Chapter 2 Alkanes and Cycloalkanes Section 1 Alkanes






- Slides: 61

Chapter 2 Alkanes and Cycloalkanes Section 1 Alkanes Section 2 Cycloalkanes

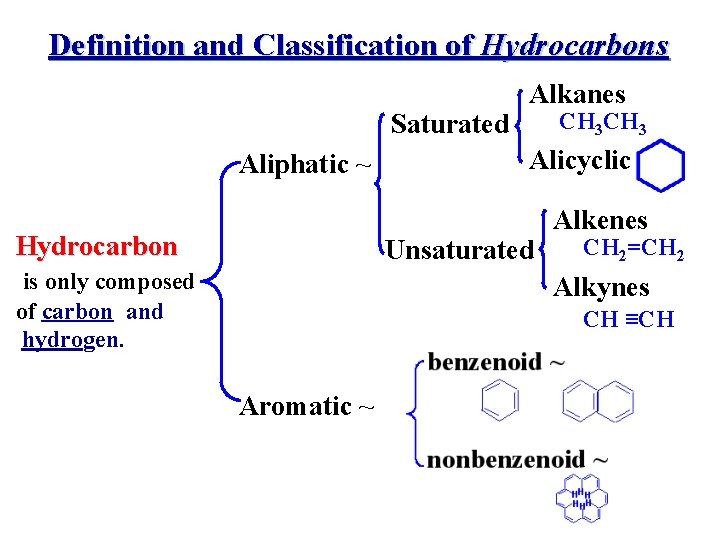
Definition and Classification of Hydrocarbons Saturated Aliphatic ~ Hydrocarbon Alkanes CH 3 Alicyclic Unsaturated is only composed of carbon and hydrogen. Alkenes CH 2=CH 2 Alkynes CH ≡CH Aromatic ~

Section 1 Alkanes Alkane is one kind of hydrocarbon in which all of the carbon are bound to each other and hydrogen by single (s) bonds. I. Structure II. Nomenclature III. Properties

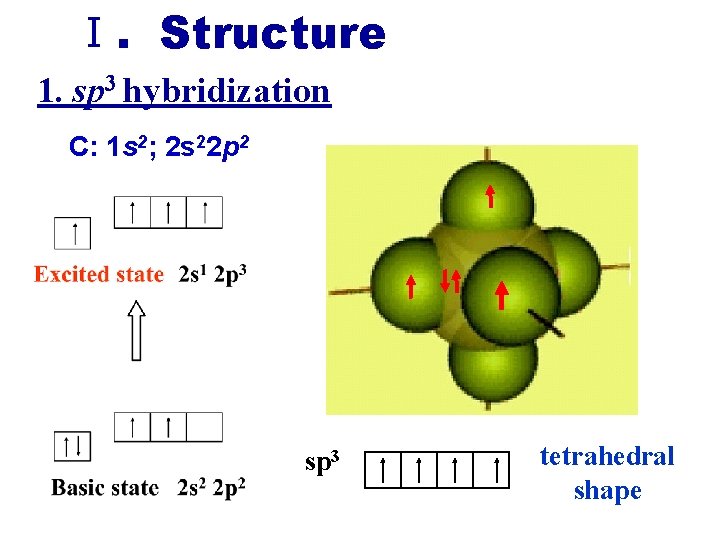
Ⅰ. Structure 1. sp 3 hybridization C: 1 s 2; 2 s 22 p 2 sp 3 tetrahedral shape

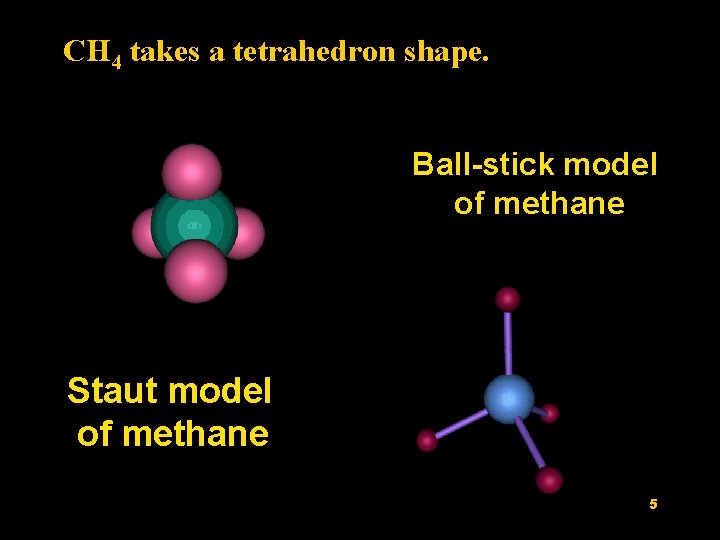
CH 4 takes a tetrahedron shape. Ball-stick model of methane Staut model of methane 5

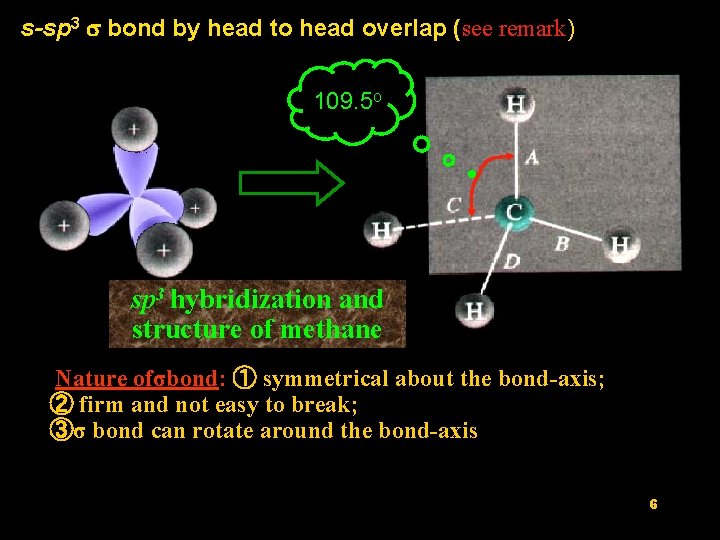
s-sp 3 s bond by head to head overlap (see remark) 109. 5 o sp 3 hybridization and structure of methane Nature ofσbond: ① symmetrical about the bond-axis; ② firm and not easy to break; ③σ bond can rotate around the bond-axis 6

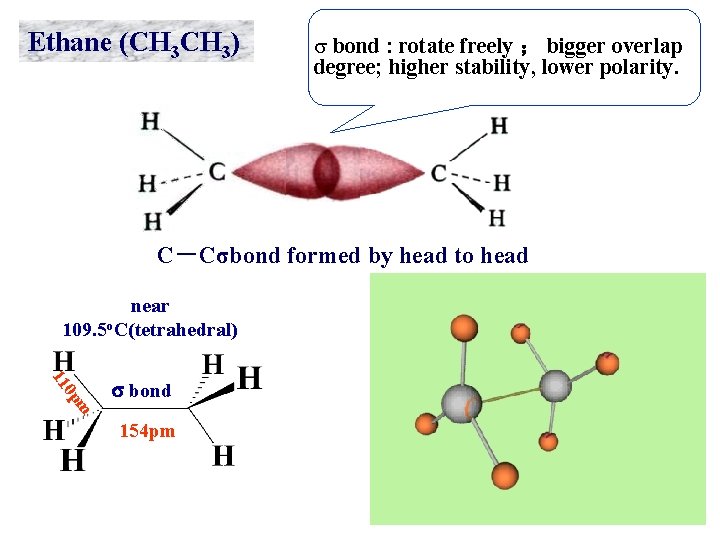
Ethane (CH 3) s bond : rotate freely ; bigger overlap degree; higher stability, lower polarity. C-Cσbond formed by head to head near 109. 5 o. C(tetrahedral) m 0 p 11 s bond 154 pm

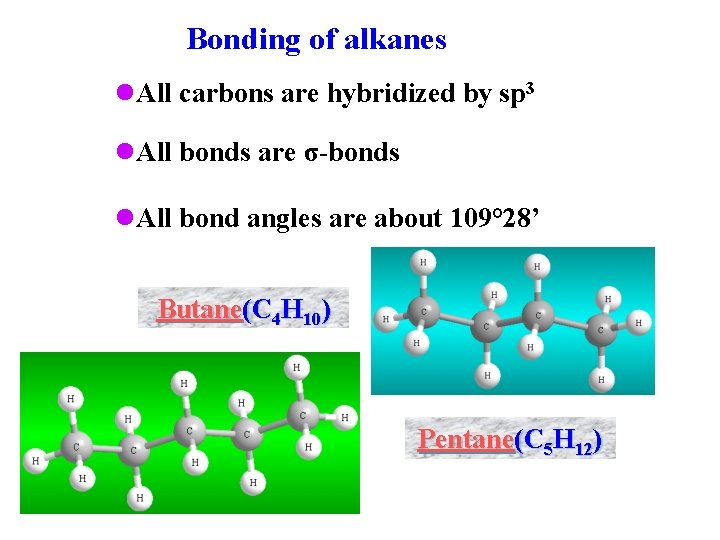
Bonding of alkanes l. All carbons are hybridized by sp 3 l. All bonds are σ-bonds l. All bond angles are about 109° 28’ Butane(C 4 H 10) Pentane(C 5 H 12)

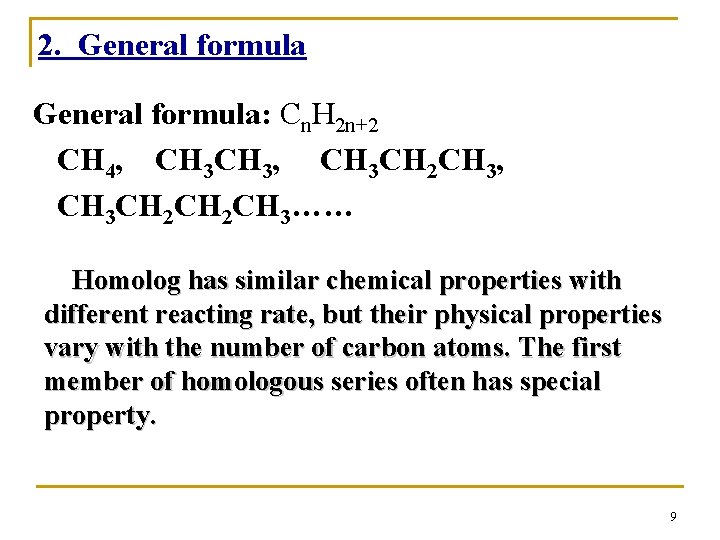
2. General formula: Cn. H 2 n+2 CH 4, CH 3, CH 3 CH 2 CH 3…… Homolog has similar chemical properties with different reacting rate, but their physical properties vary with the number of carbon atoms. The first member of homologous series often has special property. 9

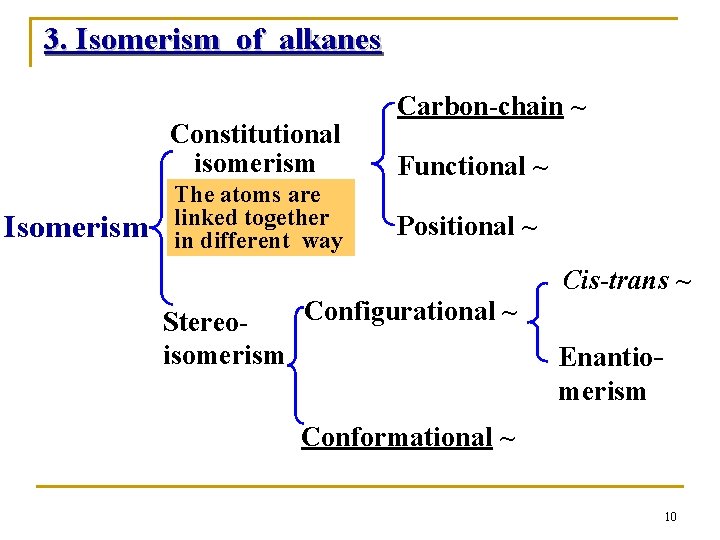
3. Isomerism of alkanes Constitutional isomerism Isomerism The atoms are linked together in different way Carbon-chain ~ Functional ~ Positional ~ Cis-trans ~ Configurational ~ Stereoisomerism Enantiomerism Conformational ~ 10

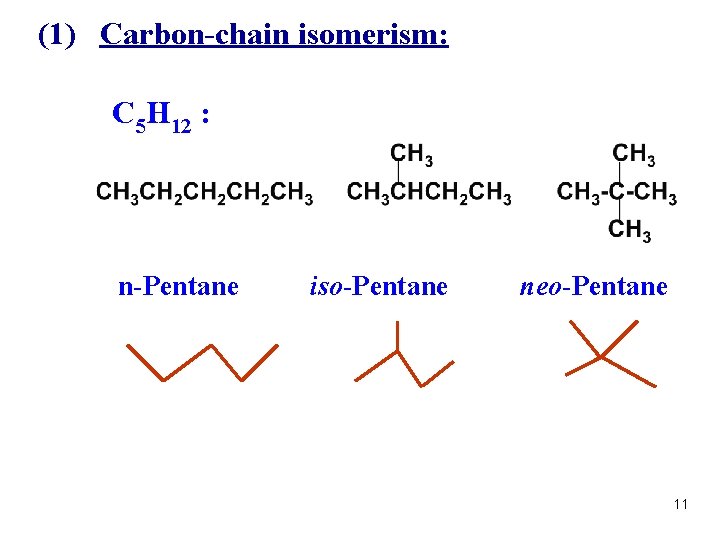
(1) Carbon-chain isomerism: C 5 H 12 : n-Pentane iso-Pentane neo-Pentane 11

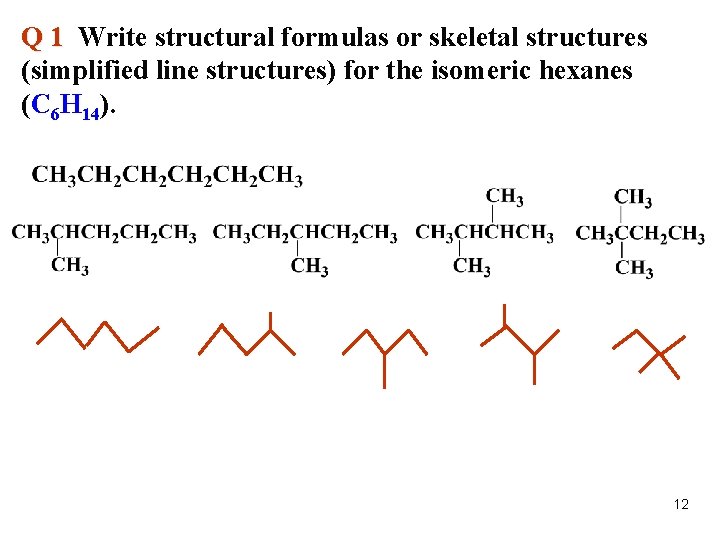
Q 1 Write structural formulas or skeletal structures (simplified line structures) for the isomeric hexanes (C 6 H 14). 12

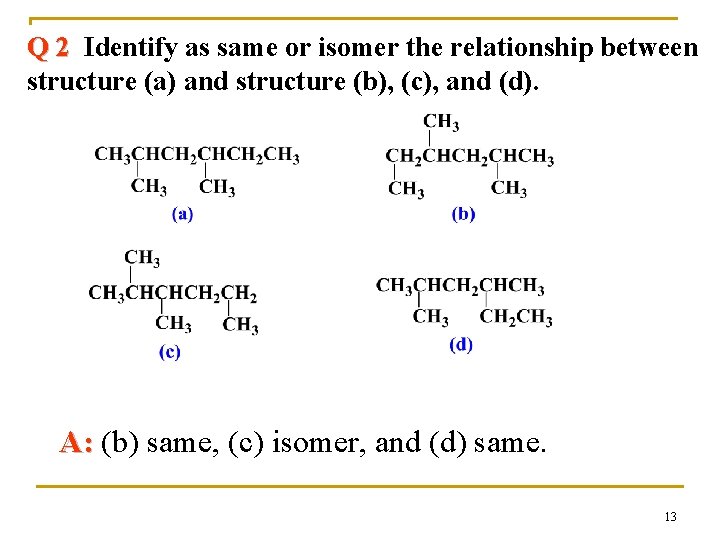
Q 2 Identify as same or isomer the relationship between structure (a) and structure (b), (c), and (d). A: (b) same, (c) isomer, and (d) same. 13

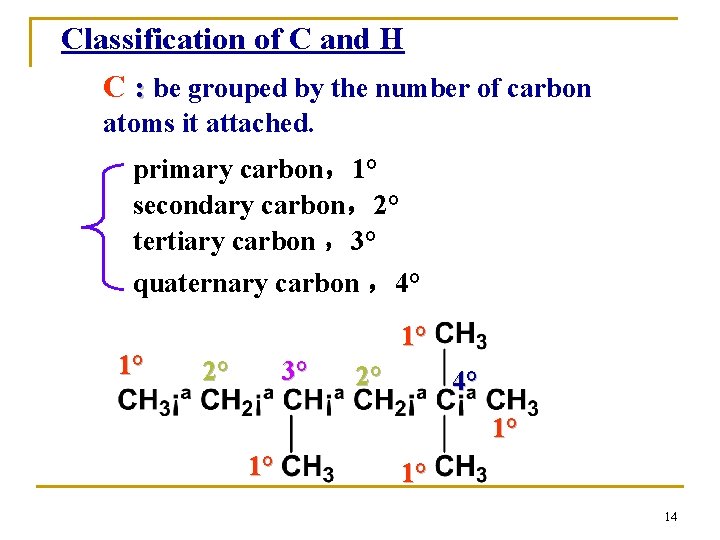
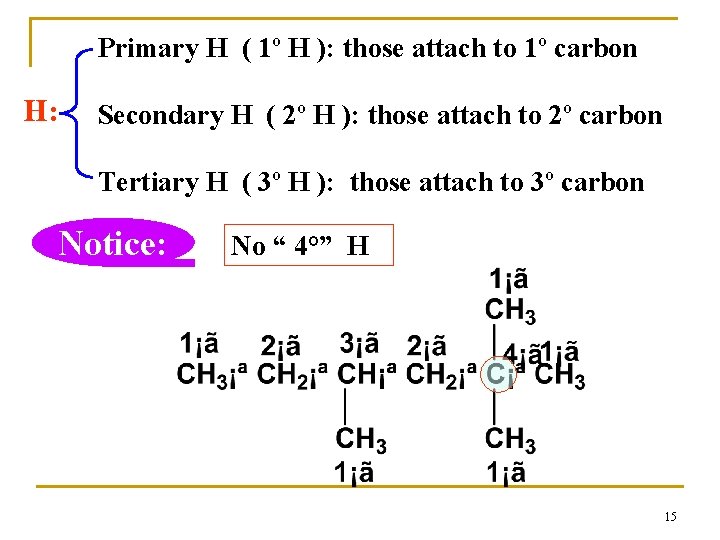
Classification of C and H C : be grouped by the number of carbon atoms it attached. primary carbon,1° secondary carbon,2° tertiary carbon ,3° quaternary carbon ,4° 1° 1° 2° 3° 2° 4° 1° 14

Primary H ( 1º H ): those attach to 1º carbon H: Secondary H ( 2º H ): those attach to 2º carbon Tertiary H ( 3º H ): those attach to 3º carbon Notice: No “ 4°” H 15

n n n Which of the following compound contains 1°, 2°, 3°and 4°carbon atoms? A、2,2,3 -trimethylbutane B、2,2,3 -trimethylpentane C、2,3,4 -trimethylpentane D、3,3 -dimethylpentane Answer: B 16

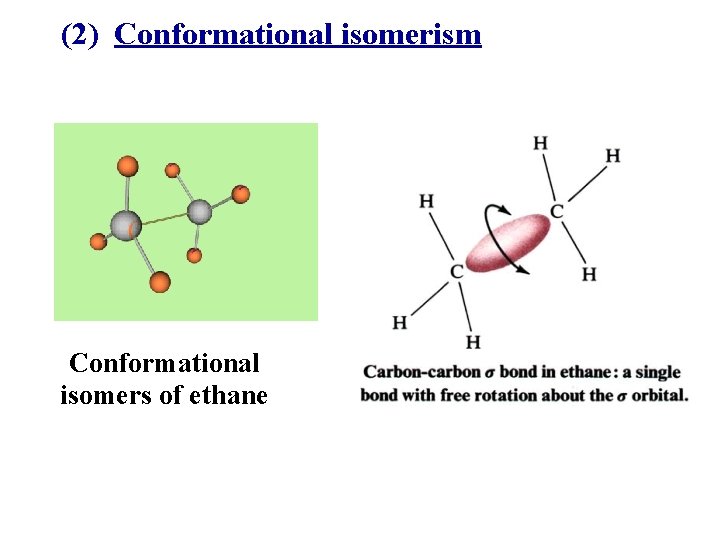
(2) Conformational isomerism Conformational isomers of ethane


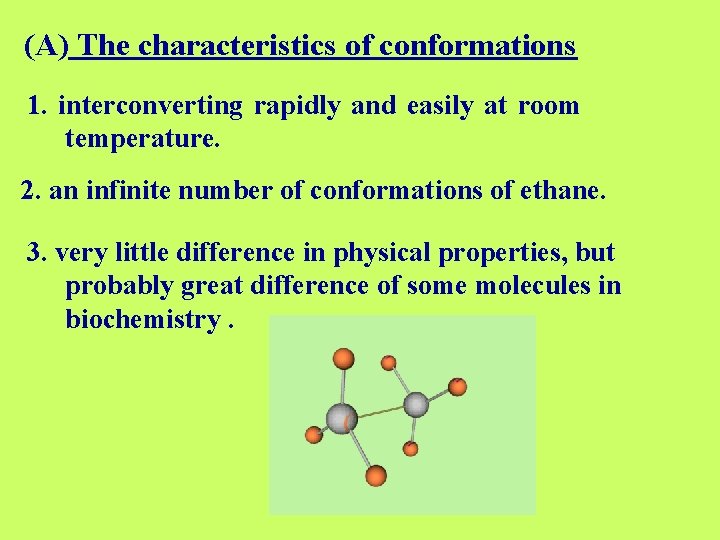
(A) The characteristics of conformations 1. interconverting rapidly and easily at room temperature. 2. an infinite number of conformations of ethane. 3. very little difference in physical properties, but probably great difference of some molecules in biochemistry.

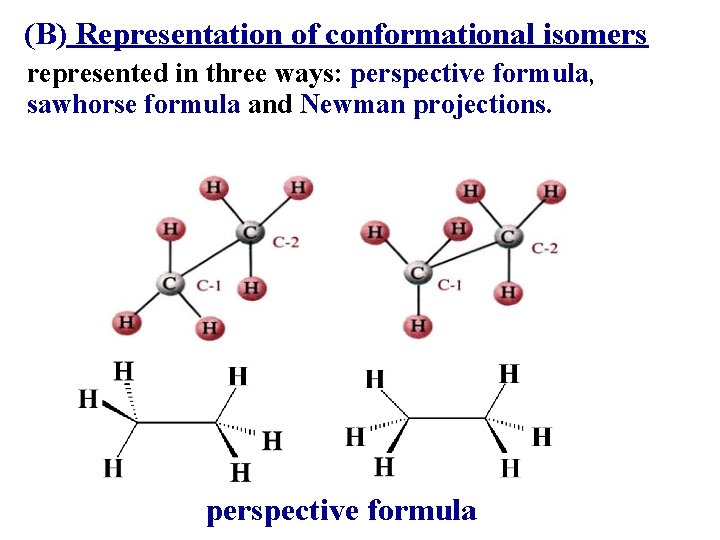
(B) Representation of conformational isomers represented in three ways: perspective formula, sawhorse formula and Newman projections. perspective formula

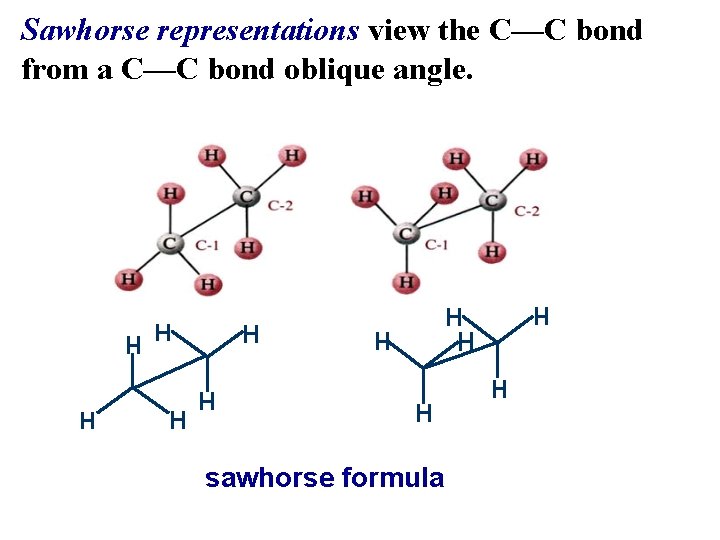
Sawhorse representations view the C—C bond from a C—C bond oblique angle. sawhorse formula

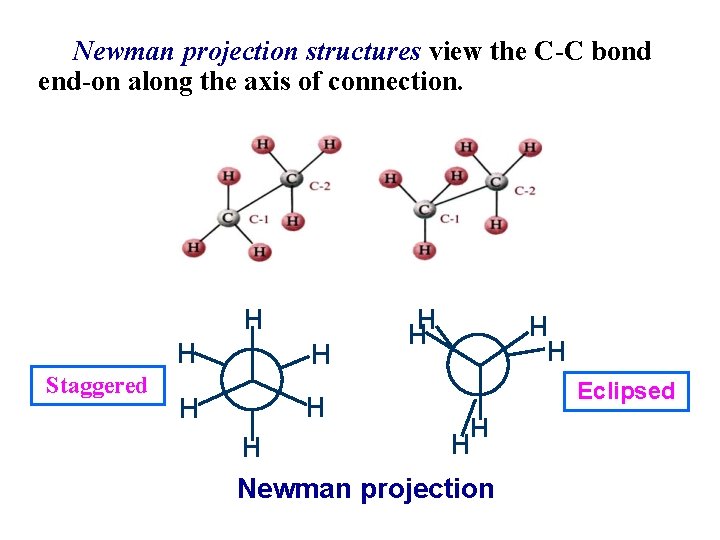
Newman projection structures view the C-C bond end-on along the axis of connection. Staggered Eclipsed Newman projection

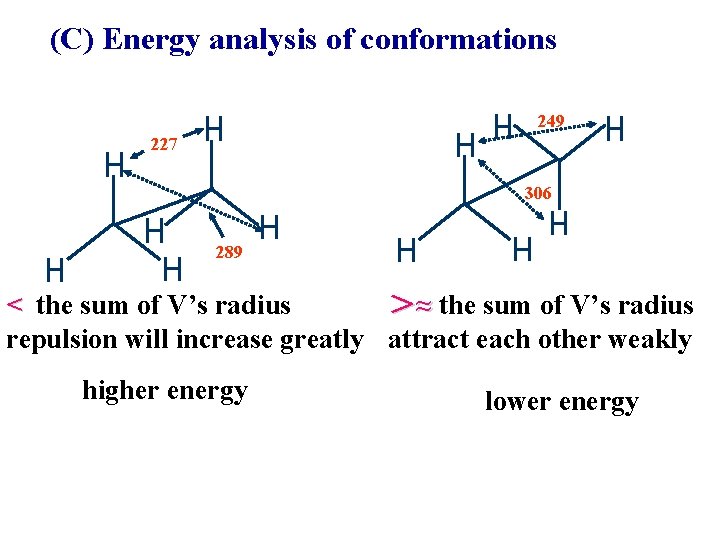
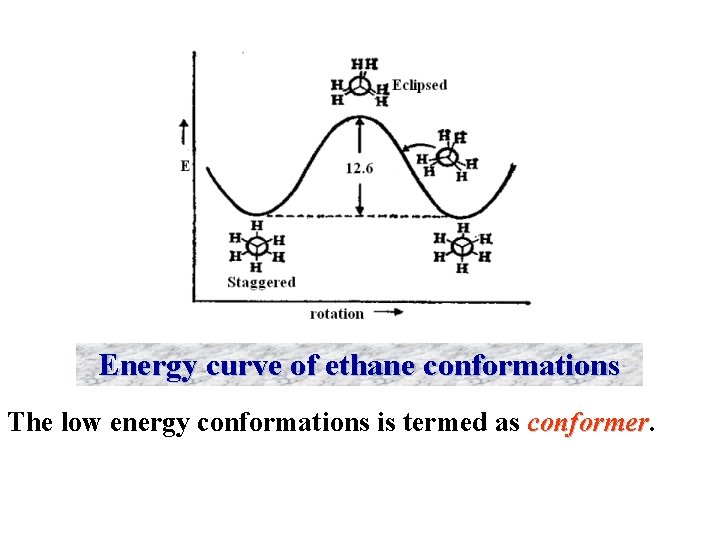
(C) Energy analysis of conformations 249 227 306 289 < the sum of V’s radius >≈ the sum of V’s radius repulsion will increase greatly attract each other weakly higher energy lower energy

Energy curve of ethane conformations The low energy conformations is termed as conformer

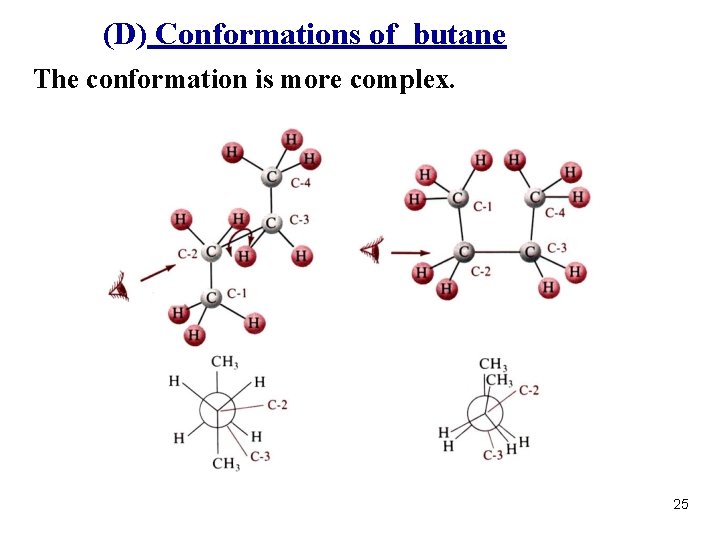
(D) Conformations of butane The conformation is more complex. 25

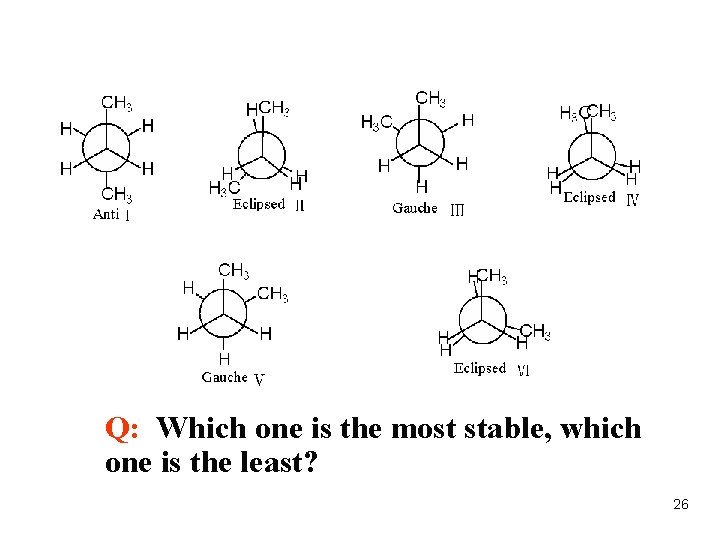
Q: Which one is the most stable, which one is the least? 26

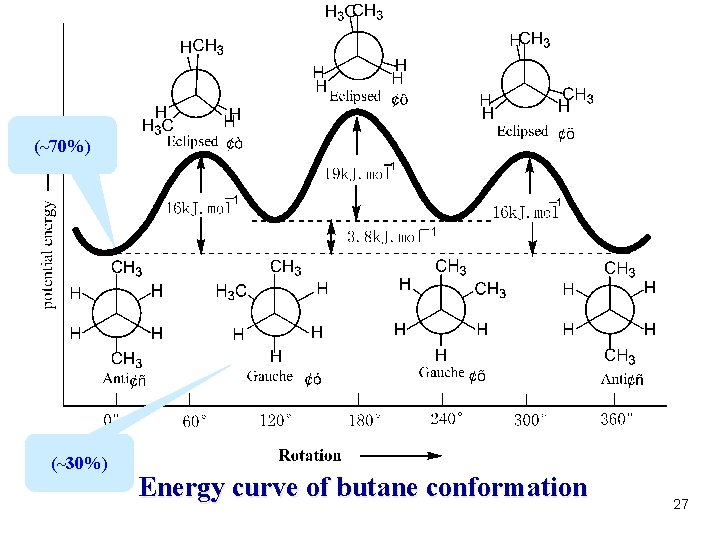
(~70%) (~30%) Energy curve of butane conformation 27

Notice: l. At room temperature, butane has many conformational isomers l. The most stable one is staggered l. The most unstable one is eclipsed Conclusion The energy of four typical conformations: Anticonformation < Gouche conformation < Eclipsed(partial ) conformation < Eclipsed conformation

in zigzag chain

¡ Q: How many conformations of butane are there? ¡ A. 2 B. 4 C. >4 D. infinite 30

II. Nomenclature 31

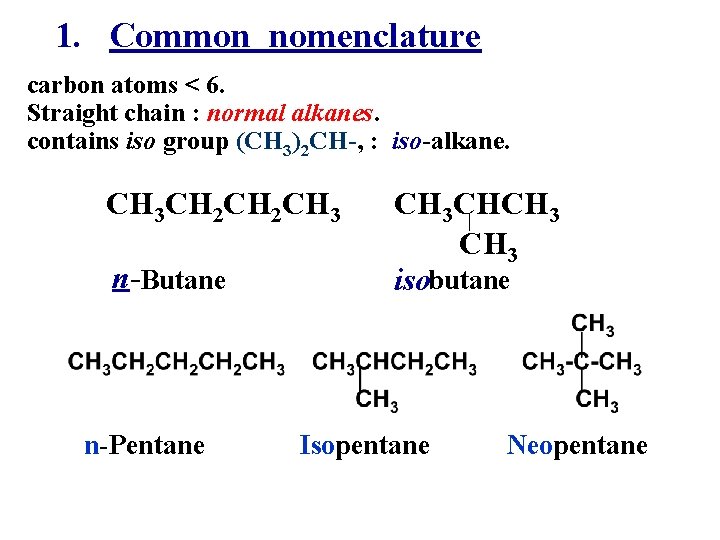
1. Common nomenclature carbon atoms < 6. Straight chain : normal alkanes. contains iso group (CH 3)2 CH-, : iso-alkane. CH 3 CH 2 CH 3 n-Butane n-Pentane CH 3 CHCH 3 isobutane Isopentane Neopentane

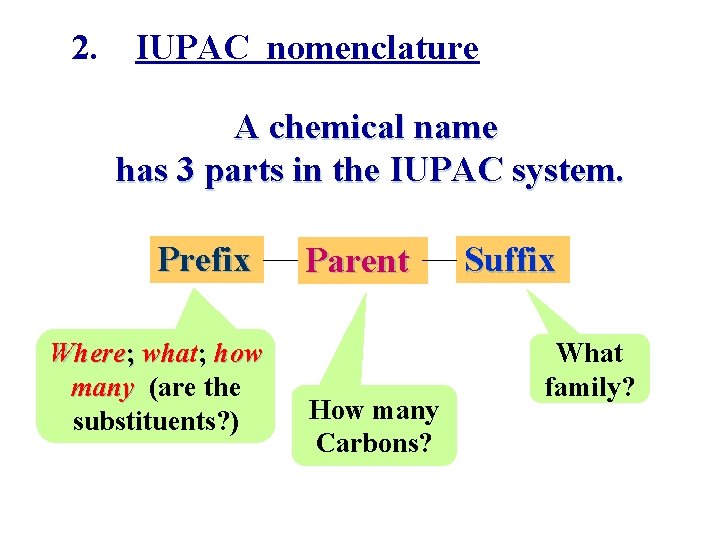
2. IUPAC nomenclature A chemical name has 3 parts in the IUPAC system. Prefix Where; what; wha how many (are the substituents? ) Parent How many Carbons? Suffix What family?

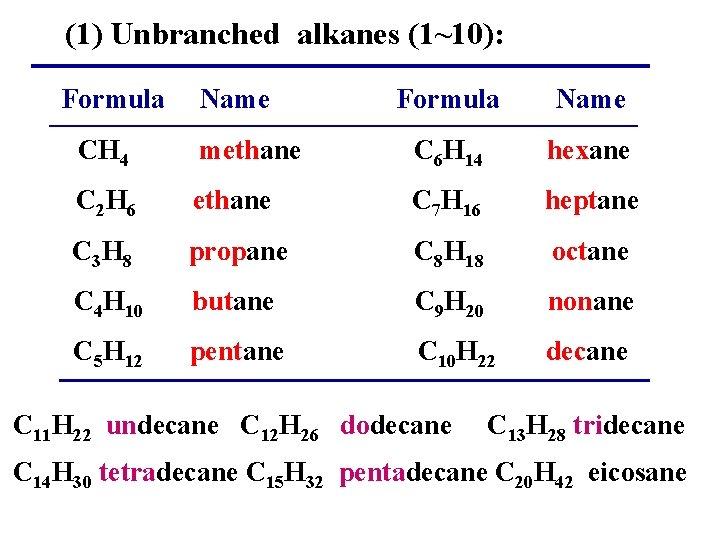
(1) Unbranched alkanes (1~10): Formula Name CH 4 methane C 6 H 14 hexane C 2 H 6 ethane C 7 H 16 heptane C 3 H 8 propane C 8 H 18 octane C 4 H 10 butane C 9 H 20 nonane C 5 H 12 pentane C 10 H 22 decane C 11 H 22 undecane C 12 H 26 dodecane C 13 H 28 tridecane C 14 H 30 tetradecane C 15 H 32 pentadecane C 20 H 42 eicosane

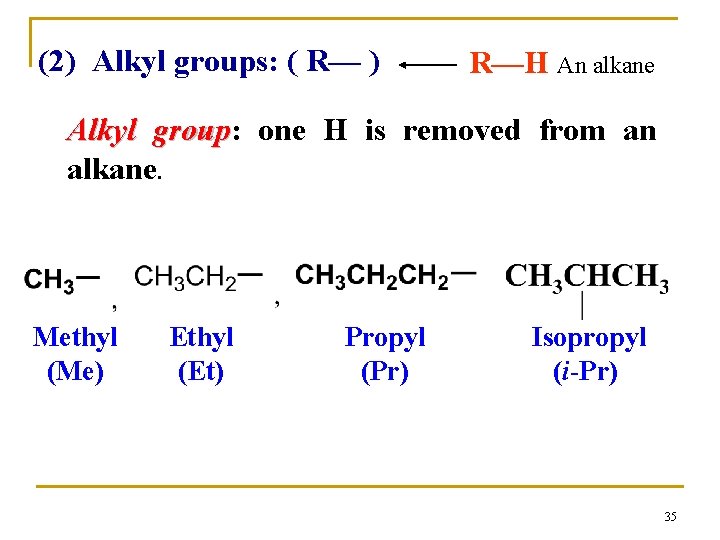
(2) Alkyl groups: ( R— ) R—H An alkane Alkyl group: group one H is removed from an alkane. Methyl (Me) Ethyl (Et) Propyl (Pr) Isopropyl (i-Pr) 35

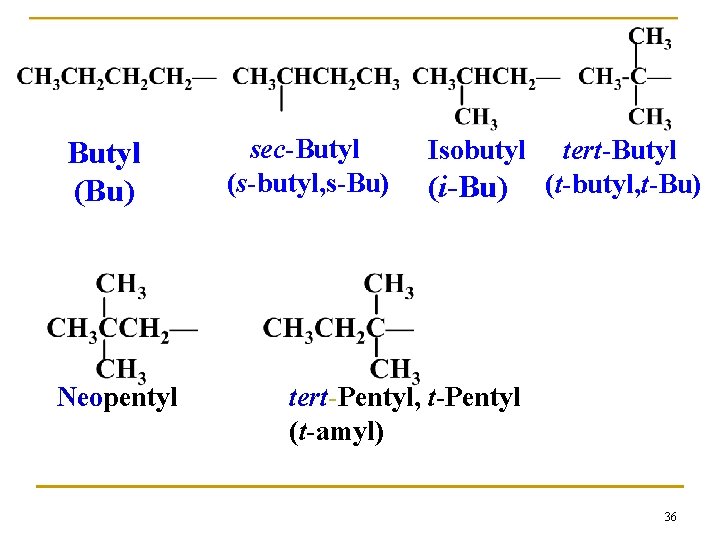
Butyl (Bu) Neopentyl sec-Butyl (s-butyl, s-Bu) Isobutyl (i-Bu) tert-Butyl (t-butyl, t-Bu) tert-Pentyl, t-Pentyl (t-amyl) 36

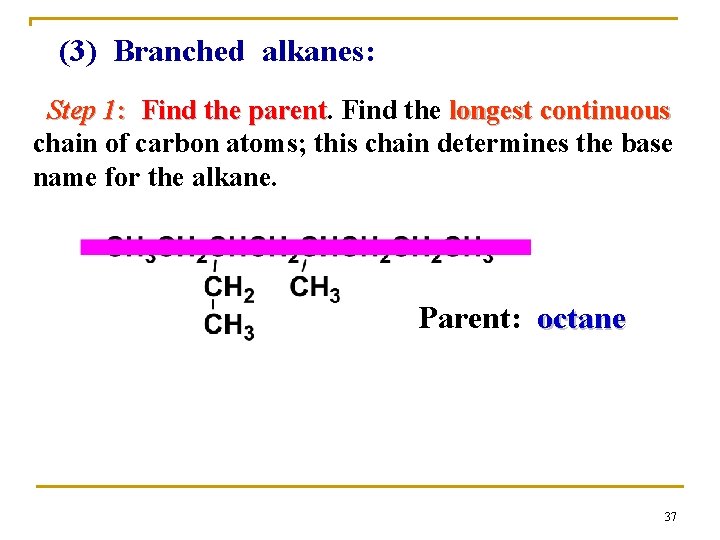
(3) Branched alkanes: Step 1: Find the parent Find the longest continuous chain of carbon atoms; this chain determines the base name for the alkane. Parent: octane 37

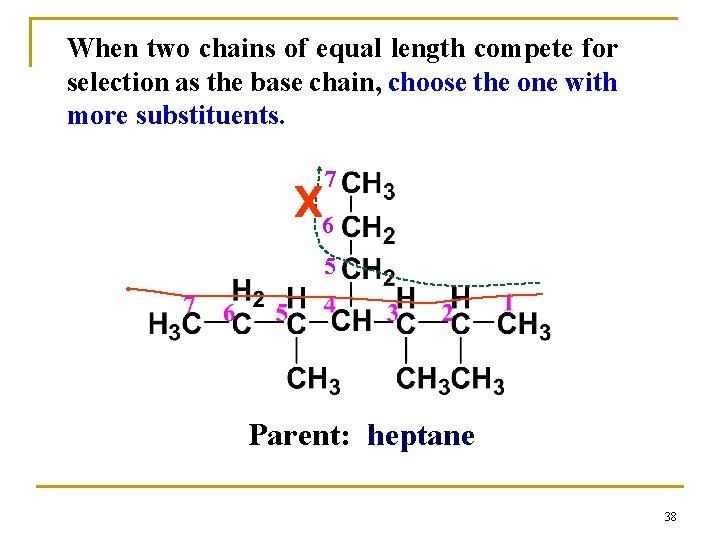
When two chains of equal length compete for selection as the base chain, choose the one with more substituents. 7 x 6 5 Parent: heptane 38

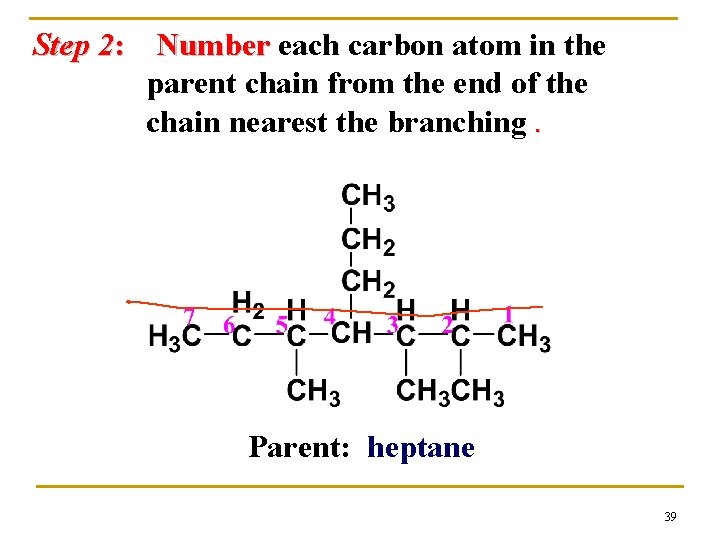
Step 2: Number each carbon atom in the parent chain from the end of the chain nearest the branching. Parent: heptane 39

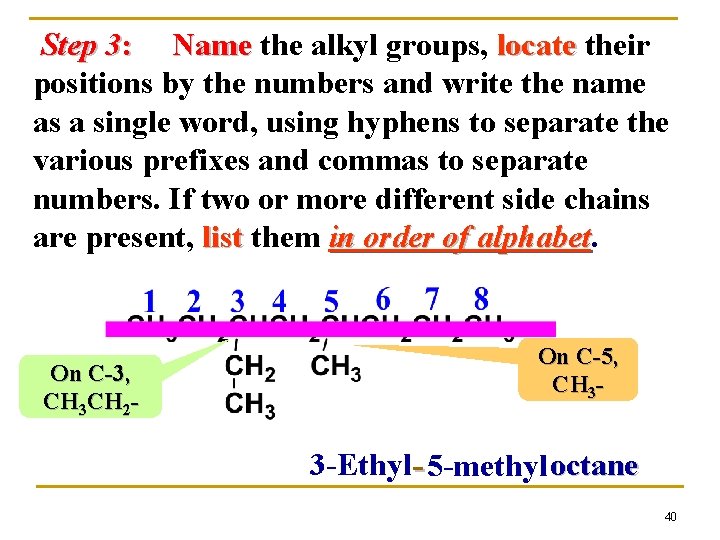
Step 3: Name the alkyl groups, locate their positions by the numbers and write the name as a single word, using hyphens to separate the various prefixes and commas to separate numbers. If two or more different side chains are present, list them in order of alphabet On C-3, CH 3 CH 2 - On C-5, CH 3 - 3 -Ethyl- 5 -methyl octane 40

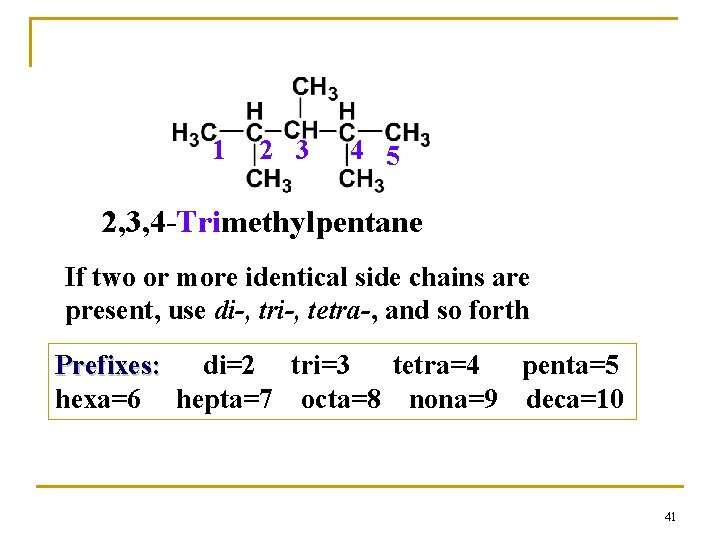
1 2 3 4 5 2, 3, 4 -Trimethylpentane If two or more identical side chains are present, use di-, tri-, tetra-, and so forth Prefixes: di=2 tri=3 tetra=4 penta=5 hexa=6 hepta=7 octa=8 nona=9 deca=10 41

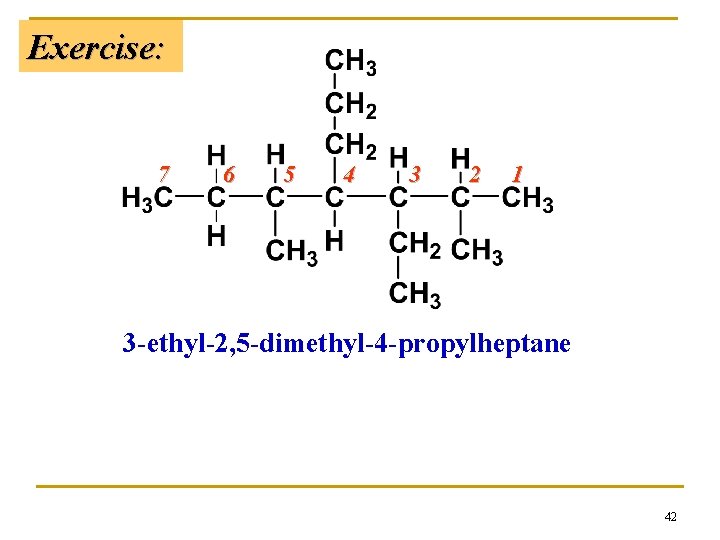
Exercise: 7 6 5 4 3 2 1 3 -ethyl-2, 5 -dimethyl-4 -propylheptane 42

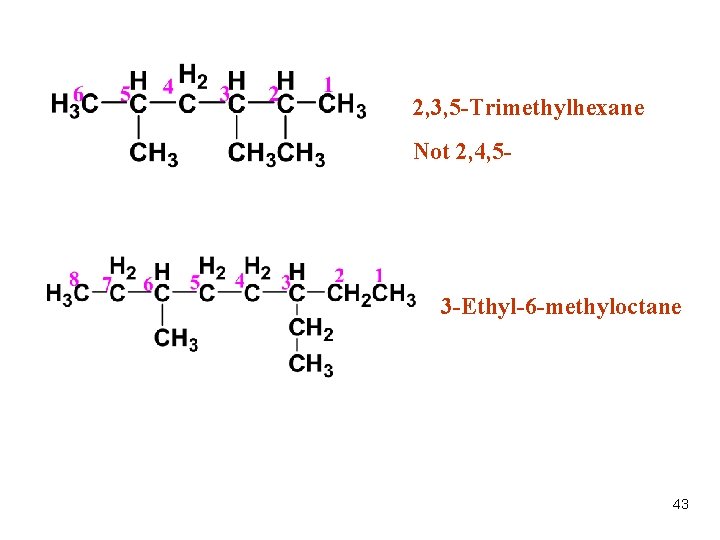
2, 3, 5 -Trimethylhexane Not 2, 4, 5 - 3 -Ethyl-6 -methyloctane 43

Notice: ① Prefixes: n-, sec-, tert- and iso, neo, are common names; ② When alphabetizing the substitutents, some prefixes (di, tri, tetra and n-, sec-, tert- and so on) should be ignored; but some prefixes ( iso, neo, cyclo) shouldn’t be ignored; ③ Be careful not to mix the two nomenclature systems. E. g. 2 -methylisobutane is wrong. 44

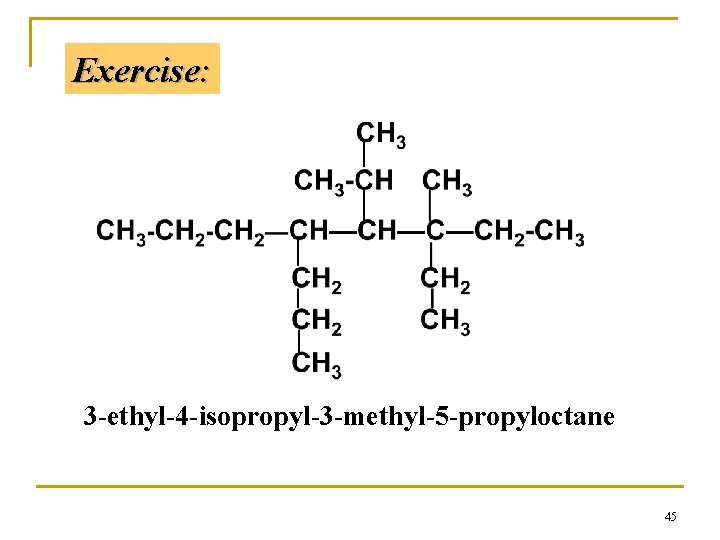
Exercise: 3 -ethyl-4 -isopropyl-3 -methyl-5 -propyloctane 45

III. Properties 1. Physical properties of alkanes 2. Chemical properties of alkanes 46

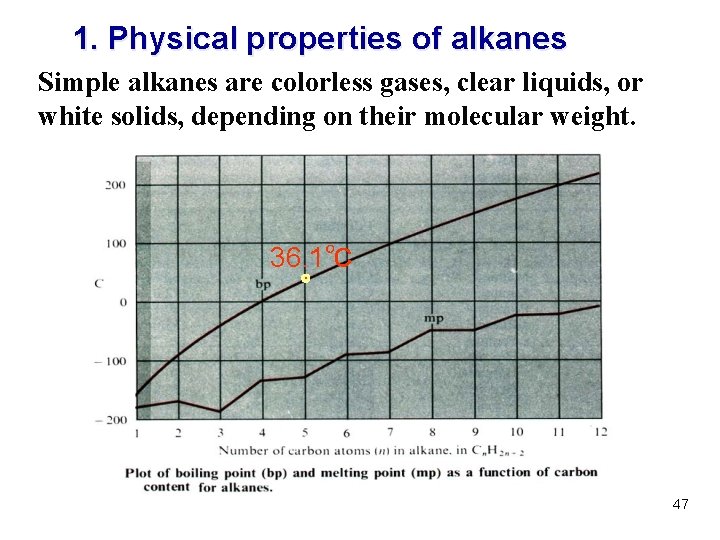
1. Physical properties of alkanes Simple alkanes are colorless gases, clear liquids, or white solids, depending on their molecular weight. 36. 1℃ 47

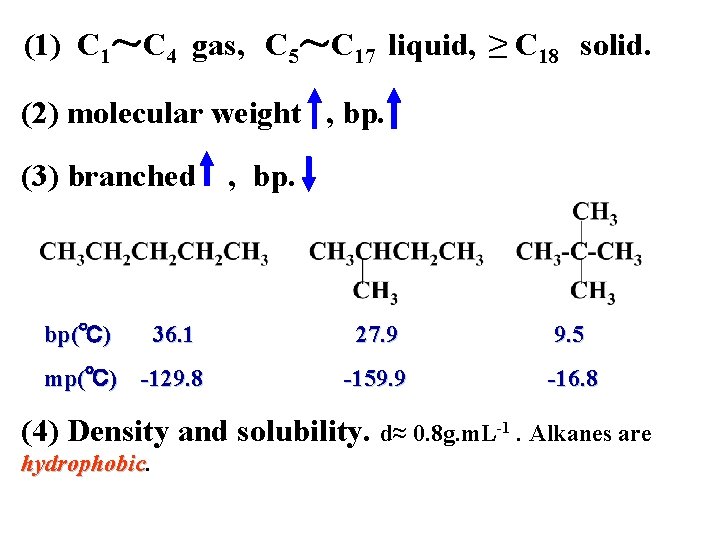
(1) C 1~C 4 gas, C 5~C 17 liquid, ≥ C 18 solid. (2) molecular weight , bp. (3) branched bp(℃) 36. 1 mp(℃) -129. 8 , bp. 27. 9 9. 5 -159. 9 -16. 8 (4) Density and solubility. d≈ 0. 8 g. m. L-1. Alkanes are hydrophobic

2. Chemical properties of alkanes Stability: C-C and C-H bonds are quite strong, so alkanes are inert to many chemical reagents. Unaffected by bases, acids, reductants, or oxidants. Q 4 Why are alkanes so inert? A reactive site in a molecule usually has one or more unshared pairs of electrons, a polar bond, an electron-deficient atom or an atom with an expandable octet. Alkanes have none of these. 49

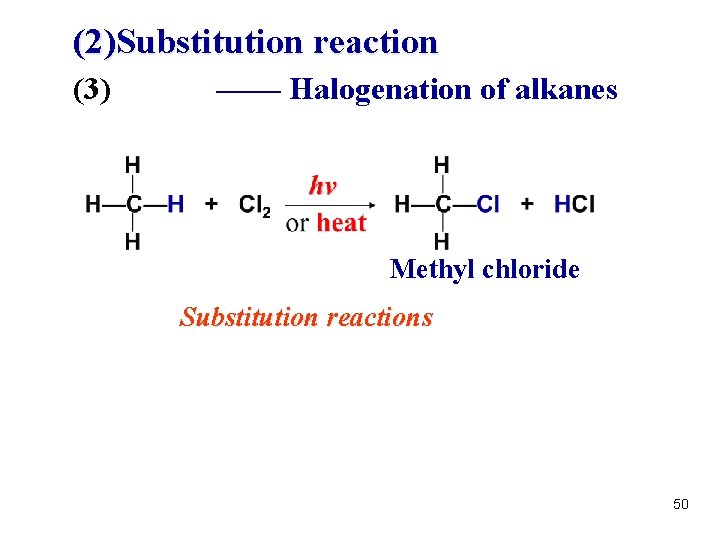
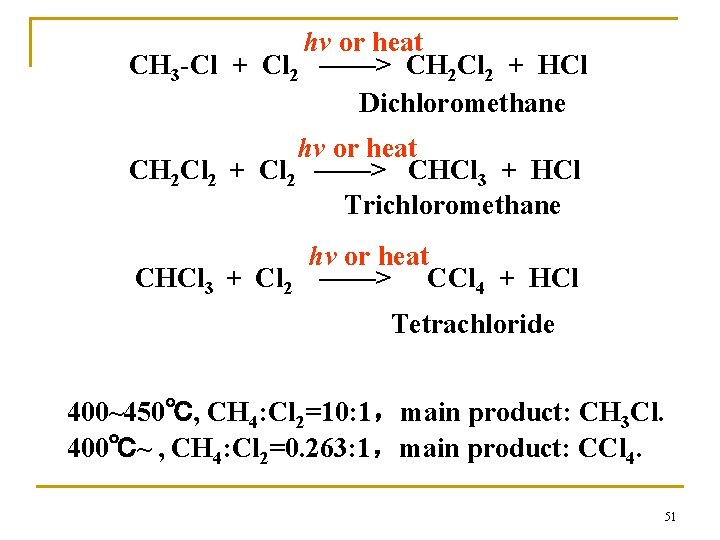
(2)Substitution reaction (3) —— Halogenation of alkanes Methyl chloride Substitution reactions 50

hv or heat CH 3 -Cl + Cl 2 ——> CH 2 Cl 2 + HCl Dichloromethane hv or heat CH 2 Cl 2 + Cl 2 ——> CHCl 3 + HCl Trichloromethane hv or heat CHCl 3 + Cl 2 ——> CCl 4 + HCl Tetrachloride 400~450℃, CH 4: Cl 2=10: 1,main product: CH 3 Cl. 400℃~ , CH 4: Cl 2=0. 263: 1,main product: CCl 4. 51

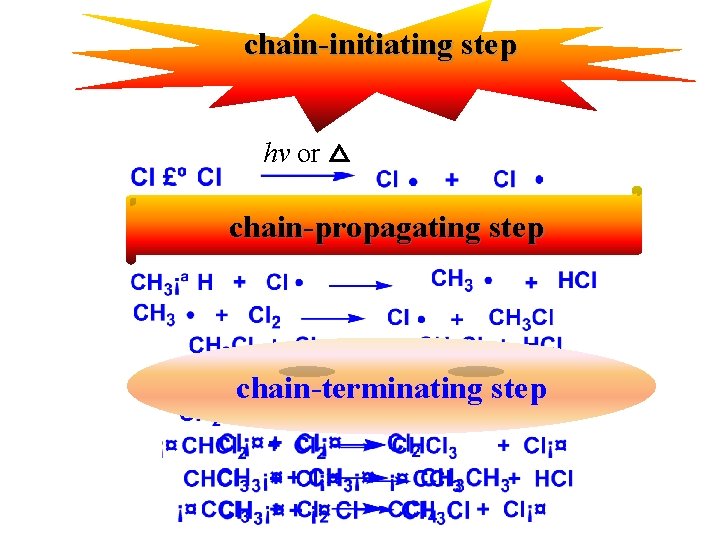
A. Reaction mechanism : How does the reaction take place? Free radical chain reaction 52

chain-initiating step hv or △ chain-propagating step chain-terminating step

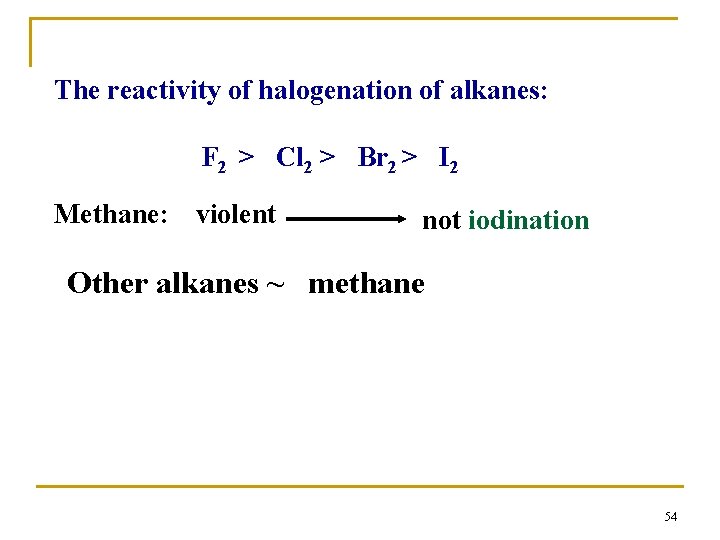
The reactivity of halogenation of alkanes: F 2 > Cl 2 > Br 2 > I 2 Methane: violent not iodination Other alkanes ~ methane 54

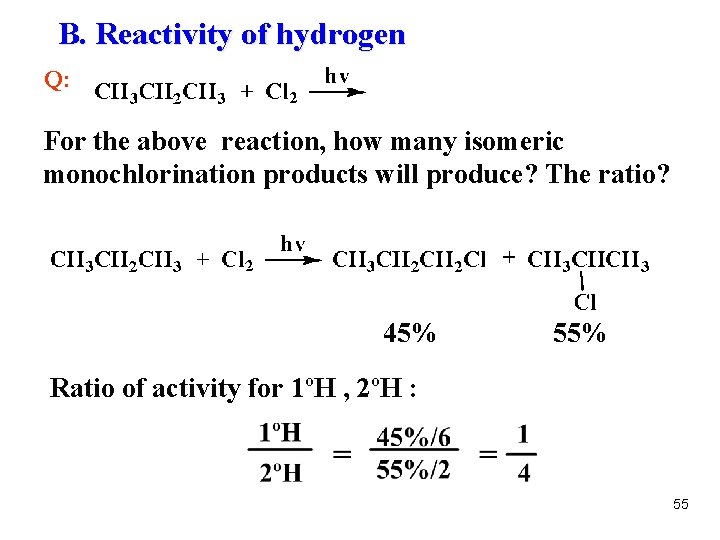
B. Reactivity of hydrogen Q: For the above reaction, how many isomeric monochlorination products will produce? The ratio? 45% 55% Ratio of activity for 1ºH , 2ºH : 55

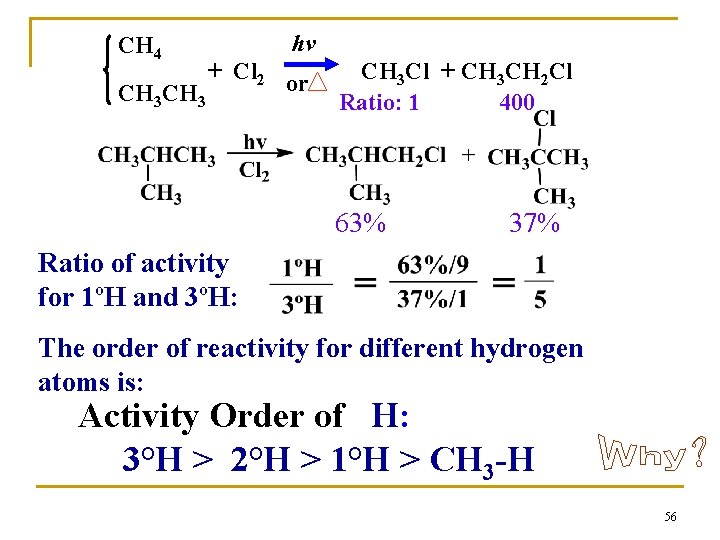
CH 4 hv + Cl 2 or CH 3 Cl + CH 3 CH 2 Cl Ratio: 1 400 63% 37% Ratio of activity for 1ºH and 3ºH: The order of reactivity for different hydrogen atoms is: Activity Order of H: 3°H > 2°H > 1°H > CH 3 -H 56

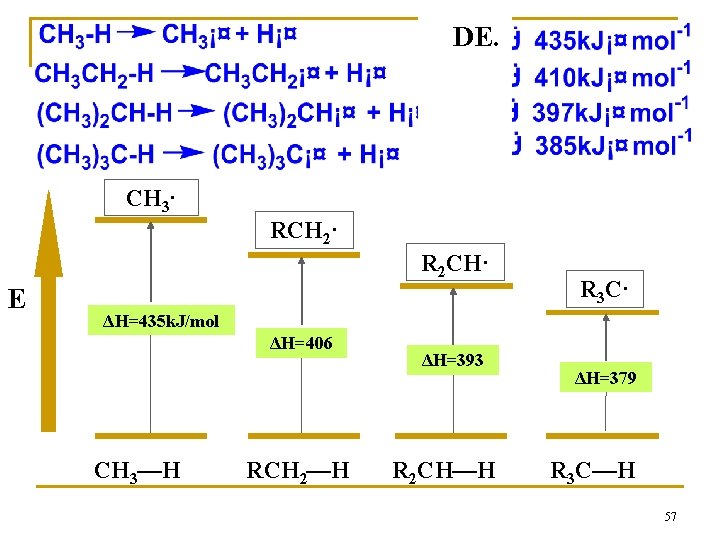
DE. CH 3· RCH 2· R 2 CH· E R 3 C· ΔH=435 k. J/mol ΔH=406 CH 3—H RCH 2—H ΔH=393 R 2 CH—H ΔH=379 R 3 C—H 57

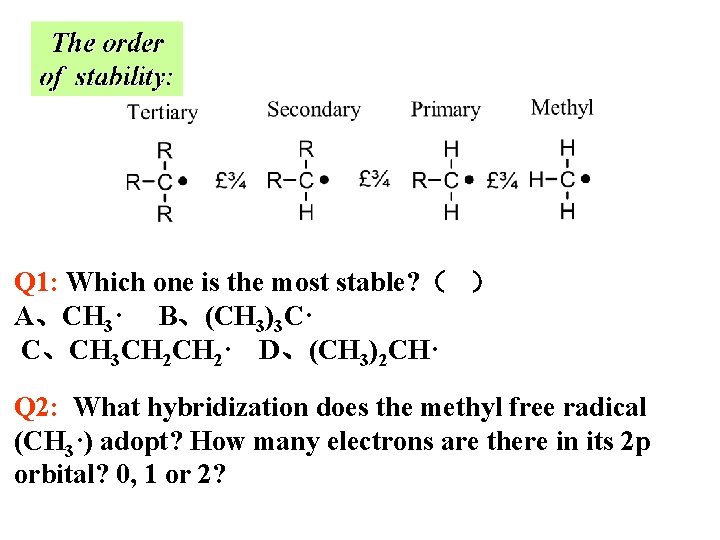
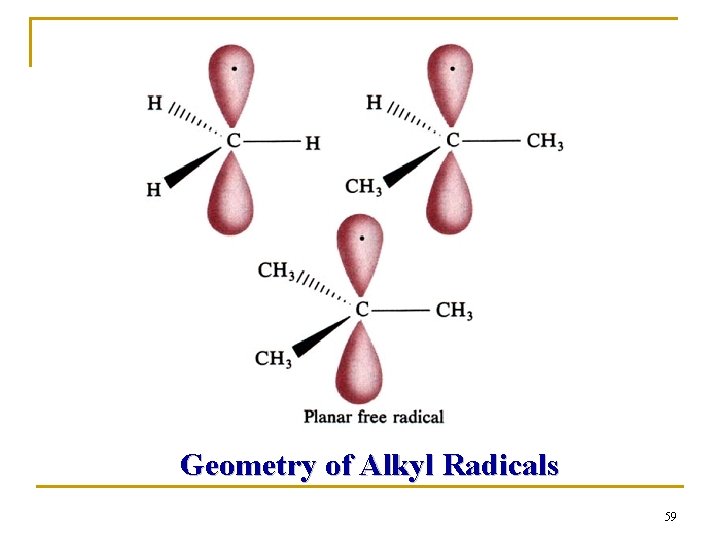
Q 1: Which one is the most stable? ( ) A、CH 3· B、(CH 3)3 C· C、CH 3 CH 2· D、(CH 3)2 CH· Q 2: What hybridization does the methyl free radical (CH 3·) adopt? How many electrons are there in its 2 p orbital? 0, 1 or 2?

Geometry of Alkyl Radicals 59

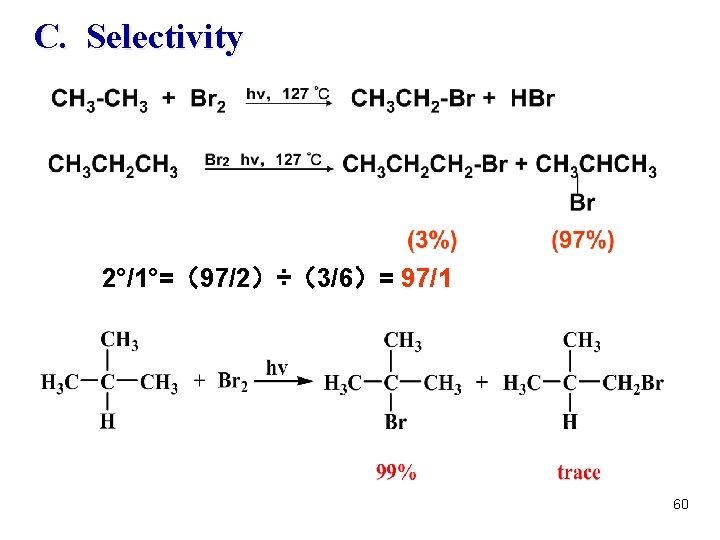
C. Selectivity 2°/1°=(97/2)÷(3/6)= 97/1 60

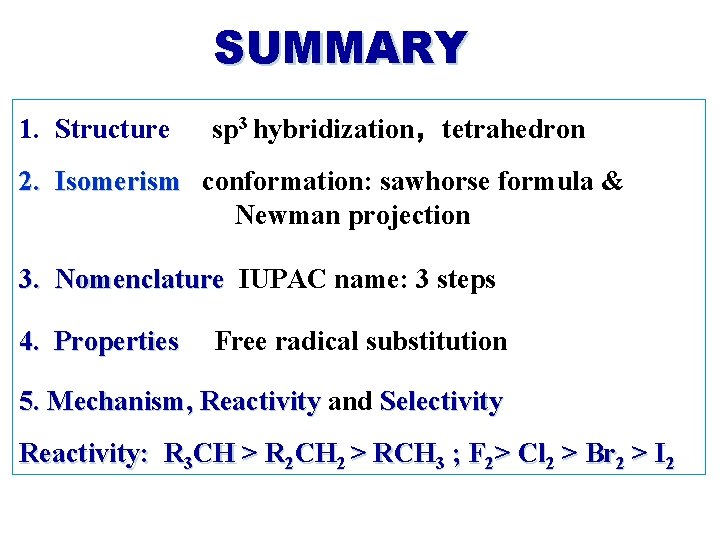
SUMMARY 1. Structure sp 3 hybridization,tetrahedron 2. Isomerism conformation: sawhorse formula & Newman projection 3. Nomenclature IUPAC name: 3 steps 4. Properties Free radical substitution 5. Mechanism, Reactivity and Selectivity Reactivity: R 3 CH > R 2 CH 2 > RCH 3 ; F 2> Cl 2 > Br 2 > I 2