2 6 Cycloalkanes Cycloalkanes are alkanes that have

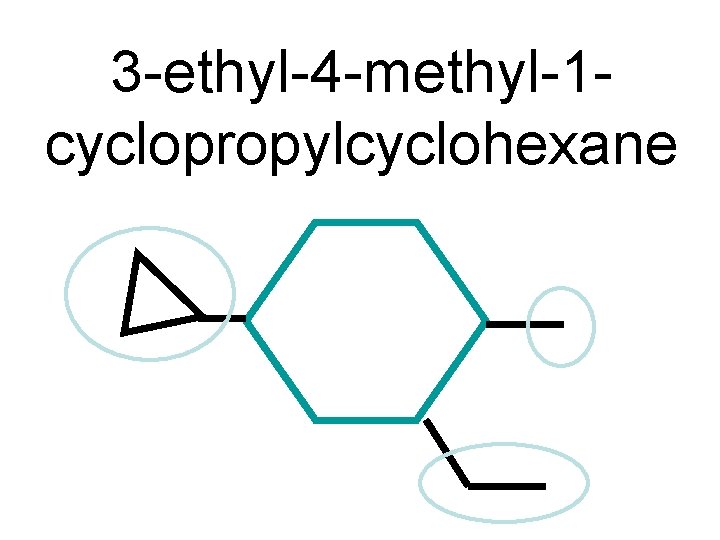






- Slides: 45

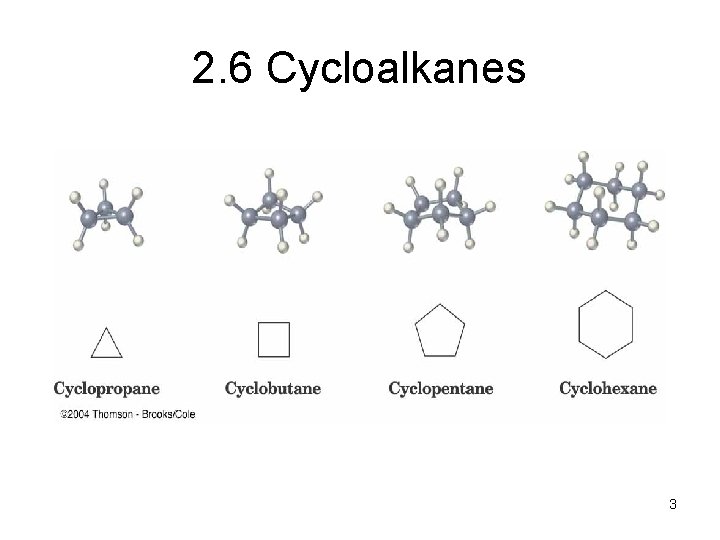
2. 6 Cycloalkanes • Cycloalkanes are alkanes that have carbon atoms that form a ring (called alicyclic compounds) • Simple cycloalkanes are rings of CH 2 units, (CH 2)n, or Cn. H 2 n • Structure is shown as a regular polygon with the number of vertices equal to the number of C’s (a projection of the actual structure) 1

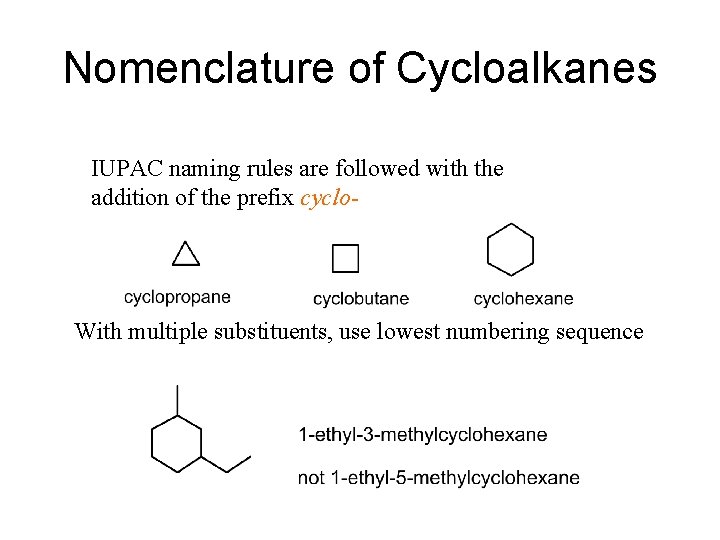
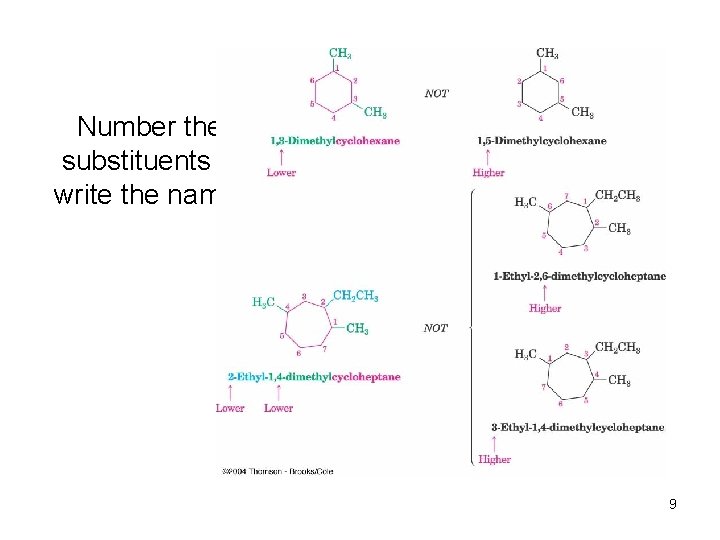
Nomenclature of Cycloalkanes IUPAC naming rules are followed with the addition of the prefix cyclo- With multiple substituents, use lowest numbering sequence

2. 6 Cycloalkanes 3

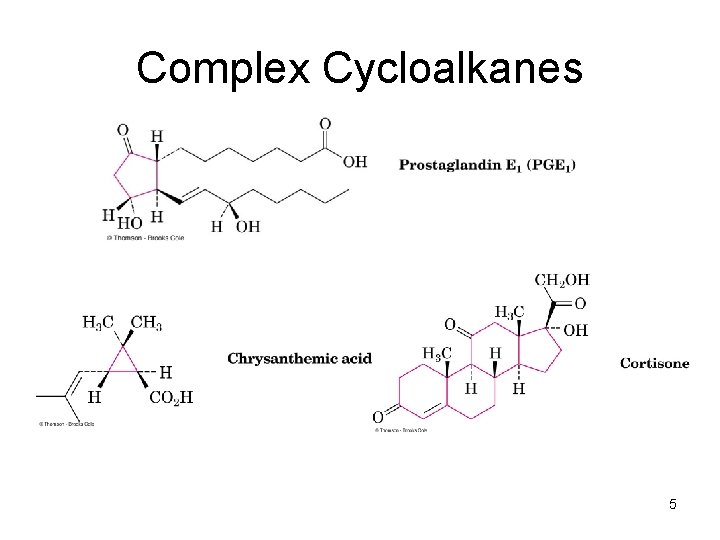
Complex Cycloalkanes • Naturally occurring materials contain cycloalkane structures • Examples: – chrysanthemic acid (cyclopropane), – prostaglandins (cyclopentane), – steroids (cyclohexanes and cyclopentane) 4

Complex Cycloalkanes 5

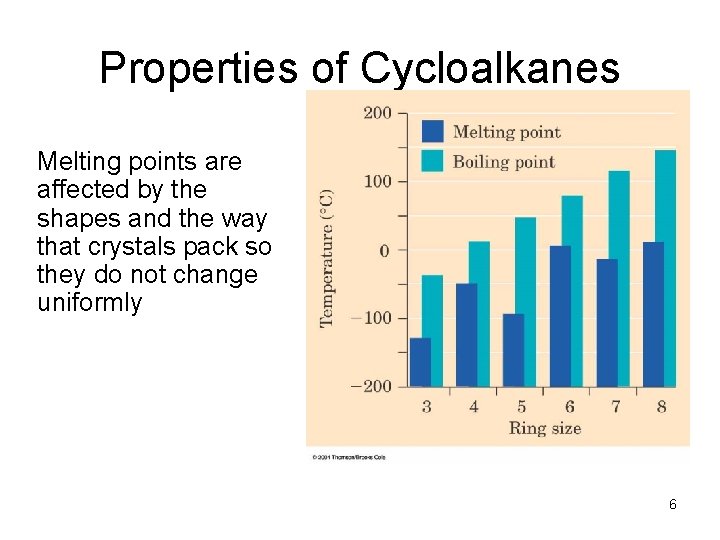
Properties of Cycloalkanes Melting points are affected by the shapes and the way that crystals pack so they do not change uniformly 6

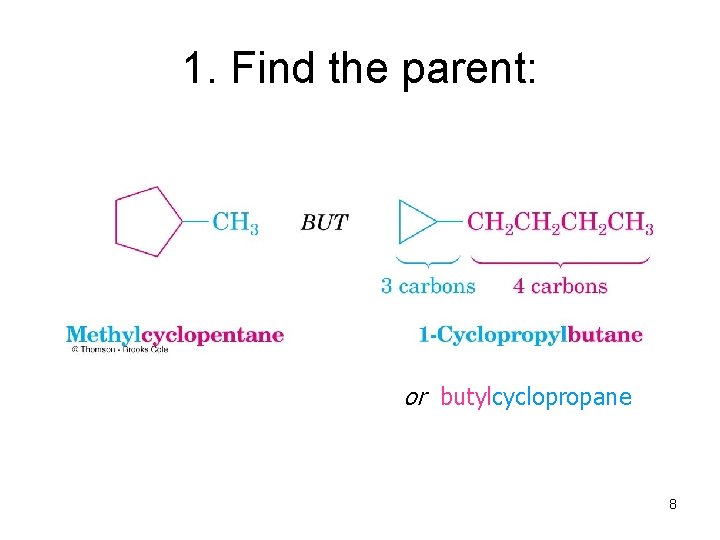
2. 7 Naming Cycloalkanes • Count the number of carbon atoms in the ring and the number in the largest substituent chain. If the number of carbon atoms in the ring is equal to or greater than the number in the substituent, the compound is named as an alkyl-substituted cycloalkane. • For an alkyl- or halo-substituted cycloalkane, start at a point of attachment as C 1 and number the substituents on the ring so that the second substituent has as low a number as possible. When two substituents are present, we number the ring beginning with the substituent first in the alphabet. • Wenn three or more substituents are present, we begin at the substituent that leads to the lowest set of locants • Number the substituents and write the name 7

1. Find the parent: or butylcyclopropane 8

Number the substituents & write the name: 9

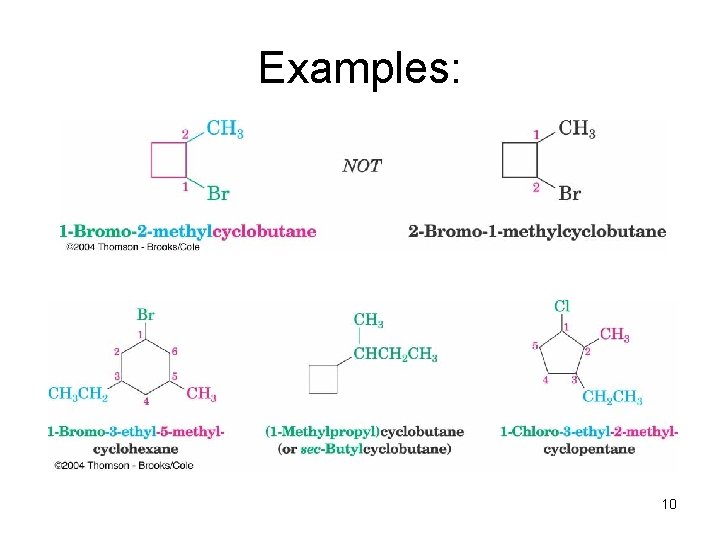
Examples: 10

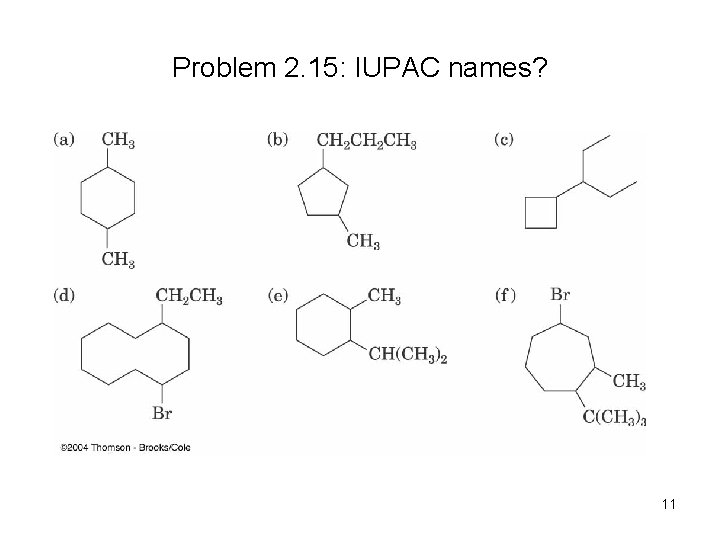
Problem 2. 15: IUPAC names? 11

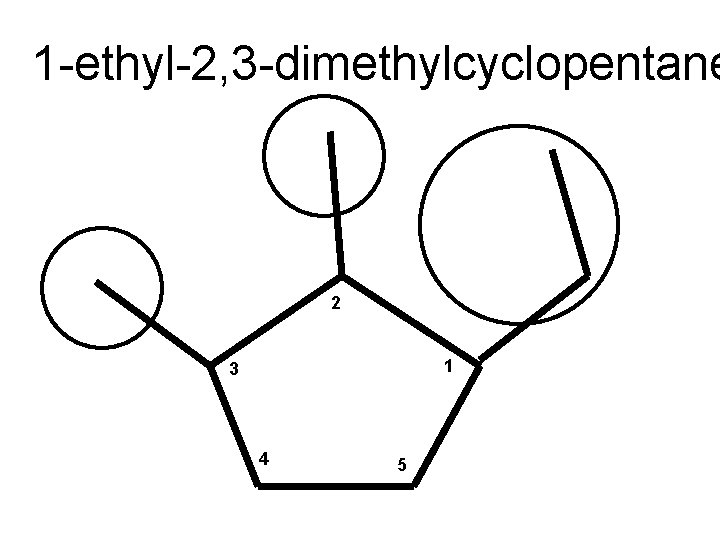
1 -ethyl-2, 3 -dimethylcyclopentane 2 1 3 4 5
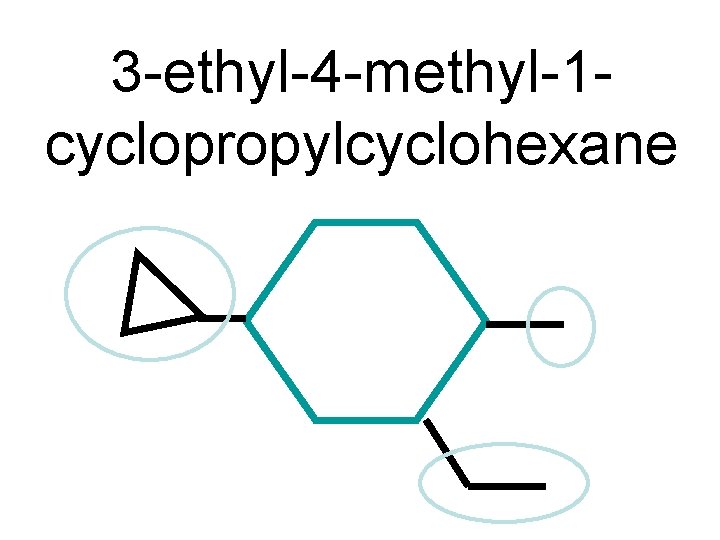
3 -ethyl-4 -methyl-1 cyclopropylcyclohexane

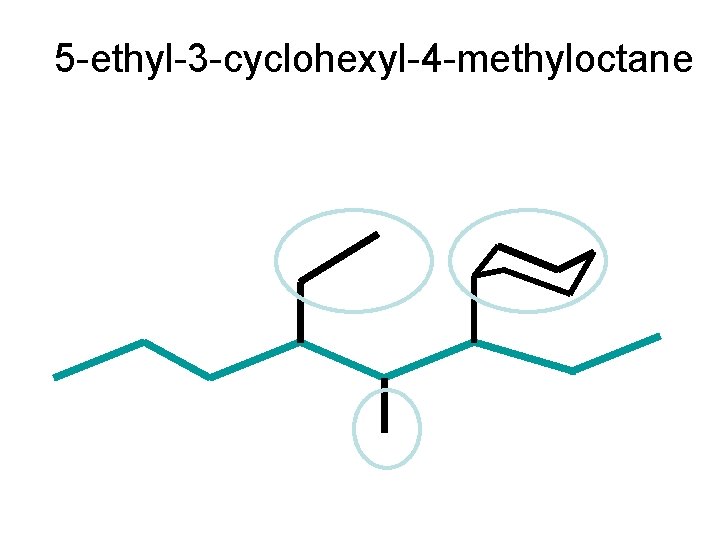
5 -ethyl-3 -cyclohexyl-4 -methyloctane

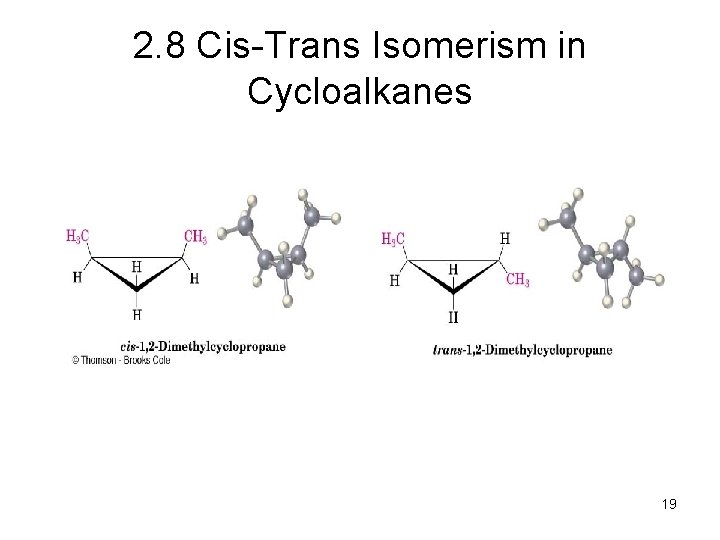
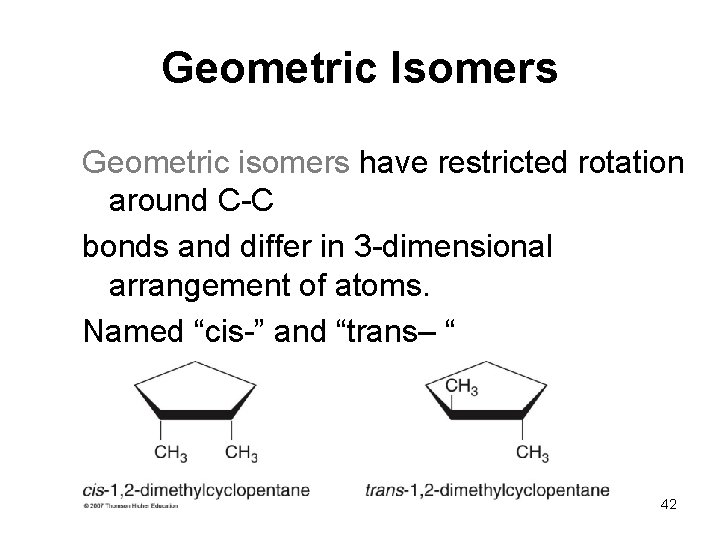
2. 8 Cis-Trans Isomerism in Cycloalkanes • Rotation about C-C bonds in cycloalkanes is limited by the ring structure • Rings have two “faces” and substituents are labeled as to their relative facial positions • There are two different 1, 2 -dimethyl-cyclopropane isomers, one with the two methyls on the same side (cis) of the ring and one with the methyls on opposite sides (trans) 15

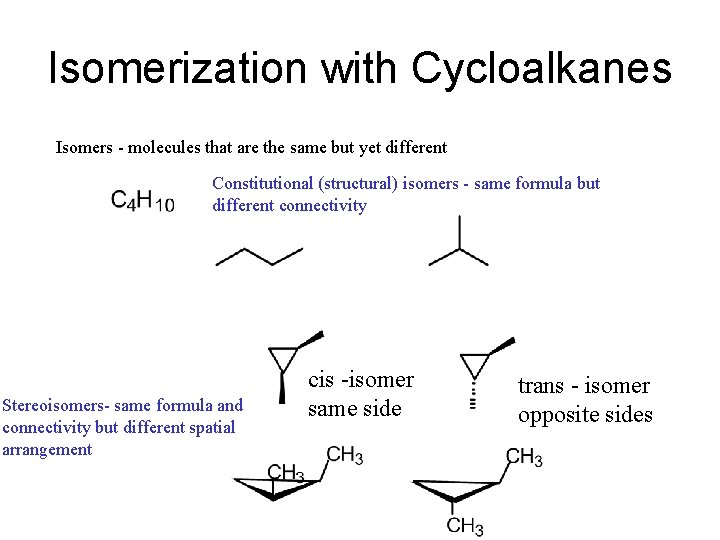
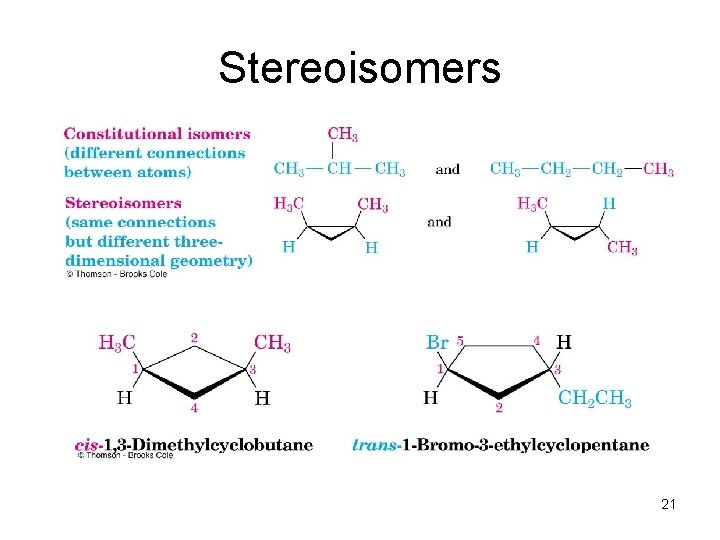
Isomerization with Cycloalkanes Isomers - molecules that are the same but yet different Constitutional (structural) isomers - same formula but different connectivity Stereoisomers- same formula and connectivity but different spatial arrangement cis -isomer same side trans - isomer opposite sides

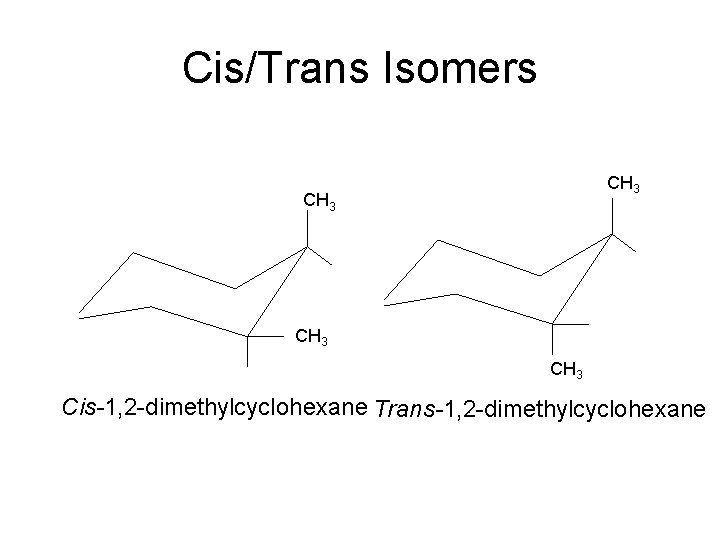
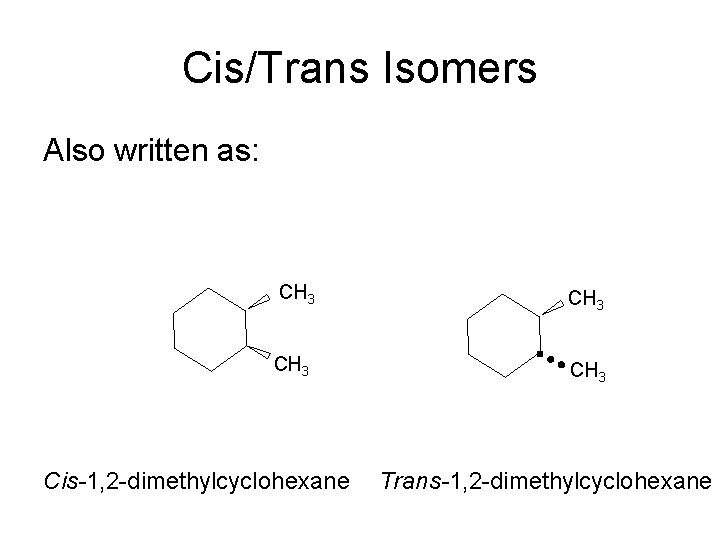
Cis/Trans Isomers CH 3 Cis-1, 2 -dimethylcyclohexane Trans-1, 2 -dimethylcyclohexane

Cis/Trans Isomers Also written as: CH 3 Cis-1, 2 -dimethylcyclohexane Trans-1, 2 -dimethylcyclohexane

2. 8 Cis-Trans Isomerism in Cycloalkanes 19

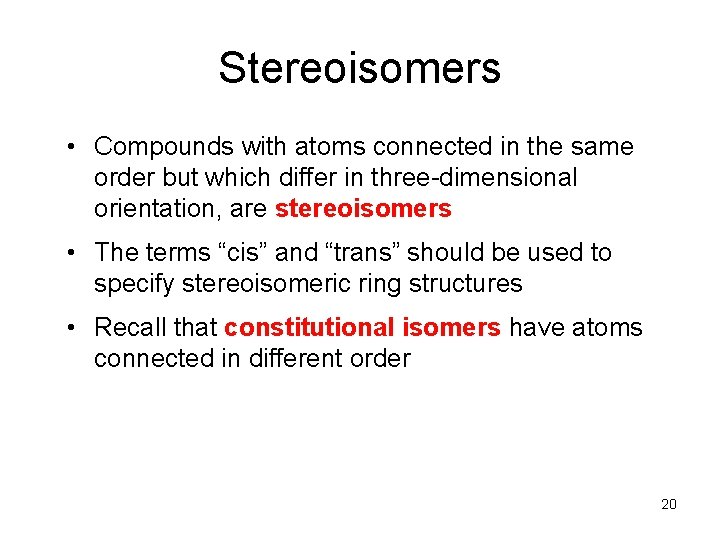
Stereoisomers • Compounds with atoms connected in the same order but which differ in three-dimensional orientation, are stereoisomers • The terms “cis” and “trans” should be used to specify stereoisomeric ring structures • Recall that constitutional isomers have atoms connected in different order 20

Stereoisomers 21

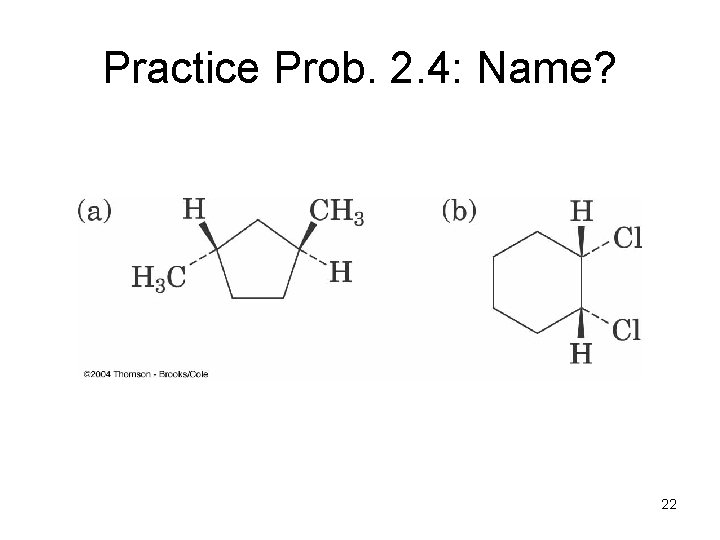
Practice Prob. 2. 4: Name? 22

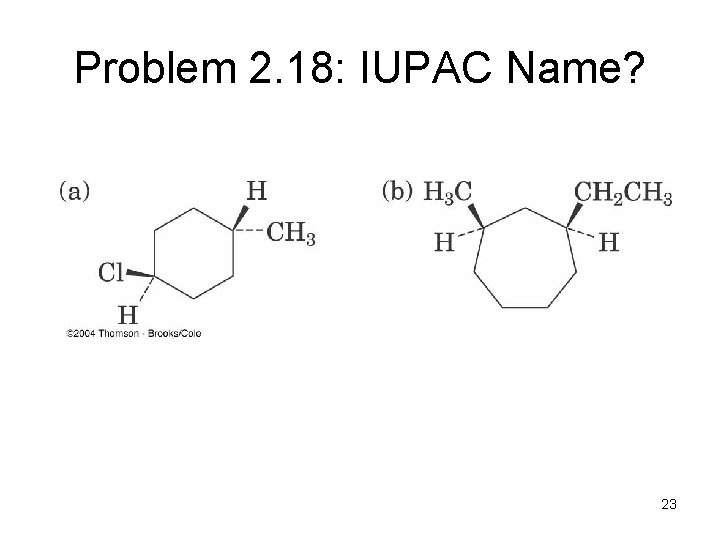
Problem 2. 18: IUPAC Name? 23

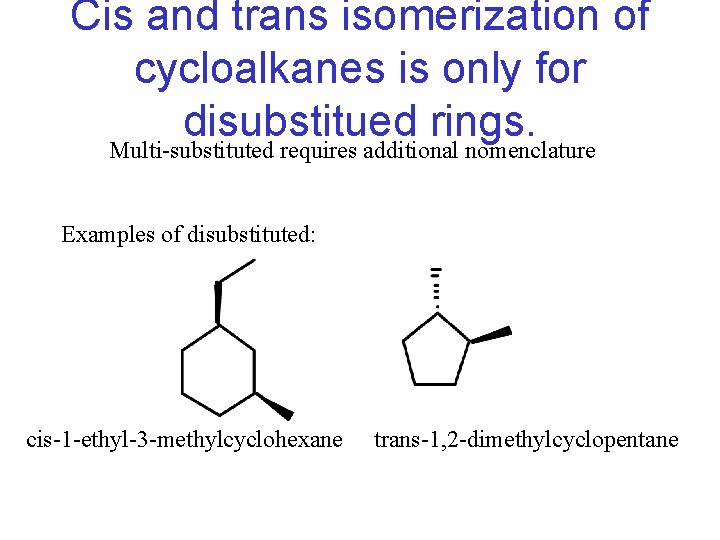
Cis and trans isomerization of cycloalkanes is only for disubstitued rings. Multi-substituted requires additional nomenclature Examples of disubstituted: cis-1 -ethyl-3 -methylcyclohexane trans-1, 2 -dimethylcyclopentane

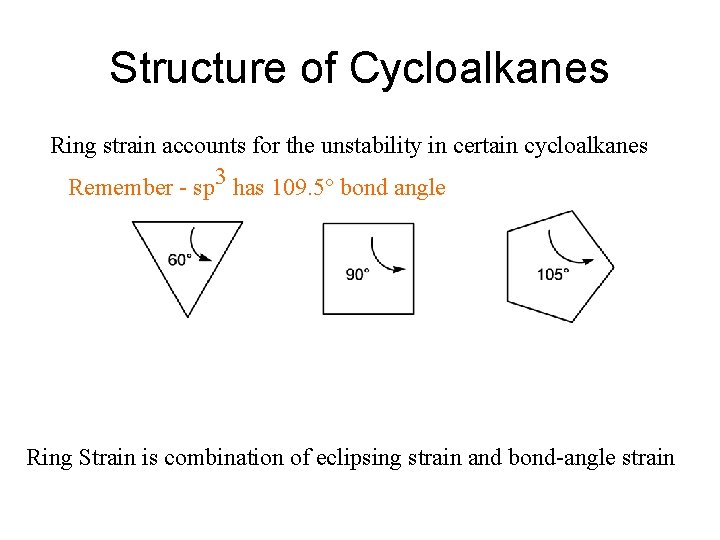
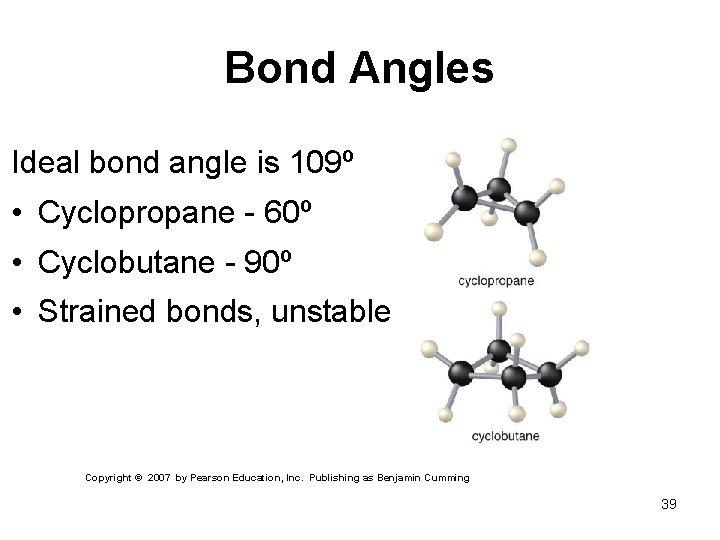
Structure of Cycloalkanes Ring strain accounts for the unstability in certain cycloalkanes Remember - sp 3 has 109. 5° bond angle Ring Strain is combination of eclipsing strain and bond-angle strain

Each cycloalkane adopts a conformation that tries to alleviate ring strain as much as possible cylcopropane “banana” bonds that do not have end-to-end overlap of typical σbonds Highly unstable ring-system that will “open” or react readily

Cyclobutane Instead of flat conformation, a “puckered” conformation reduces eclipsing strain

Cyclopentane An “envelope” conformation reduces eclipsing strain

Ring Conformation • Cyclopentane puckers

Cyclohexane The “chair” conformation eliminates all eclipsing strain and nearly all bond-angle strain

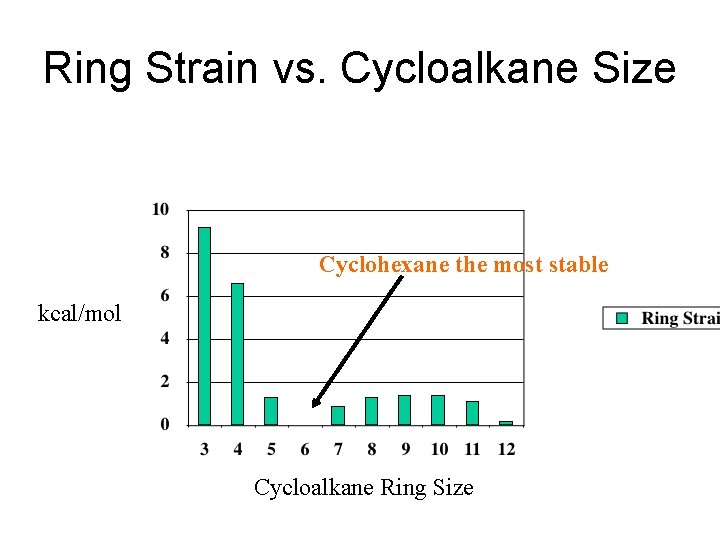
Ring Strain vs. Cycloalkane Size Cyclohexane the most stable kcal/mol Cycloalkane Ring Size

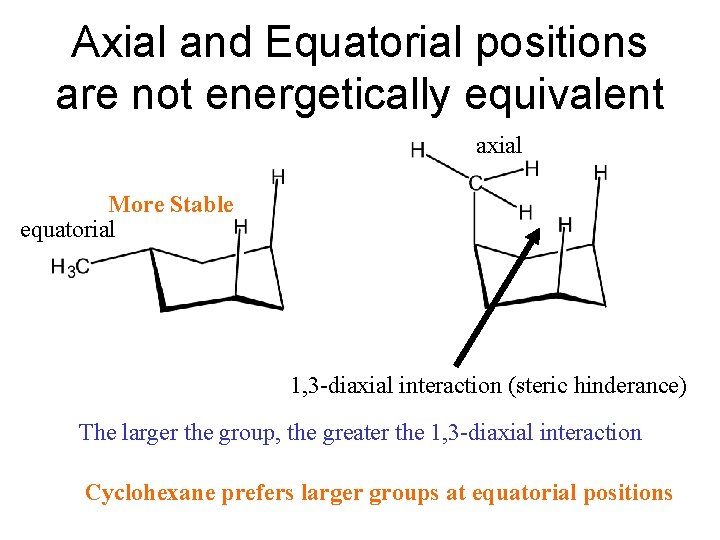
Axial and Equatorial positions are not energetically equivalent axial More Stable equatorial 1, 3 -diaxial interaction (steric hinderance) The larger the group, the greater the 1, 3 -diaxial interaction Cyclohexane prefers larger groups at equatorial positions

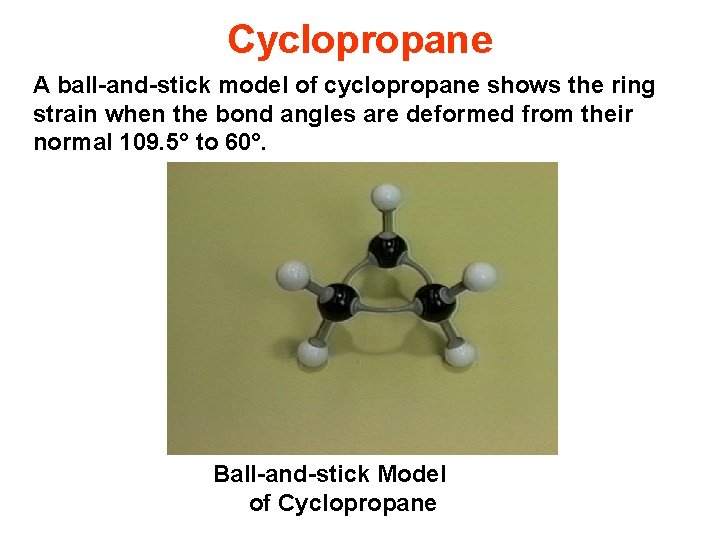
Cyclopropane A ball-and-stick model of cyclopropane shows the ring strain when the bond angles are deformed from their normal 109. 5° to 60°. Ball-and-stick Model of Cyclopropane

Cyclobutane A ball-and-stick model of cyclobutane shows the ring strain when the bond angles are deformed from their normal 109. 5° to approximately 90°.

Cyclopentane The ball-and-stick model of cyclopentane reveals a nearly planar molecule with bond angles of 108°.

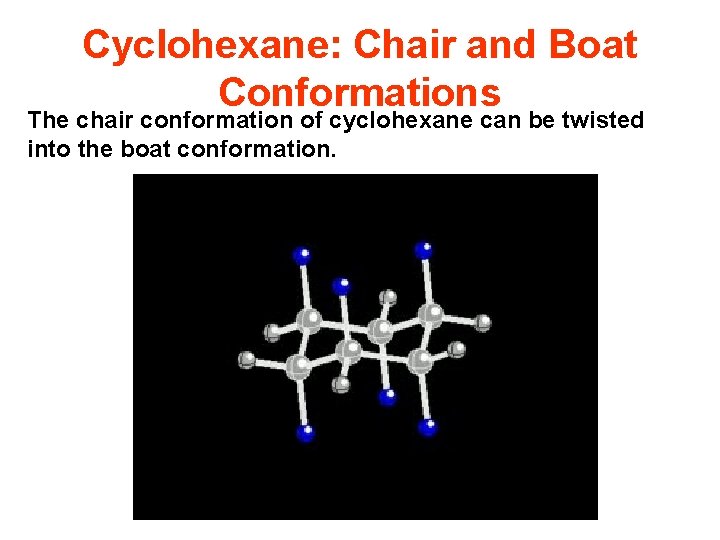
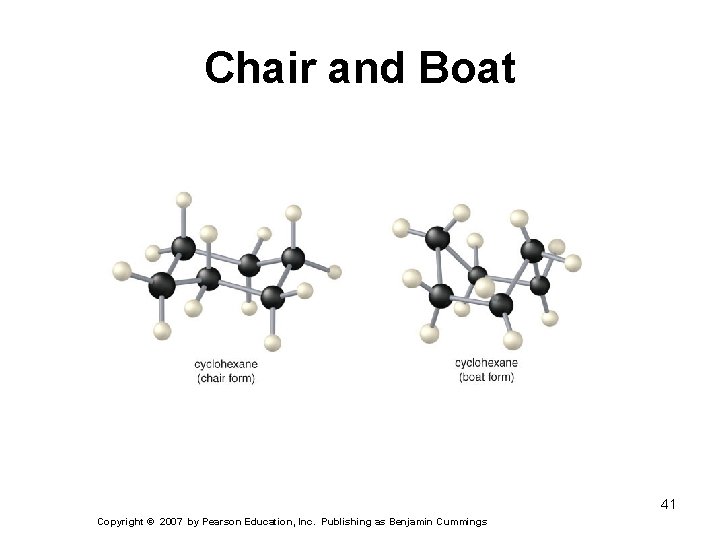
Cyclohexane: Chair and Boat Conformations The chair conformation of cyclohexane can be twisted into the boat conformation.

Cyclohexane: Chair and Boat Conformations The chair conformation of cyclohexane can be twisted into the boat conformation. Further twisting results in a second chair conformation. By labeling one axial substituent with a white sphere you can see how the axial position of one chair becomes equatorial by twisting into the second chair conformation.

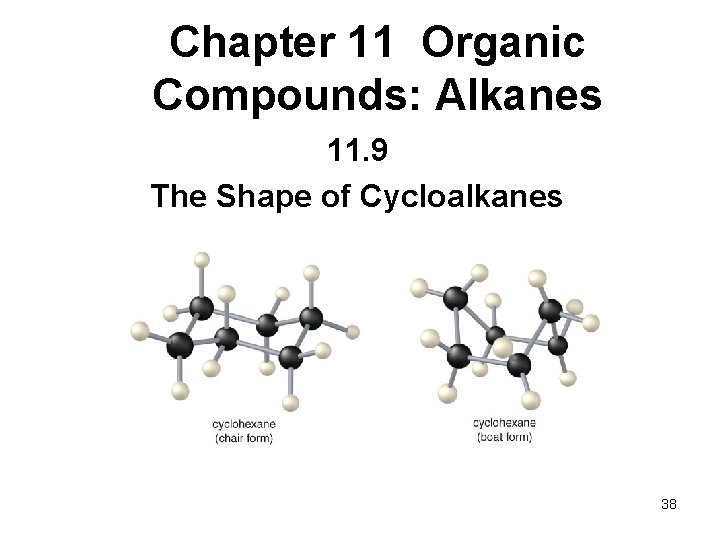
Chapter 11 Organic Compounds: Alkanes 11. 9 The Shape of Cycloalkanes 38

Bond Angles Ideal bond angle is 109º • Cyclopropane - 60º • Cyclobutane - 90º • Strained bonds, unstable Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cumming 39

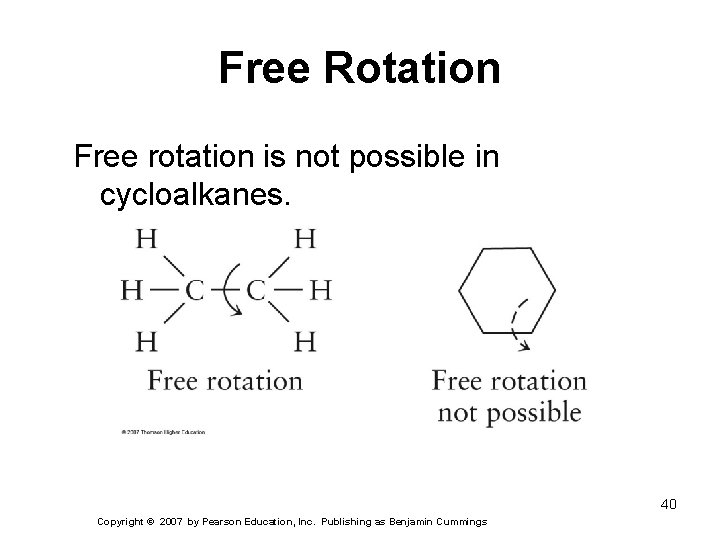
Free Rotation Free rotation is not possible in cycloalkanes. 40 Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings

Chair and Boat 41 Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings

Geometric Isomers Geometric isomers have restricted rotation around C-C bonds and differ in 3 -dimensional arrangement of atoms. Named “cis-” and “trans– “ 42

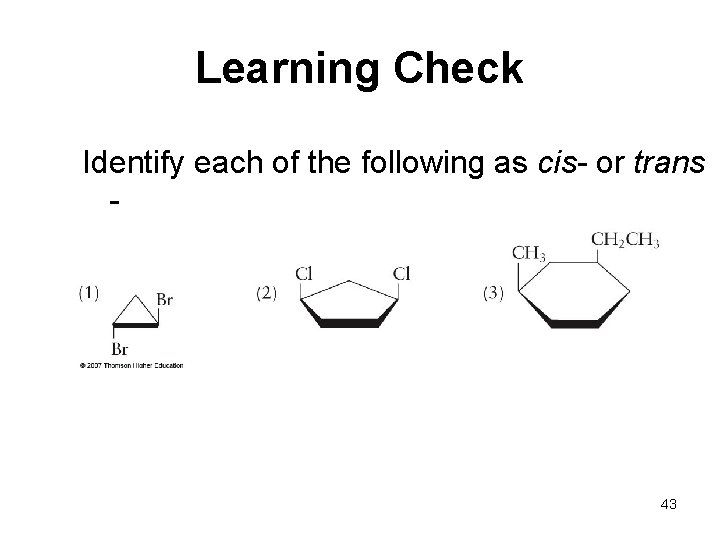
Learning Check Identify each of the following as cis- or trans - 43

Learning Check Draw and name all the isomers of dichlorolcyclobutane. (There are 5. ) 44

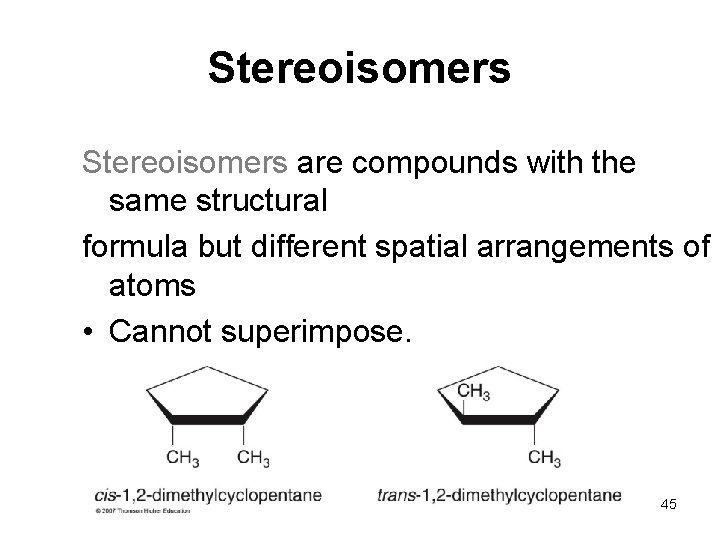
Stereoisomers are compounds with the same structural formula but different spatial arrangements of atoms • Cannot superimpose. 45
 Ethane
Ethane Antigentest åre
Antigentest åre Iupac nomenclature of cycloalkanes
Iupac nomenclature of cycloalkanes Cycloalkanes
Cycloalkanes Bromine water test
Bromine water test Cycloalkanes and their stereochemistry
Cycloalkanes and their stereochemistry Alkane methane structure
Alkane methane structure Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown
Phân độ lown Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Block nhĩ thất độ 2 mobitz 1
Block nhĩ thất độ 2 mobitz 1 Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Viscosity marble experiment
Viscosity marble experiment Bromine water test
Bromine water test Alkanes alkenes alkynes
Alkanes alkenes alkynes Combustion complete and incomplete
Combustion complete and incomplete Uses of alkanes
Uses of alkanes Order of priority of functional groups
Order of priority of functional groups 3-butyl-3-propyl-1-pentyne
3-butyl-3-propyl-1-pentyne Alkanes def
Alkanes def Cyclopentane uses
Cyclopentane uses Alkanes list
Alkanes list Alkanes
Alkanes Ir spectrum of nitrile
Ir spectrum of nitrile Alkanes solubility
Alkanes solubility Alkylation of alkanes
Alkylation of alkanes Iupac stands for
Iupac stands for General formula for alkyl group
General formula for alkyl group Chemisty
Chemisty Combustion reaction of alkanes
Combustion reaction of alkanes Unbranched carbon chain
Unbranched carbon chain Shape with 8 vertices
Shape with 8 vertices Presente simple en ingles
Presente simple en ingles What is an echinoderm
What is an echinoderm They have not rejected you
They have not rejected you Some animals are dangerous *
Some animals are dangerous * Words have meaning and names have power
Words have meaning and names have power I have resolved
I have resolved Does congress have the power to stop mail on saturdays
Does congress have the power to stop mail on saturdays Ideas have consequences bad ideas have victims
Ideas have consequences bad ideas have victims Is could past tense
Is could past tense