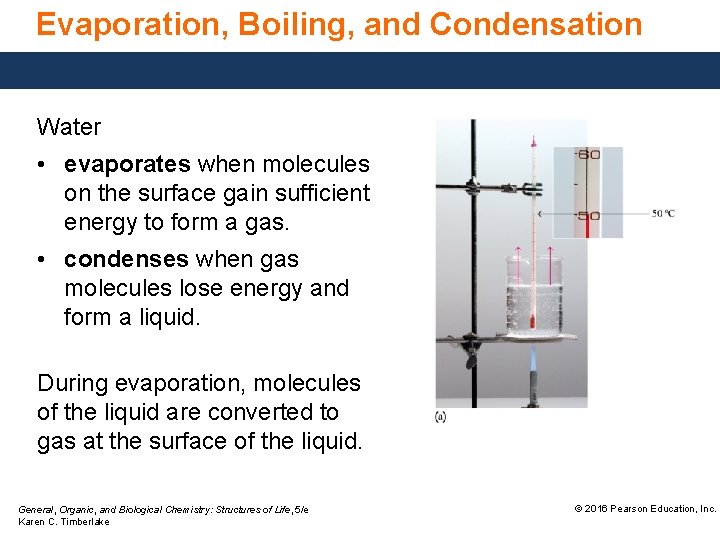
Evaporation, Boiling, and Condensation Water • evaporates when molecules on the surface gain sufficient energy to form a gas. • condenses when gas molecules lose energy and form a liquid. During evaporation, molecules of the liquid are converted to gas at the surface of the liquid. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

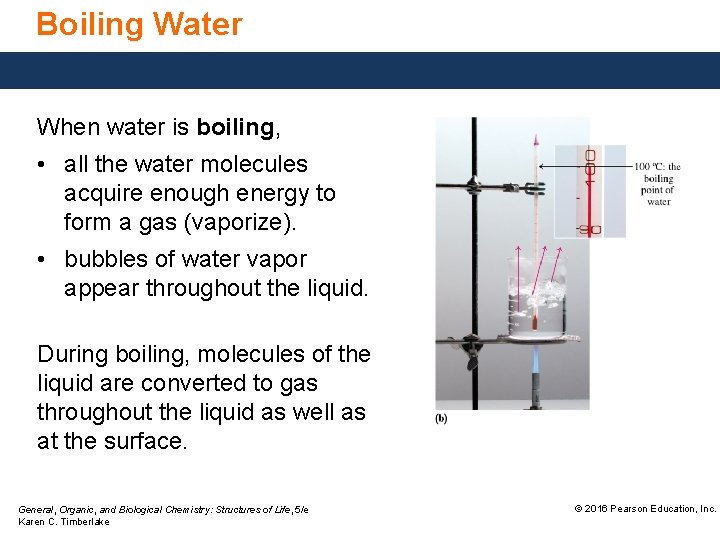
Boiling Water When water is boiling, • all the water molecules acquire enough energy to form a gas (vaporize). • bubbles of water vapor appear throughout the liquid. During boiling, molecules of the liquid are converted to gas throughout the liquid as well as at the surface. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

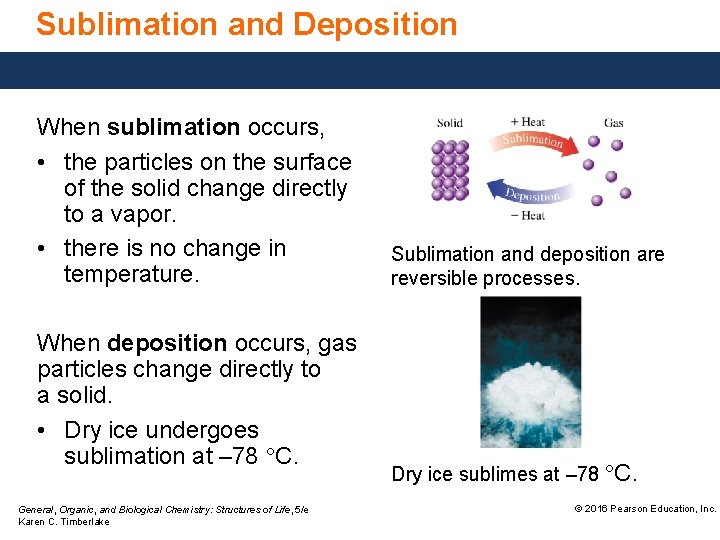
Sublimation and Deposition When sublimation occurs, • the particles on the surface of the solid change directly to a vapor. • there is no change in temperature. When deposition occurs, gas particles change directly to a solid. • Dry ice undergoes sublimation at – 78 °C. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Sublimation and deposition are reversible processes. Dry ice sublimes at – 78 °C. © 2016 Pearson Education, Inc.

Sublimation and Deposition Sublimation is used to prepare freeze-dried foods for long -term storage. Water vapor will change to solid on contact with a cold surface. Freeze-dried foods have a long shelf life because they contain no water. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

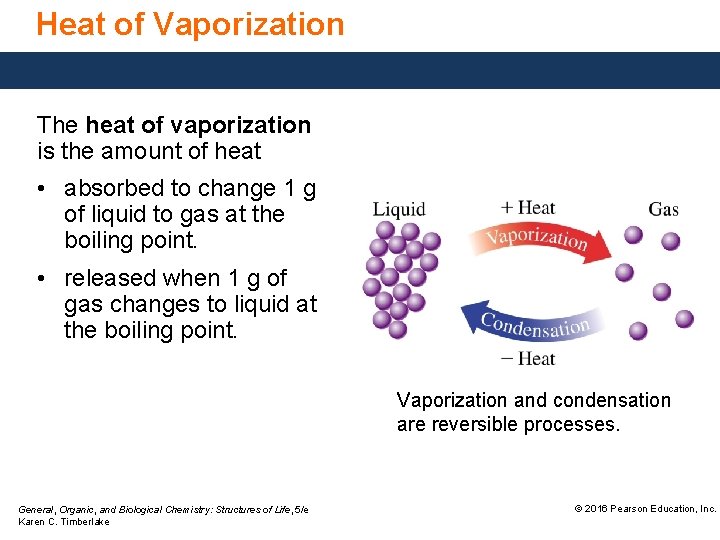
Heat of Vaporization The heat of vaporization is the amount of heat • absorbed to change 1 g of liquid to gas at the boiling point. • released when 1 g of gas changes to liquid at the boiling point. Vaporization and condensation are reversible processes. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

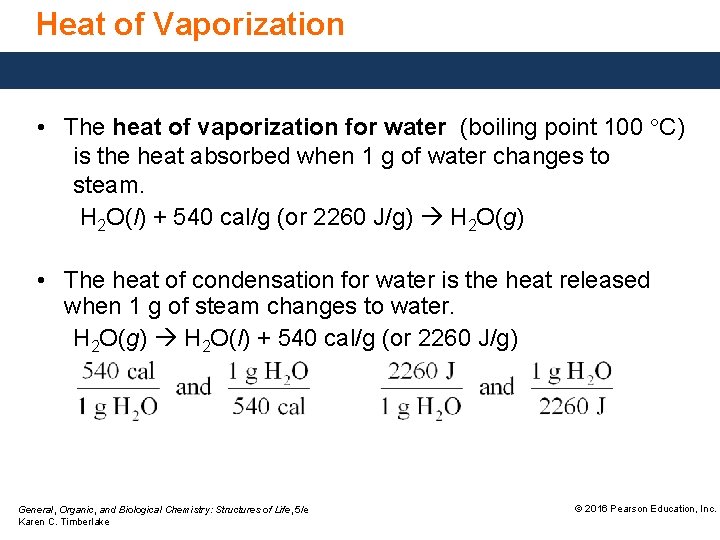
Heat of Vaporization • The heat of vaporization for water (boiling point 100 °C) is the heat absorbed when 1 g of water changes to steam. H 2 O(l) + 540 cal/g (or 2260 J/g) H 2 O(g) • The heat of condensation for water is the heat released when 1 g of steam changes to water. H 2 O(g) H 2 O(l) + 540 cal/g (or 2260 J/g) General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Study Check How many kilojoules (k. J) are released when 50. 0 g of steam from a volcano condenses at 100 °C? General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

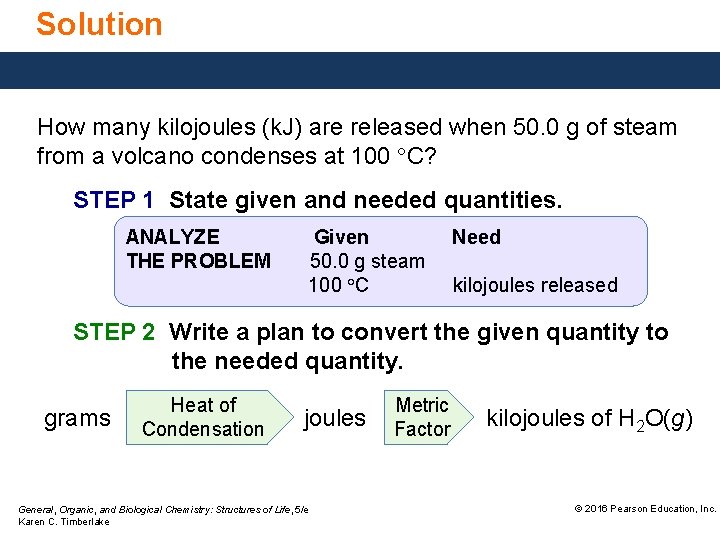
Solution How many kilojoules (k. J) are released when 50. 0 g of steam from a volcano condenses at 100 °C? STEP 1 State given and needed quantities. ANALYZE THE PROBLEM Given 50. 0 g steam 100 °C Need kilojoules released STEP 2 Write a plan to convert the given quantity to the needed quantity. grams Heat of Condensation joules General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Metric Factor kilojoules of H 2 O(g) © 2016 Pearson Education, Inc.

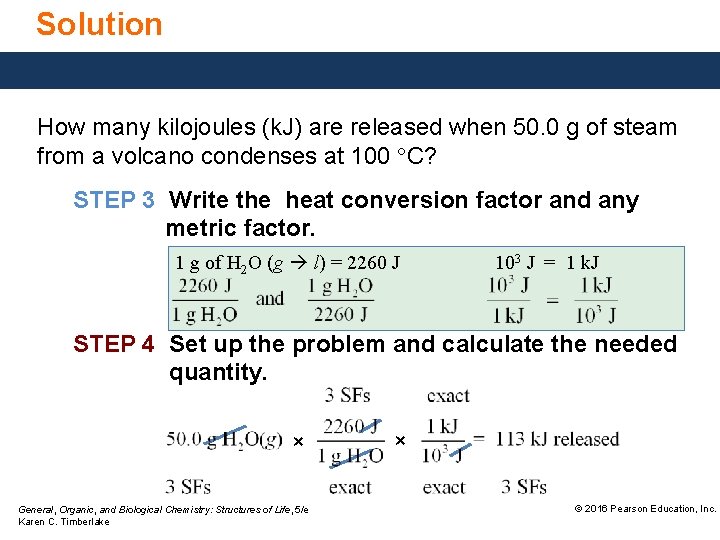
Solution How many kilojoules (k. J) are released when 50. 0 g of steam from a volcano condenses at 100 °C? STEP 3 Write the heat conversion factor and any metric factor. 1 g of H 2 O (g l) = 2260 J 103 J = 1 k. J STEP 4 Set up the problem and calculate the needed quantity. × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake × © 2016 Pearson Education, Inc.

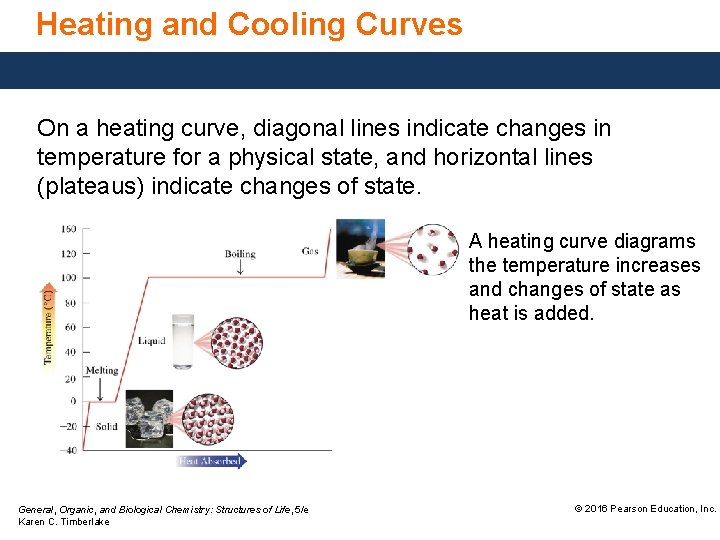
Heating and Cooling Curves On a heating curve, diagonal lines indicate changes in temperature for a physical state, and horizontal lines (plateaus) indicate changes of state. A heating curve diagrams the temperature increases and changes of state as heat is added. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Study Check 1. A plateau (horizontal line) on a heating curve represents A. a temperature change. B. a constant temperature. C. a change of state. 2. A sloped line on a heating curve represents A. a temperature change. B. a constant temperature. C. a change of state. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution 1. A plateau (horizontal line) on a heating curve represents B. a constant temperature. C. a change of state. 2. A sloped line on a heating curve represents A. a temperature change. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

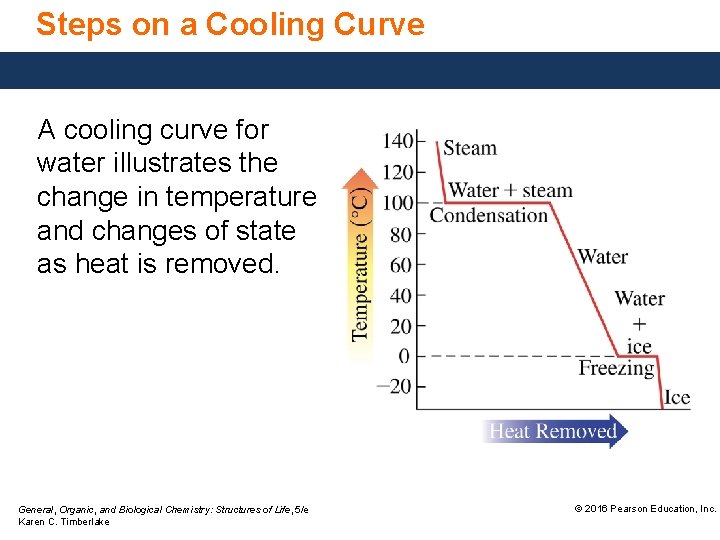
Study Check Use the cooling curve for water to answer each of the following: 1. Water condenses at a temperature of A. 0 °C. B. 50 °C. C. 100 °C. 2. At a temperature of 0 °C, liquid water A. freezes. B. melts. C. changes to a gas. 3. At 40 °C, water is a A. solid. B. liquid. C. gas. 4. When water freezes, heat is A. removed. B. added. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Solution Use the cooling curve for water to answer each of the following: 1. Water condenses at a temperature of C. 100 °C. 2. At a temperature of 0 °C, liquid water A. freezes. 3. At 40 °C, water is a B. liquid. 4. When water freezes, heat is A. removed. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

Steps on a Cooling Curve A cooling curve for water illustrates the change in temperature and changes of state as heat is removed. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

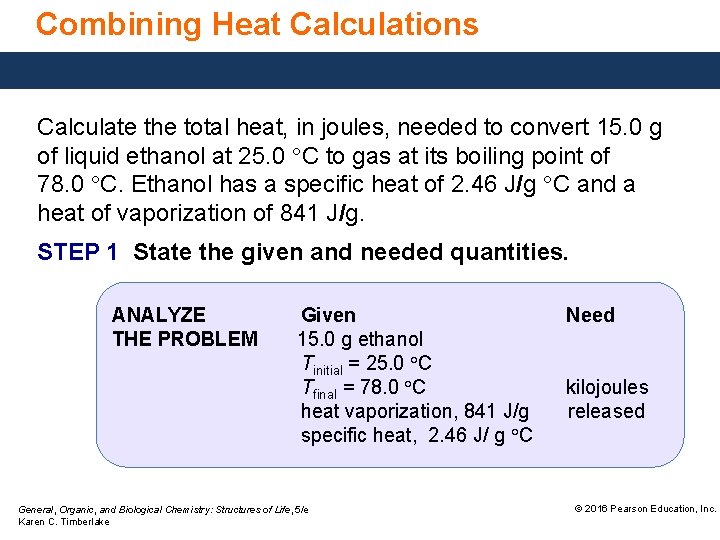
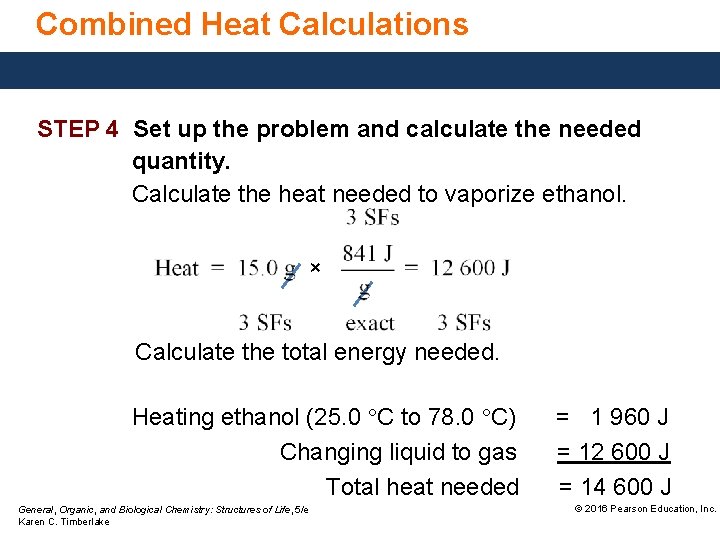
Combining Heat Calculations Calculate the total heat, in joules, needed to convert 15. 0 g of liquid ethanol at 25. 0 °C to gas at its boiling point of 78. 0 °C. Ethanol has a specific heat of 2. 46 J/g °C and a heat of vaporization of 841 J/g. STEP 1 State the given and needed quantities. ANALYZE THE PROBLEM Given 15. 0 g ethanol Tinitial = 25. 0 °C Tfinal = 78. 0 °C heat vaporization, 841 J/g specific heat, 2. 46 J/ g °C General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Need kilojoules released © 2016 Pearson Education, Inc.

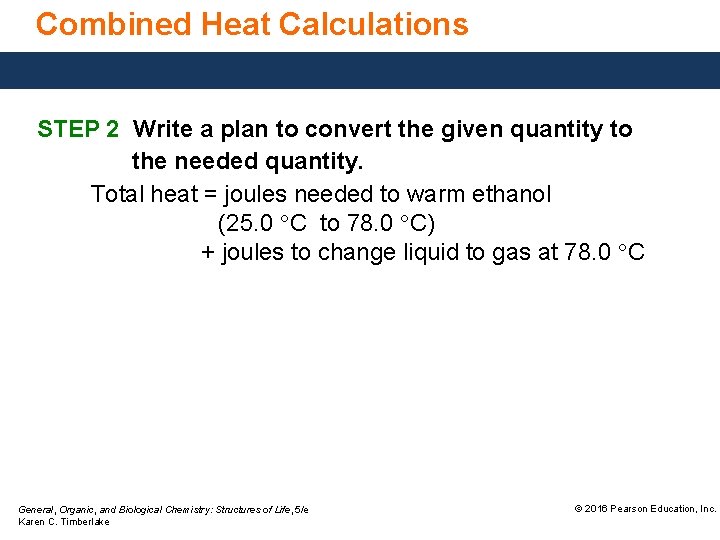
Combined Heat Calculations STEP 2 Write a plan to convert the given quantity to the needed quantity. Total heat = joules needed to warm ethanol (25. 0 °C to 78. 0 °C) + joules to change liquid to gas at 78. 0 °C General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

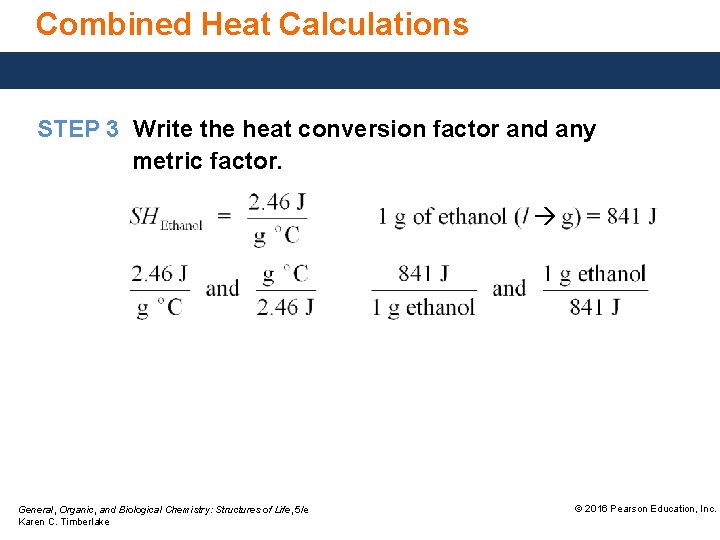
Combined Heat Calculations STEP 3 Write the heat conversion factor and any metric factor. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

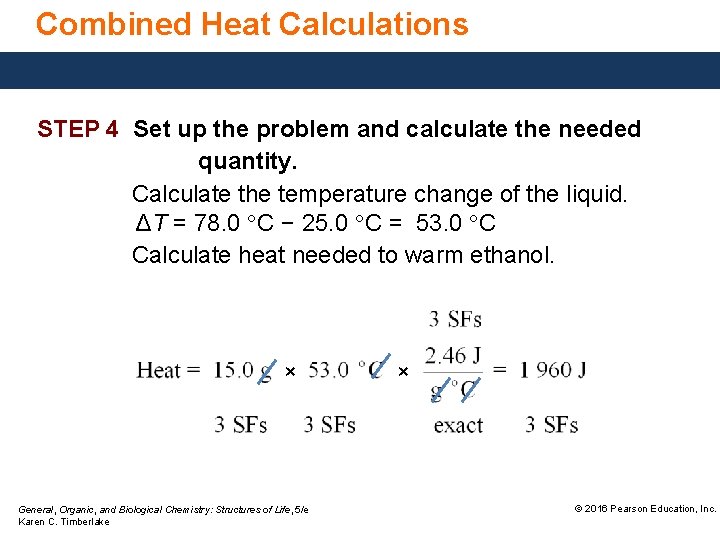
Combined Heat Calculations STEP 4 Set up the problem and calculate the needed quantity. Calculate the temperature change of the liquid. ΔT = 78. 0 °C − 25. 0 °C = 53. 0 °C Calculate heat needed to warm ethanol. × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake × © 2016 Pearson Education, Inc.

Combined Heat Calculations STEP 4 Set up the problem and calculate the needed quantity. Calculate the heat needed to vaporize ethanol. × Calculate the total energy needed. Heating ethanol (25. 0 °C to 78. 0 °C) Changing liquid to gas Total heat needed General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake = 1 960 J = 12 600 J = 14 600 J © 2016 Pearson Education, Inc.

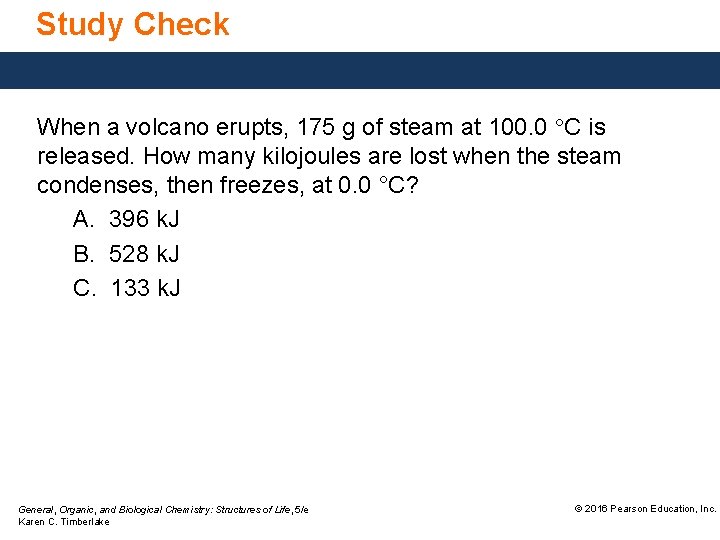
Study Check When a volcano erupts, 175 g of steam at 100. 0 °C is released. How many kilojoules are lost when the steam condenses, then freezes, at 0. 0 °C? A. 396 k. J B. 528 k. J C. 133 k. J General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

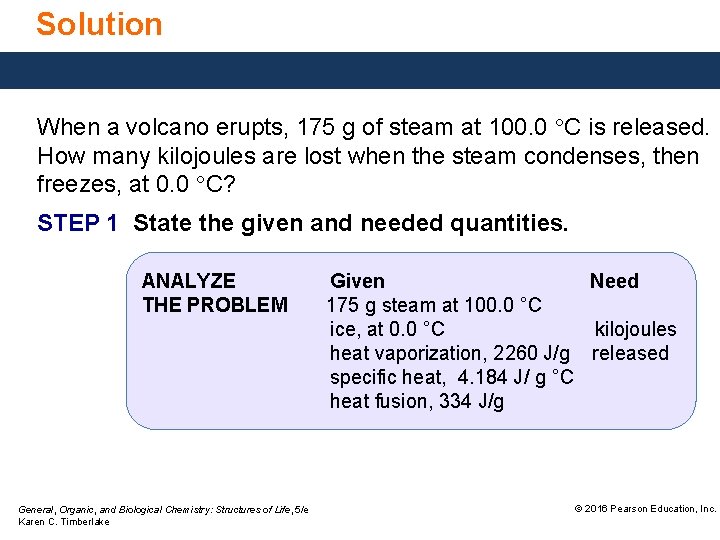
Solution When a volcano erupts, 175 g of steam at 100. 0 °C is released. How many kilojoules are lost when the steam condenses, then freezes, at 0. 0 °C? STEP 1 State the given and needed quantities. ANALYZE THE PROBLEM General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Given Need 175 g steam at 100. 0 °C ice, at 0. 0 °C kilojoules heat vaporization, 2260 J/g released specific heat, 4. 184 J/ g °C heat fusion, 334 J/g © 2016 Pearson Education, Inc.

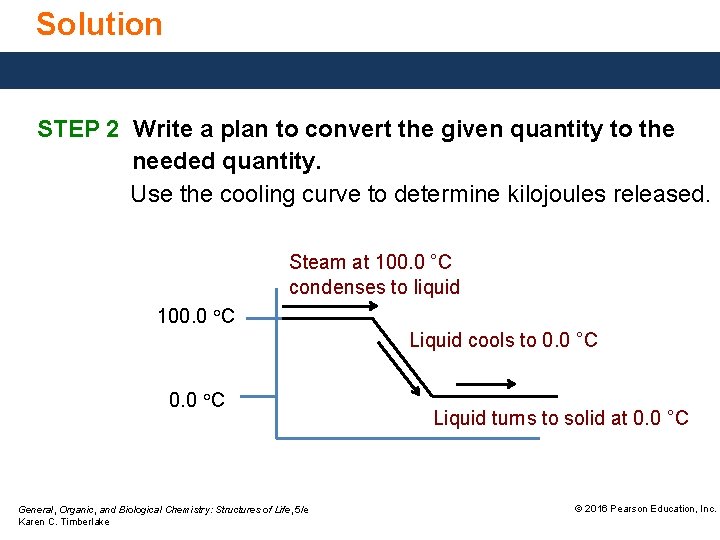
Solution STEP 2 Write a plan to convert the given quantity to the needed quantity. Use the cooling curve to determine kilojoules released. Steam at 100. 0 °C condenses to liquid 100. 0 °C Liquid cools to 0. 0 °C General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake Liquid turns to solid at 0. 0 °C © 2016 Pearson Education, Inc.

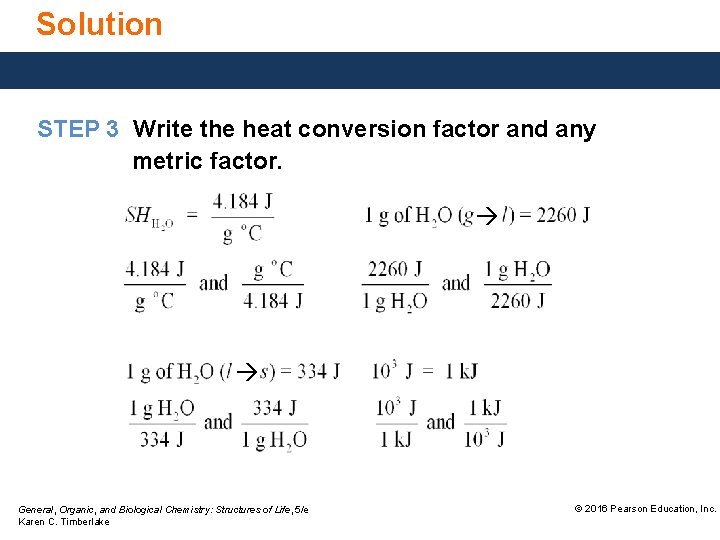
Solution STEP 3 Write the heat conversion factor and any metric factor. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

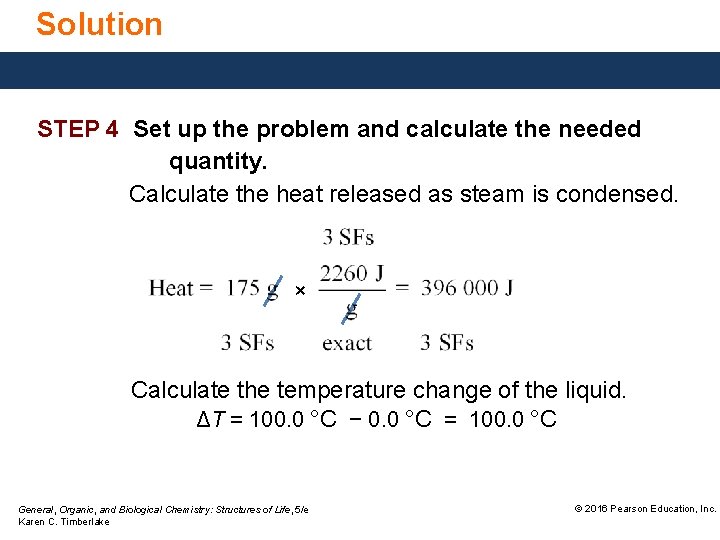
Solution STEP 4 Set up the problem and calculate the needed quantity. Calculate the heat released as steam is condensed. × Calculate the temperature change of the liquid. ΔT = 100. 0 °C − 0. 0 °C = 100. 0 °C General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

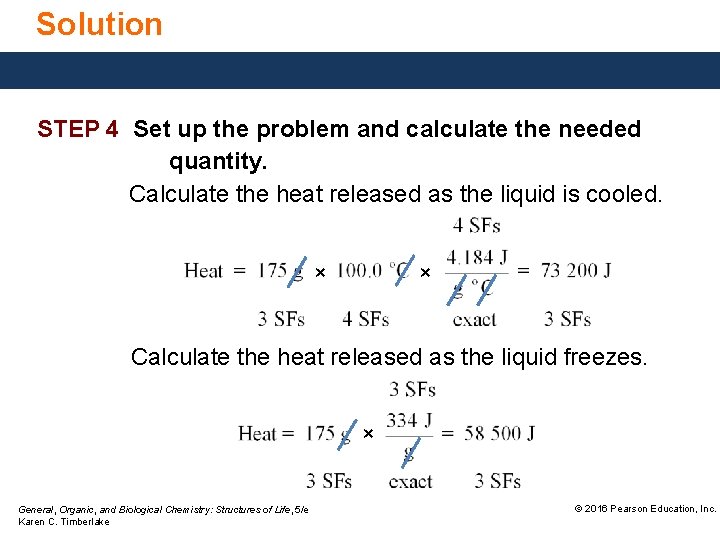
Solution STEP 4 Set up the problem and calculate the needed quantity. Calculate the heat released as the liquid is cooled. × × Calculate the heat released as the liquid freezes. × General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

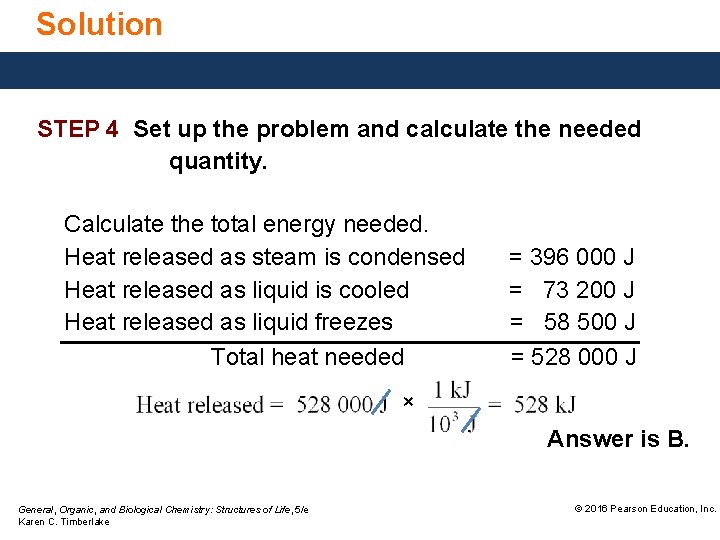
Solution STEP 4 Set up the problem and calculate the needed quantity. Calculate the total energy needed. Heat released as steam is condensed Heat released as liquid is cooled Heat released as liquid freezes Total heat needed = 396 000 J = 73 200 J = 58 500 J = 528 000 J × Answer is B. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.

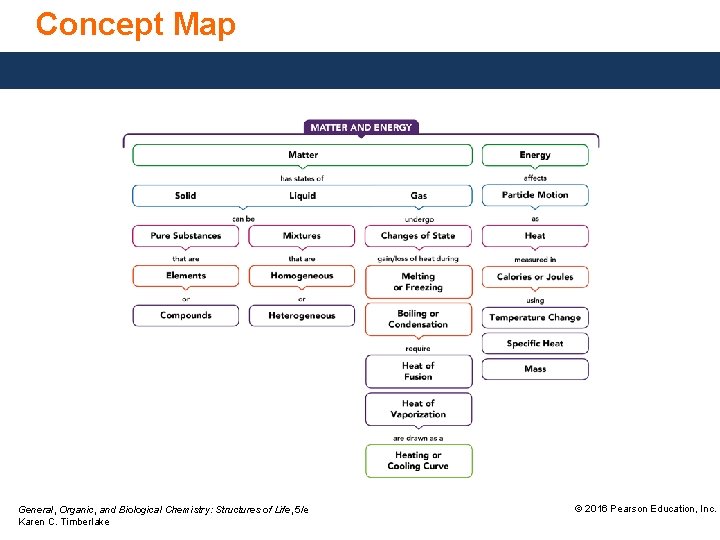
Concept Map General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.