Cycloalkanes Alkanes that form rings are called cycloalkanes




- Slides: 16

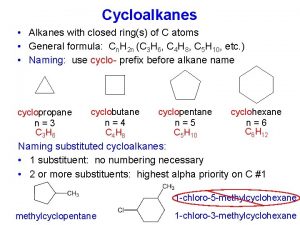
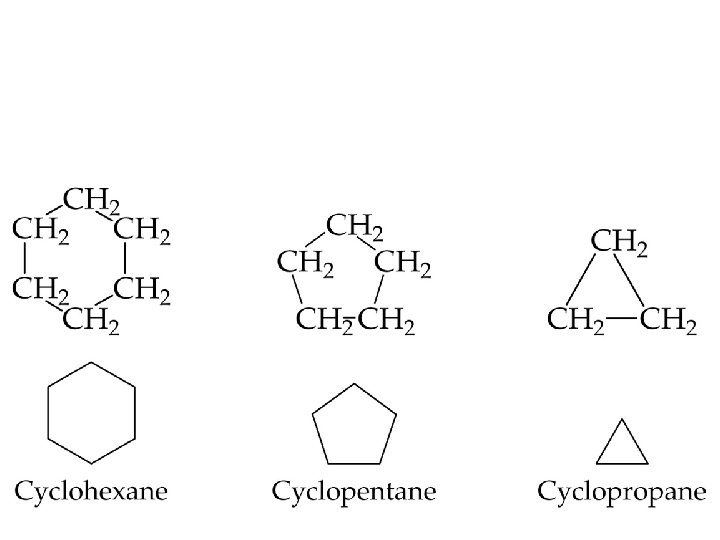
Cycloalkanes • Alkanes that form rings are called cycloalkanes. • Cyclopropane and cyclobutanes are strained because the C-C-C bond angles in the ring are less than 109. 5 required for the tetrahedral geometry. • Because of the strain in the ring, cyclopropane is very reactive.


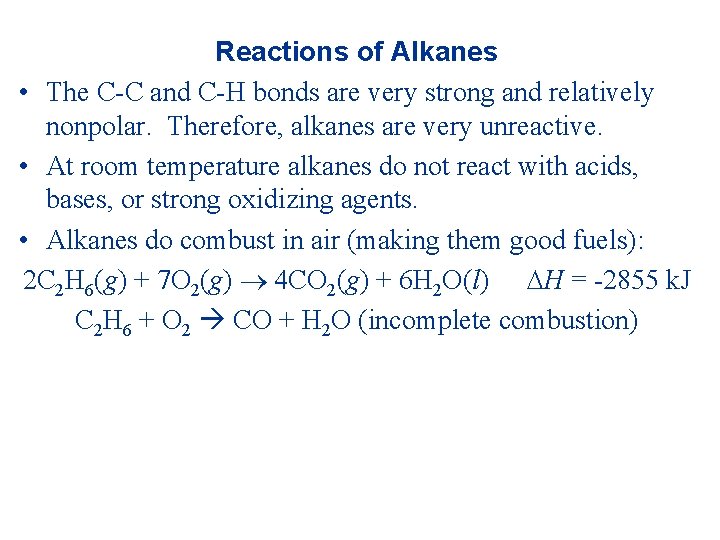
Reactions of Alkanes • The C-C and C-H bonds are very strong and relatively nonpolar. Therefore, alkanes are very unreactive. • At room temperature alkanes do not react with acids, bases, or strong oxidizing agents. • Alkanes do combust in air (making them good fuels): 2 C 2 H 6(g) + 7 O 2(g) 4 CO 2(g) + 6 H 2 O(l) H = -2855 k. J C 2 H 6 + O 2 CO + H 2 O (incomplete combustion)

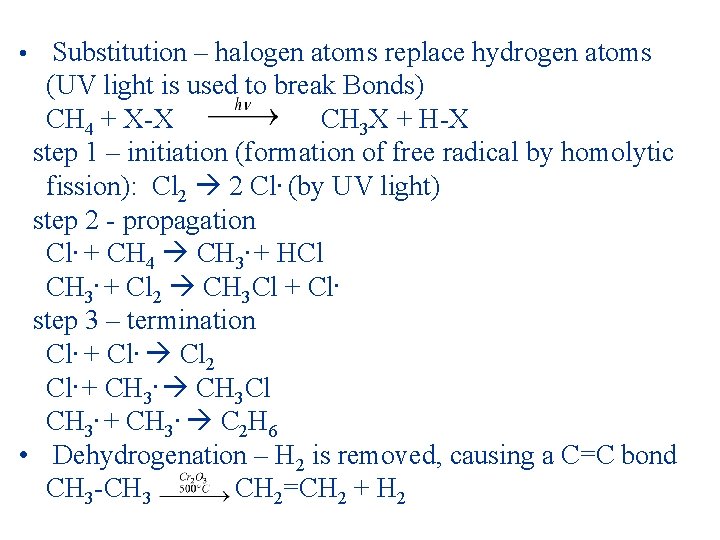
• Substitution – halogen atoms replace hydrogen atoms (UV light is used to break Bonds) CH 4 + X-X CH 3 X + H-X step 1 – initiation (formation of free radical by homolytic fission): Cl 2 2 Cl. (by UV light) step 2 - propagation Cl. + CH 4 CH 3. + HCl CH 3. + Cl 2 CH 3 Cl + Cl. step 3 – termination Cl. + Cl. Cl 2 Cl. + CH 3 Cl CH 3. + CH 3. C 2 H 6 • Dehydrogenation – H 2 is removed, causing a C=C bond CH 3 -CH 3 CH 2=CH 2 + H 2

Unsaturated Hydrocarbons Alkenes • Alkenes contain C, H atoms and single and double bonds. • The simplest alkenes are H 2 C=CH 2 (ethene) and CH 3 CH=CH 2 (propene): – their trivial names are ethylene and propylene. • Alkenes are named in the same way as alkanes with the suffix -ene replacing the -ane in alkanes. • The location of the double bond is indicated by a number.

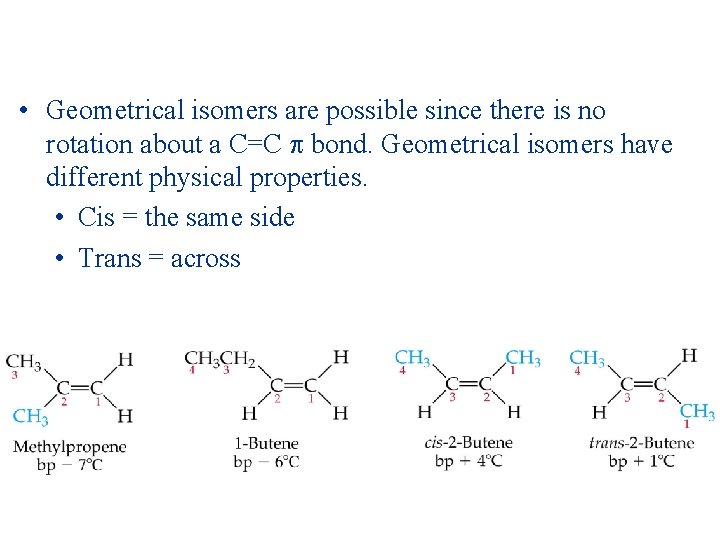
• Geometrical isomers are possible since there is no rotation about a C=C bond. Geometrical isomers have different physical properties. • Cis = the same side • Trans = across

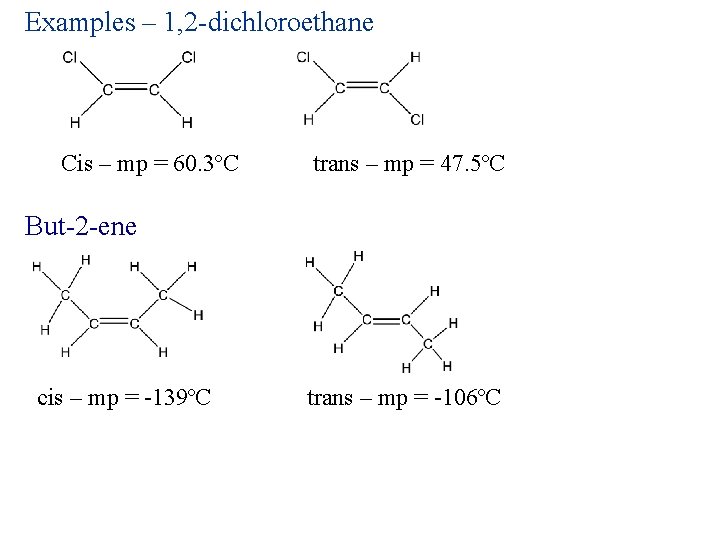
Examples – 1, 2 -dichloroethane Cis – mp = 60. 3ºC trans – mp = 47. 5ºC But-2 -ene cis – mp = -139ºC trans – mp = -106ºC

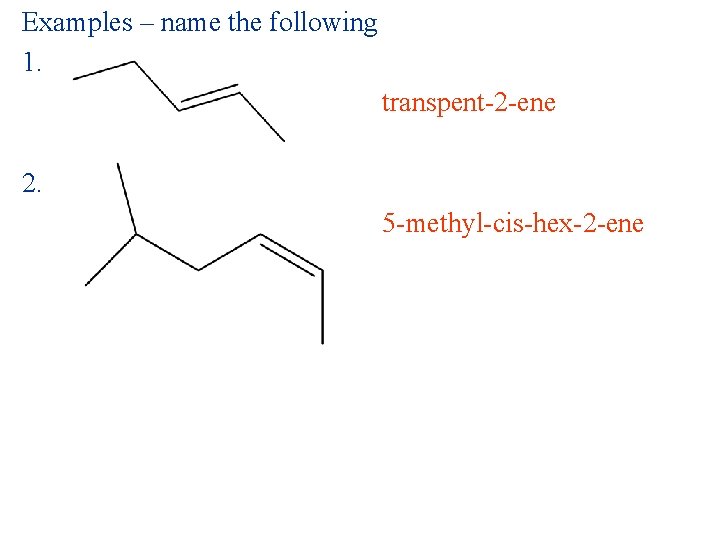
Examples – name the following 1. transpent-2 -ene 2. 5 -methyl-cis-hex-2 -ene

• • Alkynes are hydrocarbons with one or more C C bond. Therefore, alkynes have one and two bonds between two C atoms. Ethyne (acetylene) is a reactive alkyne: HC CH. When acetylene is burned in the presence of oxygen (oxyacetylene torch) the temperature is about 3200 K. Alkynes are named in the same way as alkenes with the suffix -yne replacing the -ene for alkenes.

Unsaturated Hydrocarbons Addition Reactions of Alkenes and Alkynes • The most dominant reaction for alkenes and alkynes involves the addition of something (hydrogen, halogen, water) to the two atoms which form the double bond: • Note that the C-C bond has been replaced by two C-Br bonds. • Alkenes will react with bromine water by addition, while alkanes will show no reaction with bromine water. This is one way to distinguish between alkanes and alkenes.

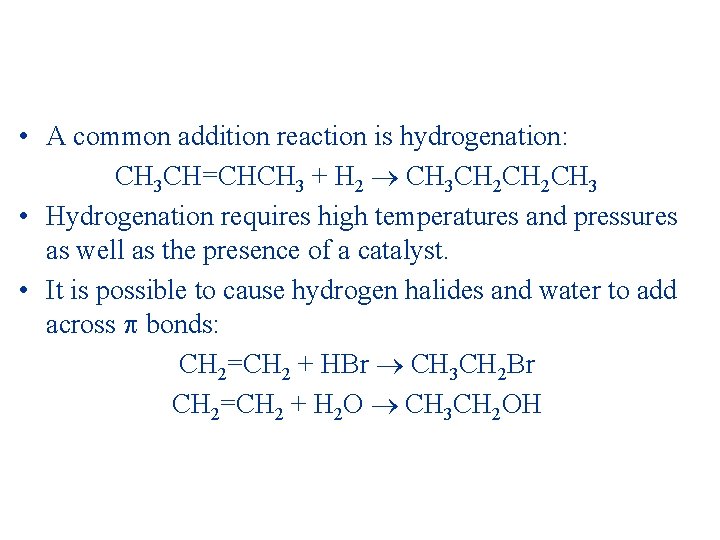
• A common addition reaction is hydrogenation: CH 3 CH=CHCH 3 + H 2 CH 3 CH 2 CH 3 • Hydrogenation requires high temperatures and pressures as well as the presence of a catalyst. • It is possible to cause hydrogen halides and water to add across bonds: CH 2=CH 2 + HBr CH 3 CH 2 Br CH 2=CH 2 + H 2 O CH 3 CH 2 OH

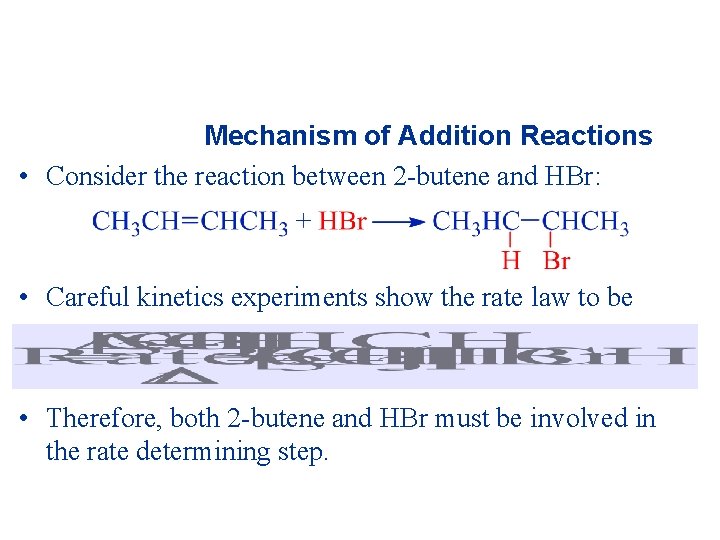
Mechanism of Addition Reactions • Consider the reaction between 2 -butene and HBr: • Careful kinetics experiments show the rate law to be • Therefore, both 2 -butene and HBr must be involved in the rate determining step.

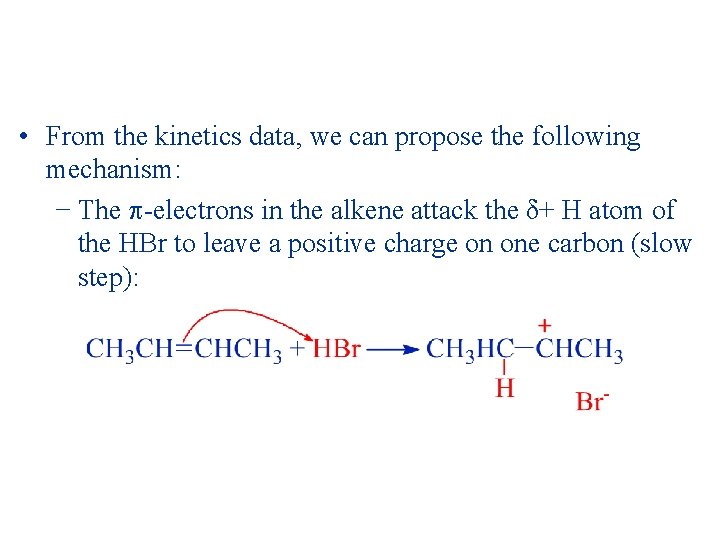
• From the kinetics data, we can propose the following mechanism: − The -electrons in the alkene attack the δ+ H atom of the HBr to leave a positive charge on one carbon (slow step):

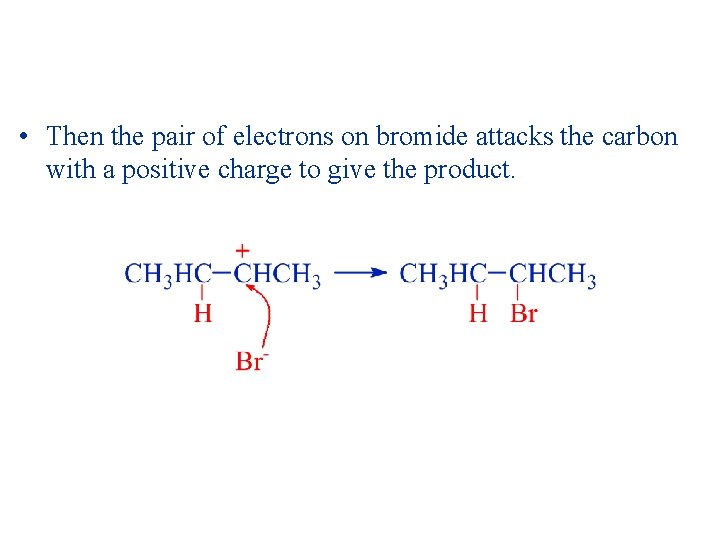
• Then the pair of electrons on bromide attacks the carbon with a positive charge to give the product.

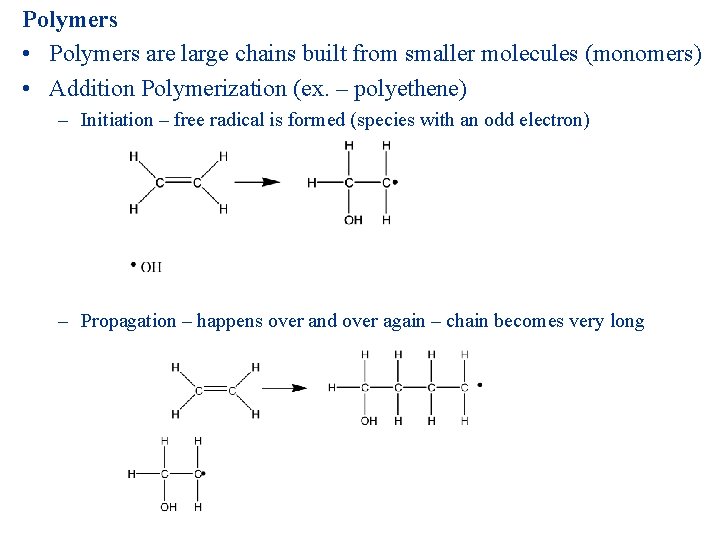
Polymers • Polymers are large chains built from smaller molecules (monomers) • Addition Polymerization (ex. – polyethene) – Initiation – free radical is formed (species with an odd electron) – Propagation – happens over and over again – chain becomes very long

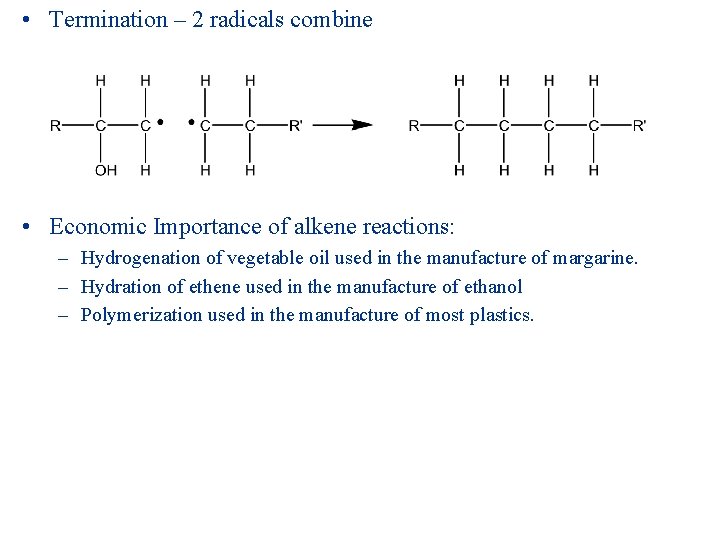
• Termination – 2 radicals combine • Economic Importance of alkene reactions: – Hydrogenation of vegetable oil used in the manufacture of margarine. – Hydration of ethene used in the manufacture of ethanol – Polymerization used in the manufacture of most plastics.
 Ethane
Ethane Mikael ferm
Mikael ferm Alkanes list
Alkanes list Cycloalkanes and their stereochemistry
Cycloalkanes and their stereochemistry Which compound is inorganic
Which compound is inorganic Founder of organic chemistry
Founder of organic chemistry Complete combustion of alkene
Complete combustion of alkene Cycloalkanes
Cycloalkanes An arrangement of fascicles in concentric rings is called
An arrangement of fascicles in concentric rings is called Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block nhĩ thất độ 1
Block nhĩ thất độ 1 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai