Chapter 3 Alkanes and Cycloalkanes Conformations and cistrans














![Spirocyclic Systems Spirocyclic compounds have two rings with one common atom. Named as spiro[number]alkane. Spirocyclic Systems Spirocyclic compounds have two rings with one common atom. Named as spiro[number]alkane.](https://slidetodoc.com/presentation_image_h/e0add6a9e9a55a591df2340fb3b2ba46/image-58.jpg)
![Bridged Compounds Two nonadjacent atoms common to two, or more, rings. Named as bicyclo[number]alkane. Bridged Compounds Two nonadjacent atoms common to two, or more, rings. Named as bicyclo[number]alkane.](https://slidetodoc.com/presentation_image_h/e0add6a9e9a55a591df2340fb3b2ba46/image-59.jpg)
- Slides: 63

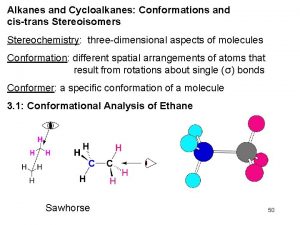
Chapter 3 Alkanes and Cycloalkanes: Conformations and cis-trans Stereoisomers

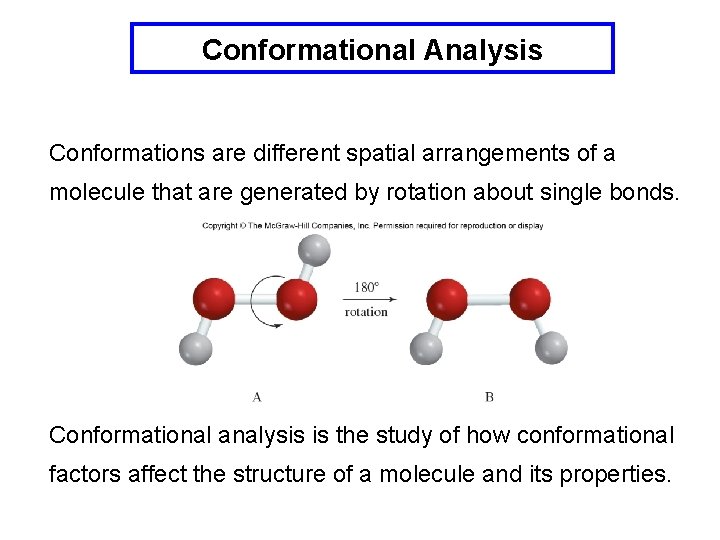
Conformational Analysis Conformations are different spatial arrangements of a molecule that are generated by rotation about single bonds. Conformational analysis is the study of how conformational factors affect the structure of a molecule and its properties.

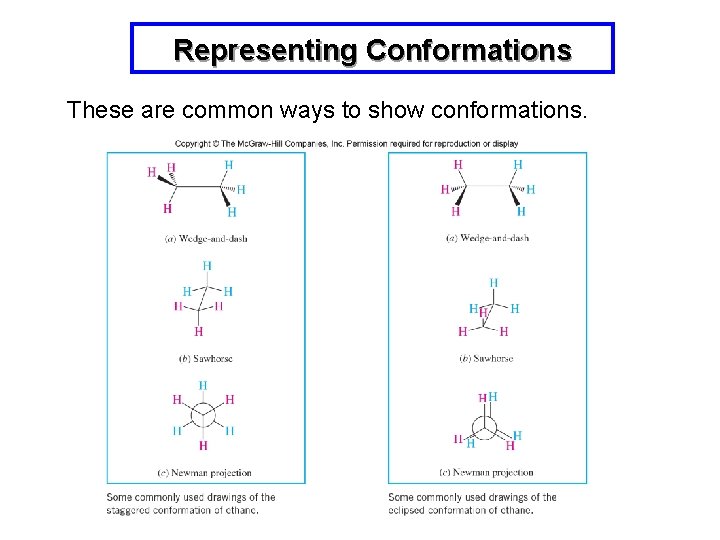
Representing Conformations These are common ways to show conformations.

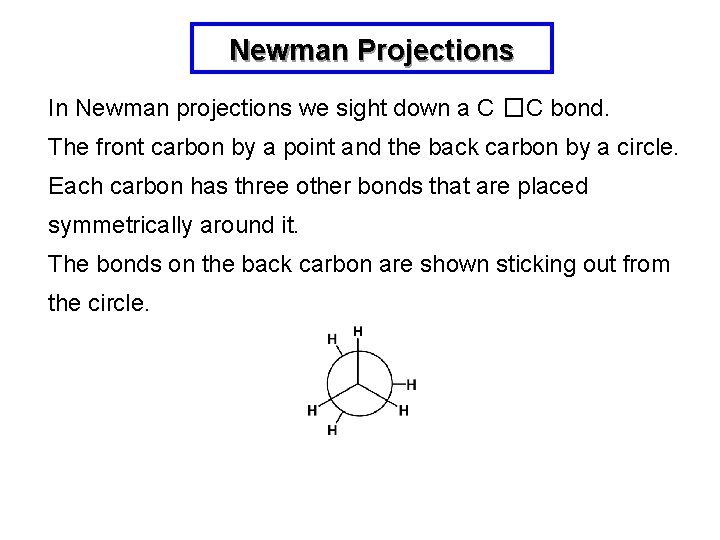
Newman Projections In Newman projections we sight down a C �C bond. The front carbon by a point and the back carbon by a circle. Each carbon has three other bonds that are placed symmetrically around it. The bonds on the back carbon are shown sticking out from the circle.

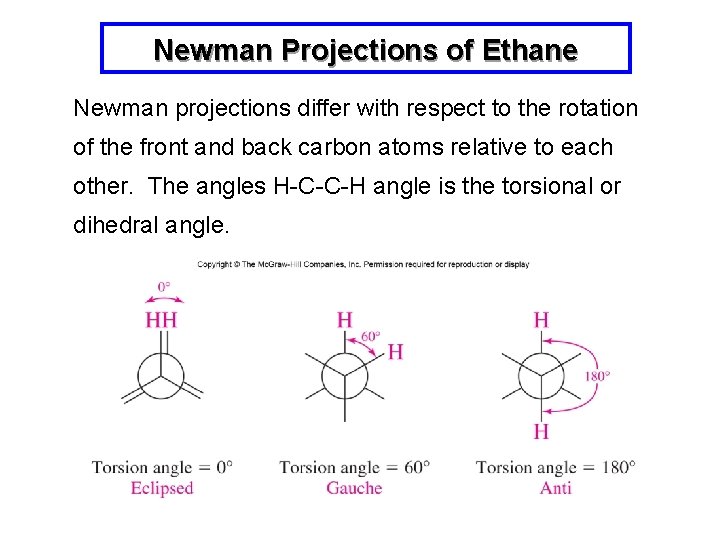
Newman Projections of Ethane Newman projections differ with respect to the rotation of the front and back carbon atoms relative to each other. The angles H-C-C-H angle is the torsional or dihedral angle.

An Important Point: The terms anti and gauche apply only to bonds (or groups) on adjacent carbons, and only to staggered conformations.

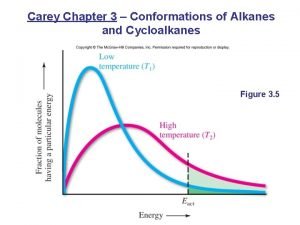
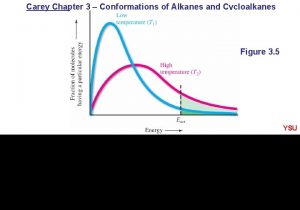
Relative Stability of Newman Projections The eclipsed conformation of ethane is the highest energy conformation. Repulsion between bonds destabilizes the eclipsed conformation. The staggered conformation is the most stable. Better electron delocalization stabilizes the staggered conformation. Conformations that are not staggered are said to have torsional strain.

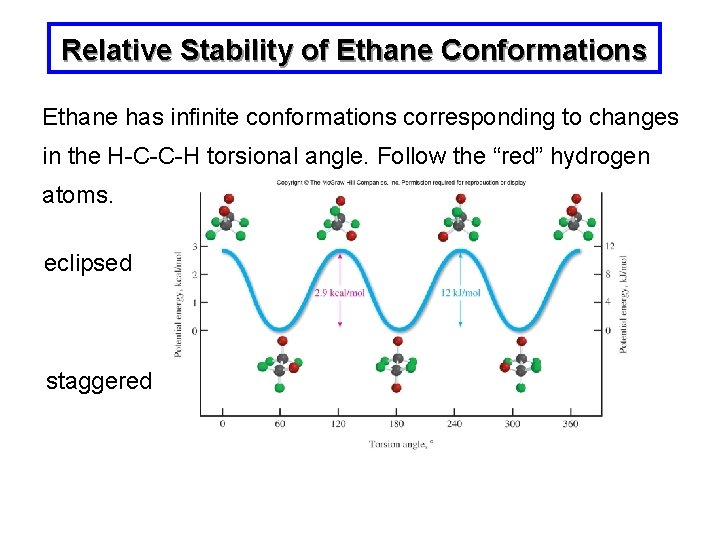
Relative Stability of Ethane Conformations Ethane has infinite conformations corresponding to changes in the H-C-C-H torsional angle. Follow the “red” hydrogen atoms. eclipsed staggered

Relative Stability of Newman Projections At any instant, almost all of the molecules are in staggered conformations; hardly any are in eclipsed conformations. The difference between these two conformations is 12 k. J/mol.

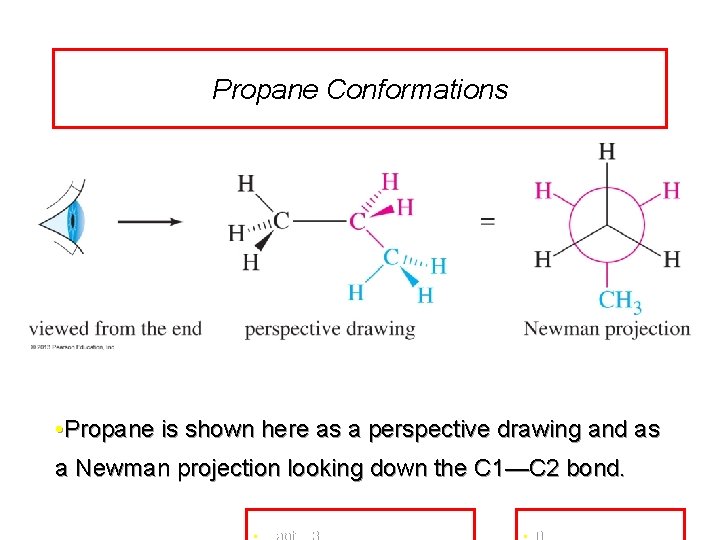
Propane Conformations • Propane is shown here as a perspective drawing and as a Newman projection looking down the C 1—C 2 bond.

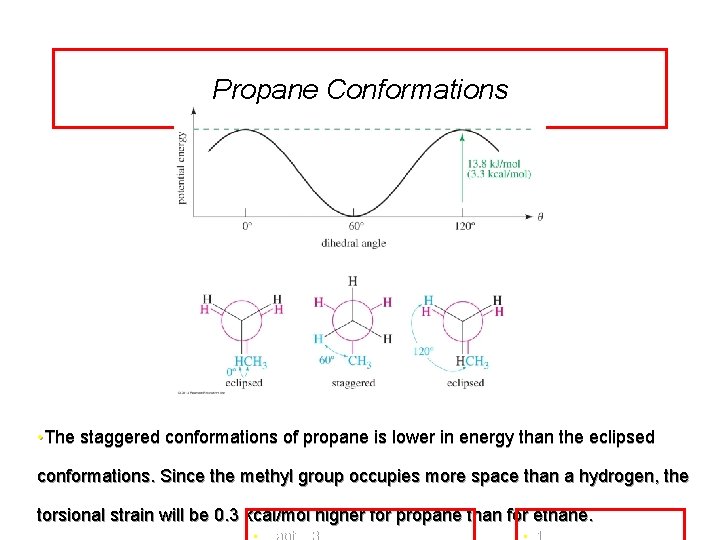
Propane Conformations • The staggered conformations of propane is lower in energy than the eclipsed conformations. Since the methyl group occupies more space than a hydrogen, the torsional strain will be 0. 3 kcal/mol higher for propane than for ethane.

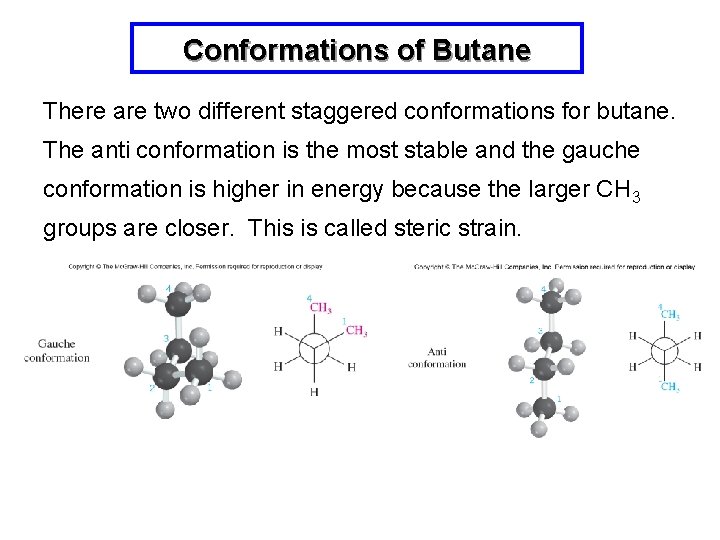
Conformations of Butane There are two different staggered conformations for butane. The anti conformation is the most stable and the gauche conformation is higher in energy because the larger CH 3 groups are closer. This is called steric strain.

Strain in Newman Projections Torsional strain is the strain that results from eclipsed bonds. Steric hindrance results when two atoms are too close together. Also called van der Waals strain. Steric strain is the combination of both of these.

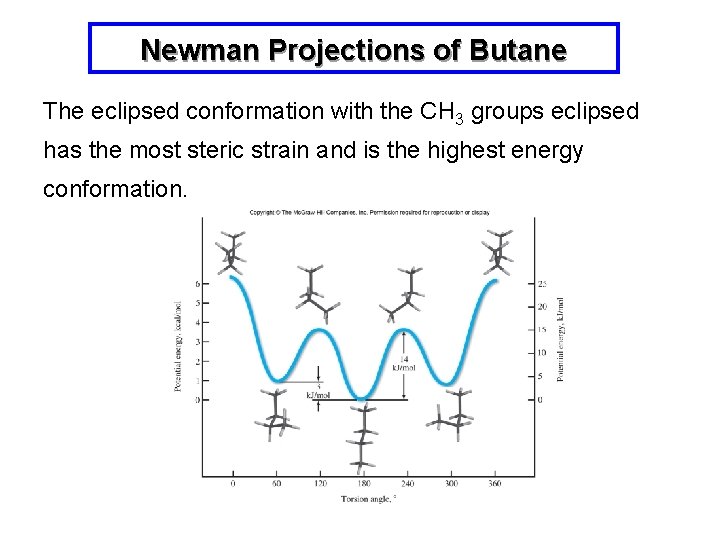
Newman Projections of Butane The eclipsed conformation with the CH 3 groups eclipsed has the most steric strain and is the highest energy conformation.

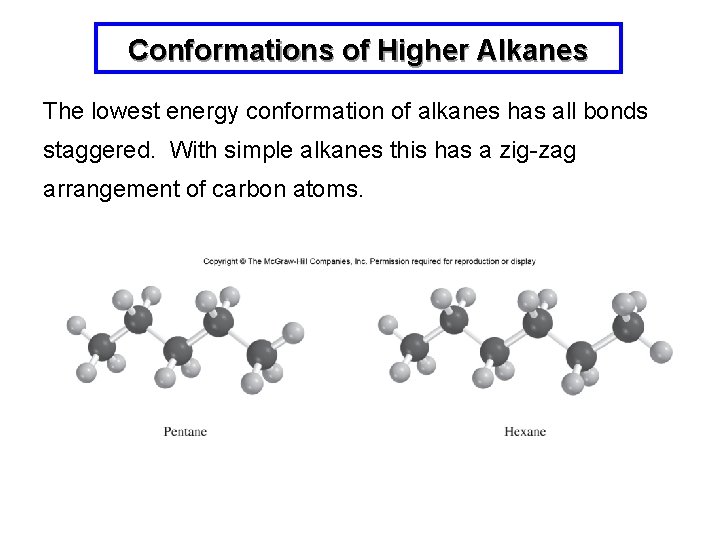
Conformations of Higher Alkanes The lowest energy conformation of alkanes has all bonds staggered. With simple alkanes this has a zig-zag arrangement of carbon atoms.

The Shapes of Cycloalkanes: Planar or Nonplanar?

Stabilities of Cycloalkanes • Five- and six-membered rings are the most common in nature. • Carbons of cycloalkanes are sp 3 hybridized and thus require an angle of 109. 5º. • When a cycloalkane carbon has an angle other than 109. 5º, there will not be optimum overlap and the compound will have angle strain. • Angle strain is sometimes called Baeyer strain in honor of Adolf von Baeyer, who first explained this phenomenon. • Torsional strain arises when all the bonds are eclipsed. © 2013 Pearson Education, Inc.

Types of Strain • Torsional strain that results from eclipsed bonds • van der Waals strain (steric strain) strain that results from atoms being too close together • angle strain that results from distortion of bond angles from normal values

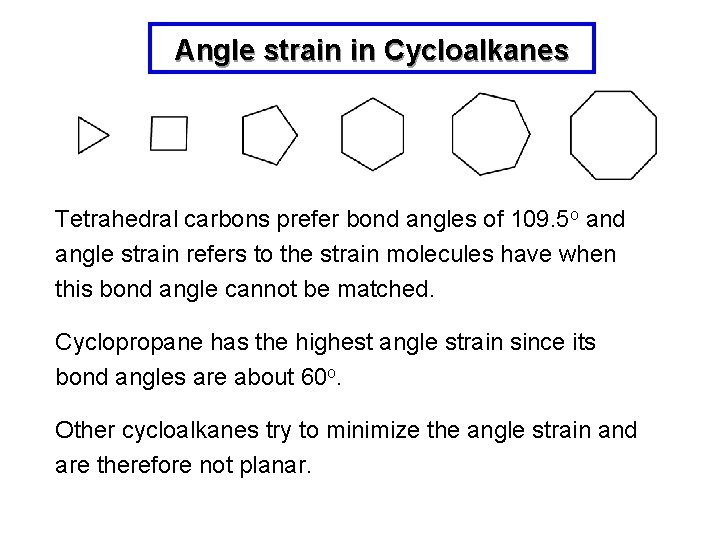
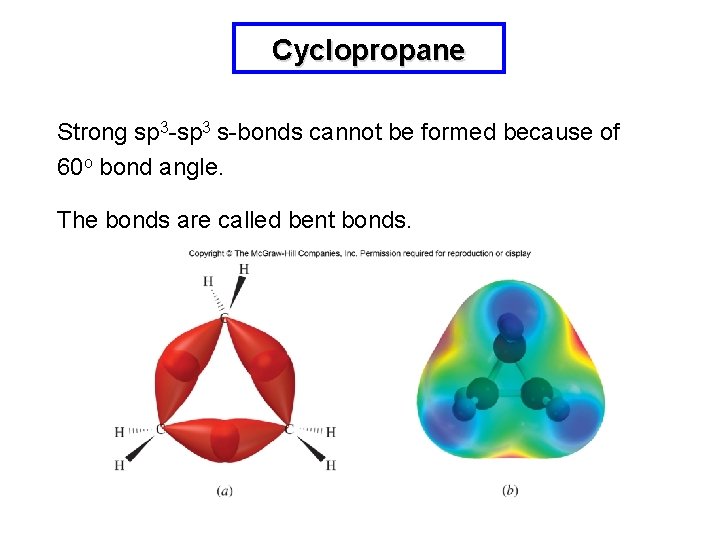
Angle strain in Cycloalkanes Tetrahedral carbons prefer bond angles of 109. 5 o and angle strain refers to the strain molecules have when this bond angle cannot be matched. Cyclopropane has the highest angle strain since its bond angles are about 60 o. Other cycloalkanes try to minimize the angle strain and are therefore not planar.

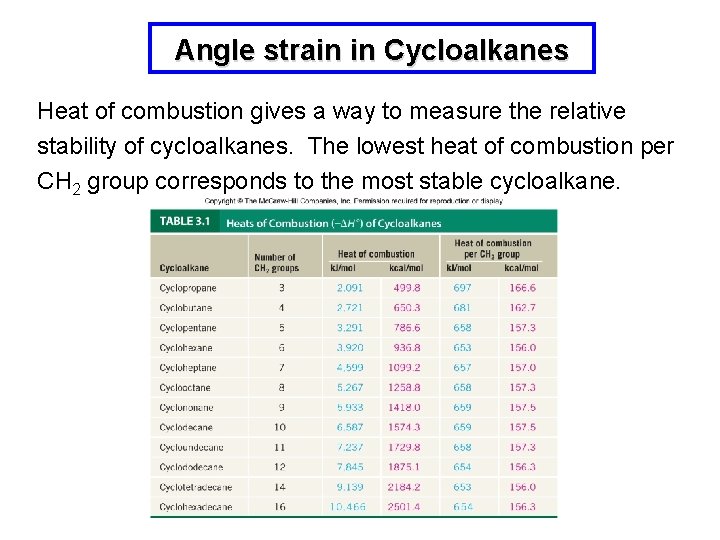
Angle strain in Cycloalkanes Heat of combustion gives a way to measure the relative stability of cycloalkanes. The lowest heat of combustion per CH 2 group corresponds to the most stable cycloalkane.

Cyclopropane Strong sp 3 -sp 3 s-bonds cannot be formed because of 60 o bond angle. The bonds are called bent bonds.

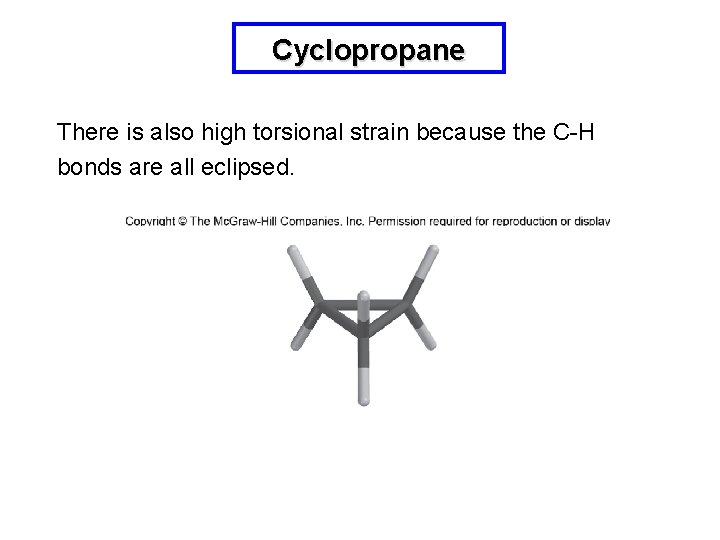
Cyclopropane There is also high torsional strain because the C-H bonds are all eclipsed.

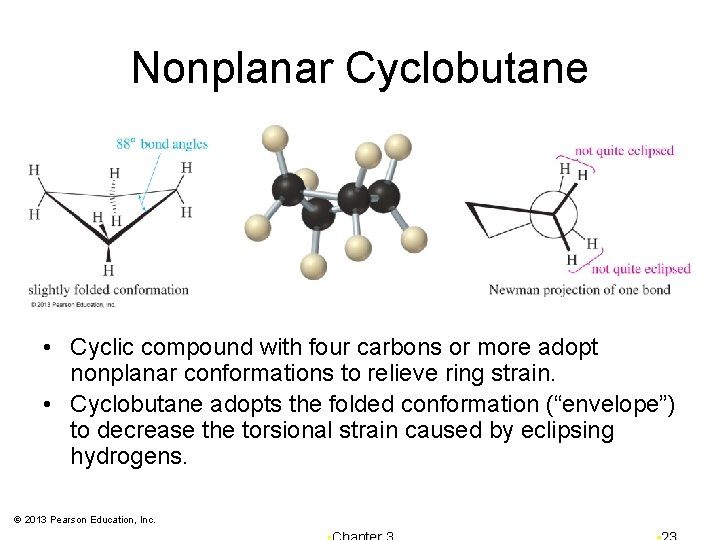
Nonplanar Cyclobutane • Cyclic compound with four carbons or more adopt nonplanar conformations to relieve ring strain. • Cyclobutane adopts the folded conformation (“envelope”) to decrease the torsional strain caused by eclipsing hydrogens. © 2013 Pearson Education, Inc.

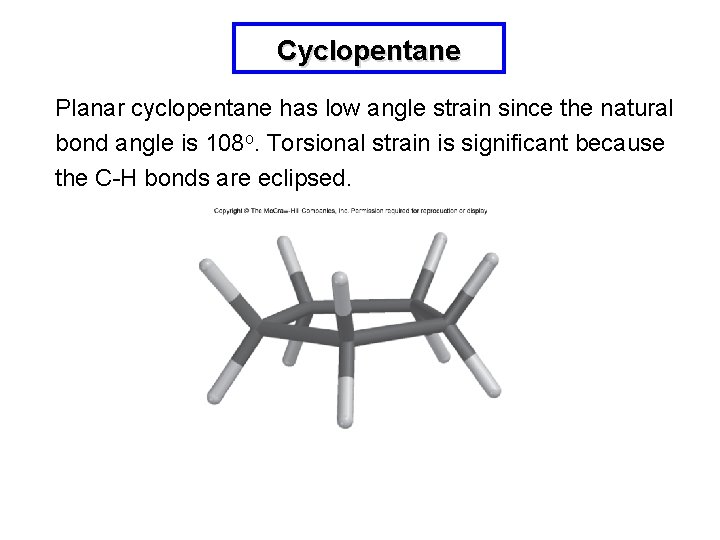
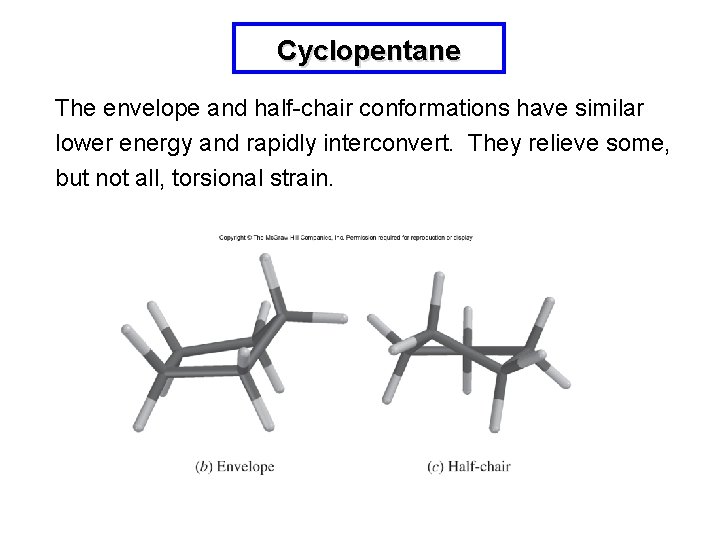
Cyclopentane Planar cyclopentane has low angle strain since the natural bond angle is 108 o. Torsional strain is significant because the C-H bonds are eclipsed.

Cyclopentane The envelope and half-chair conformations have similar lower energy and rapidly interconvert. They relieve some, but not all, torsional strain.

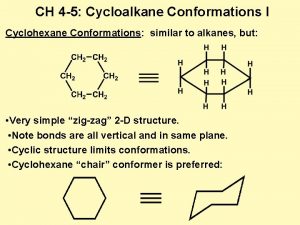
Conformations of Cyclohexane

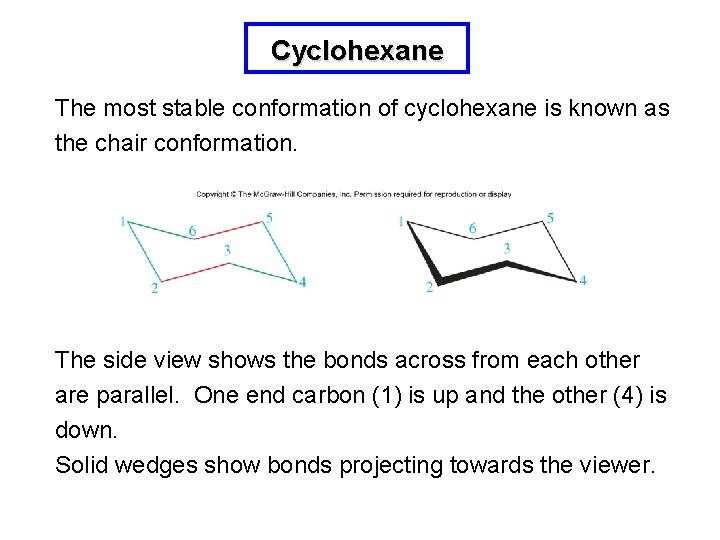
Cyclohexane The most stable conformation of cyclohexane is known as the chair conformation. The side view shows the bonds across from each other are parallel. One end carbon (1) is up and the other (4) is down. Solid wedges show bonds projecting towards the viewer.

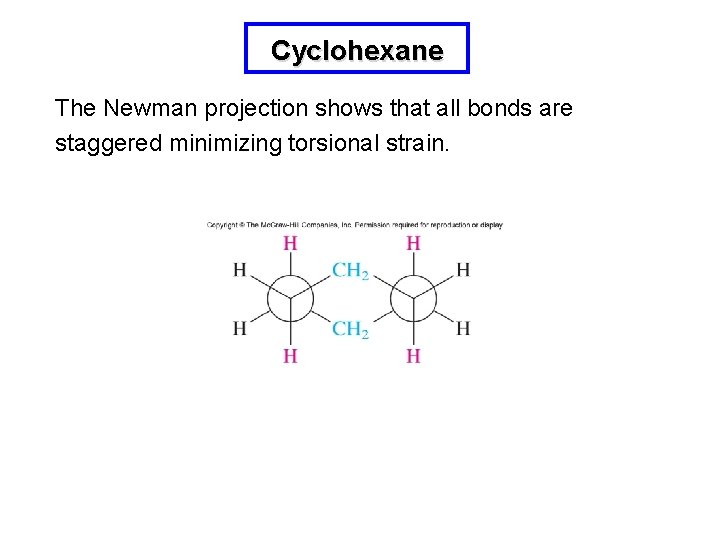
Cyclohexane The Newman projection shows that all bonds are staggered minimizing torsional strain.

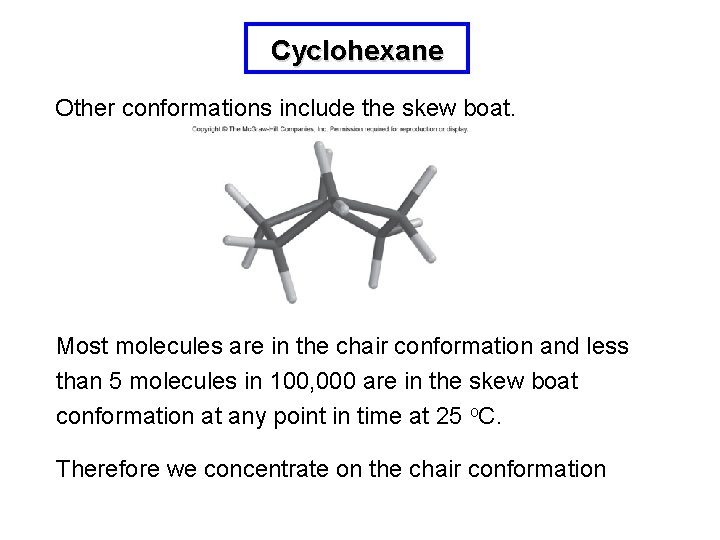
Cyclohexane Other conformations include the skew boat. Most molecules are in the chair conformation and less than 5 molecules in 100, 000 are in the skew boat conformation at any point in time at 25 o. C. Therefore we concentrate on the chair conformation

Axial and Equatorial Bonds in Cyclohexane

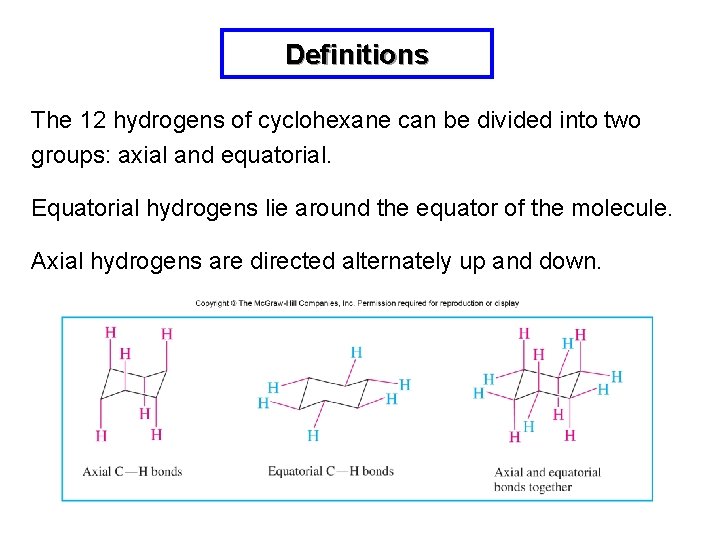
Definitions The 12 hydrogens of cyclohexane can be divided into two groups: axial and equatorial. Equatorial hydrogens lie around the equator of the molecule. Axial hydrogens are directed alternately up and down.

Conformational Inversion in Cyclohexane

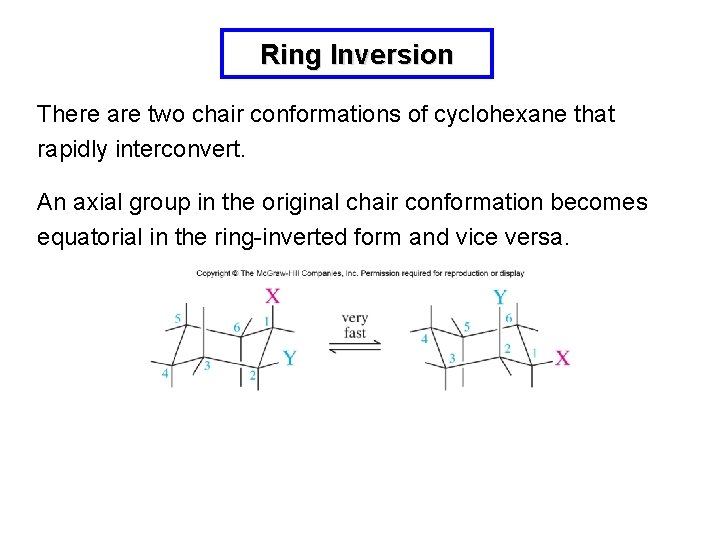
Ring Inversion There are two chair conformations of cyclohexane that rapidly interconvert. An axial group in the original chair conformation becomes equatorial in the ring-inverted form and vice versa.

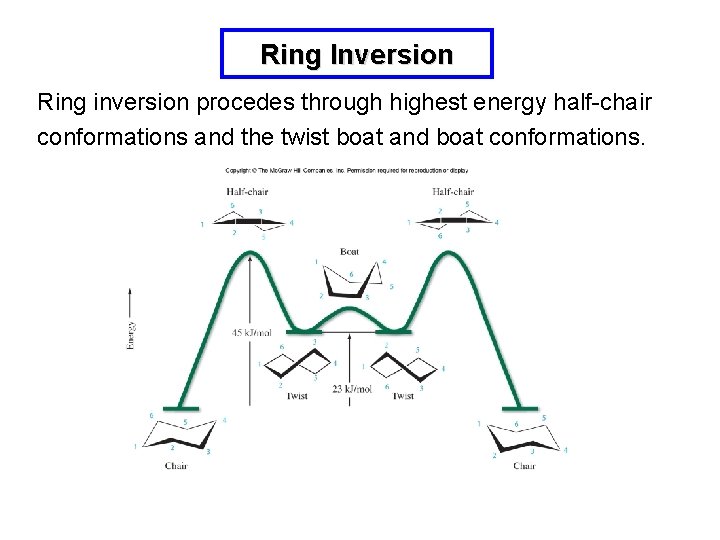
Ring Inversion Ring inversion procedes through highest energy half-chair conformations and the twist boat and boat conformations.

Conformational Analysis of Monosubstituted Cyclohexanes

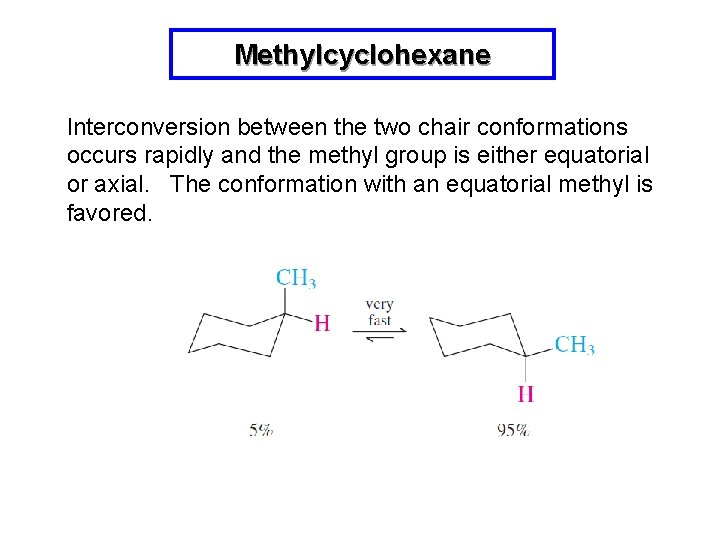
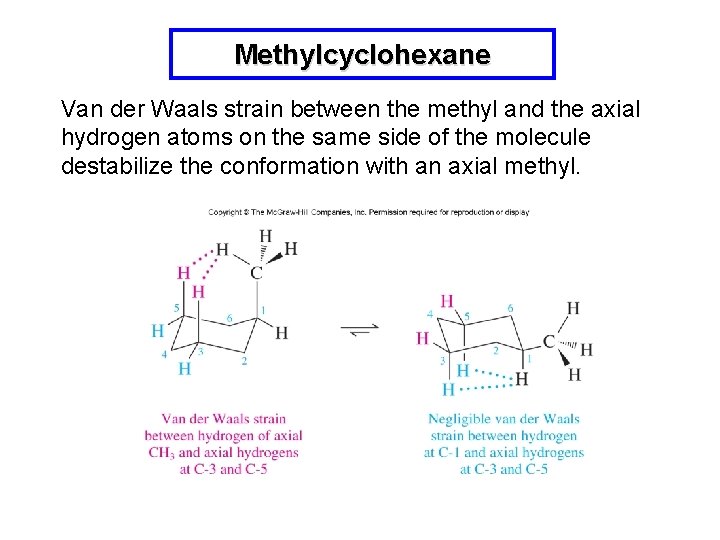
Methylcyclohexane Interconversion between the two chair conformations occurs rapidly and the methyl group is either equatorial or axial. The conformation with an equatorial methyl is favored.

Methylcyclohexane Van der Waals strain between the methyl and the axial hydrogen atoms on the same side of the molecule destabilize the conformation with an axial methyl.

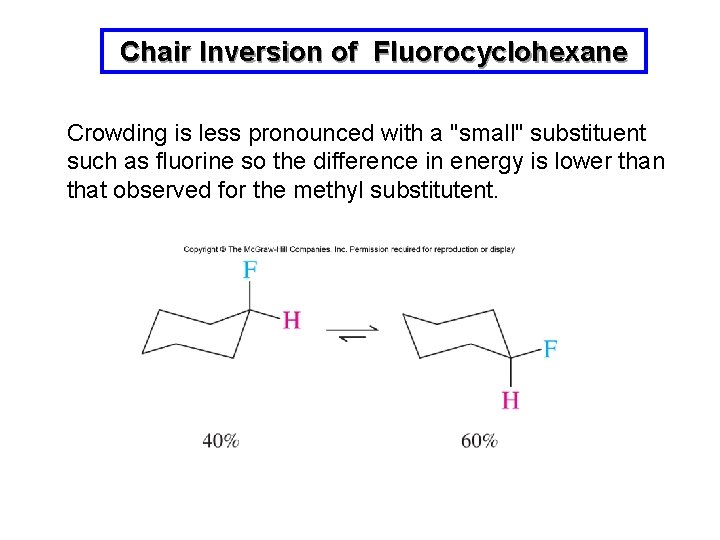
Chair Inversion of Fluorocyclohexane Crowding is less pronounced with a "small" substituent such as fluorine so the difference in energy is lower than that observed for the methyl substitutent.

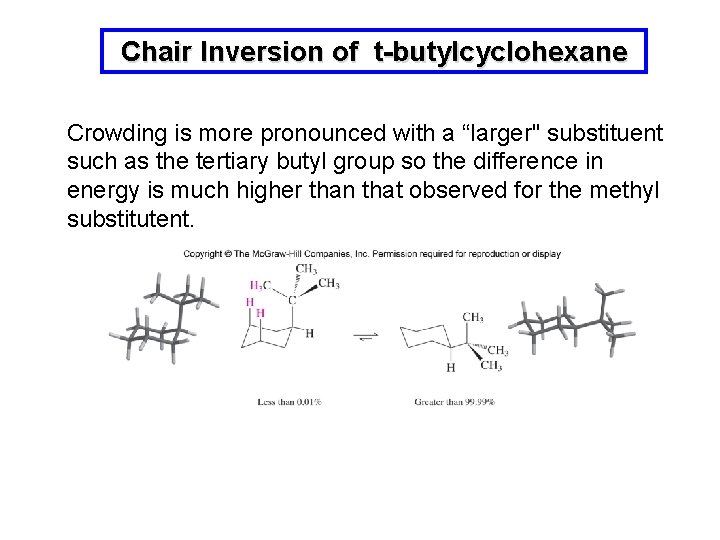
Chair Inversion of t-butylcyclohexane Crowding is more pronounced with a “larger" substituent such as the tertiary butyl group so the difference in energy is much higher than that observed for the methyl substitutent.

Disubstituted Cycloalkanes: cis-trans Stereoisomers

Definitions of Isomers are different compounds that have the same molecular formula. Constitutional isomers differ in connectivity. Stereoisomers have the same connectivity but a different arrangement of the atoms in space.

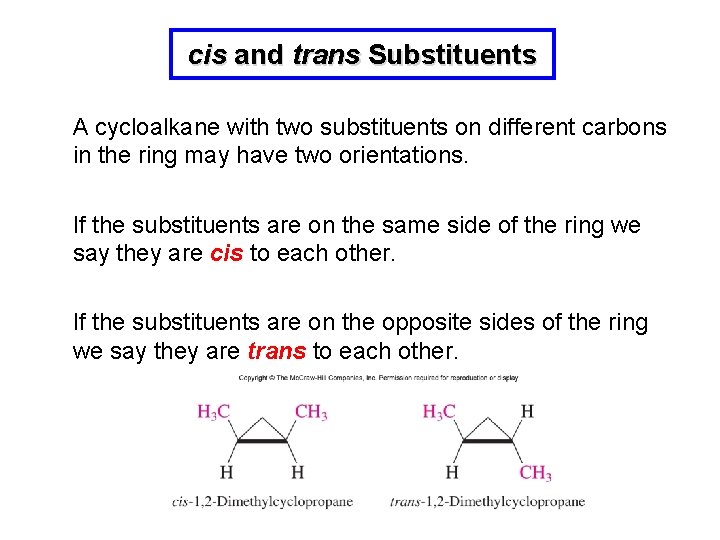
cis and trans Substituents A cycloalkane with two substituents on different carbons in the ring may have two orientations. If the substituents are on the same side of the ring we say they are cis to each other. If the substituents are on the opposite sides of the ring we say they are trans to each other.

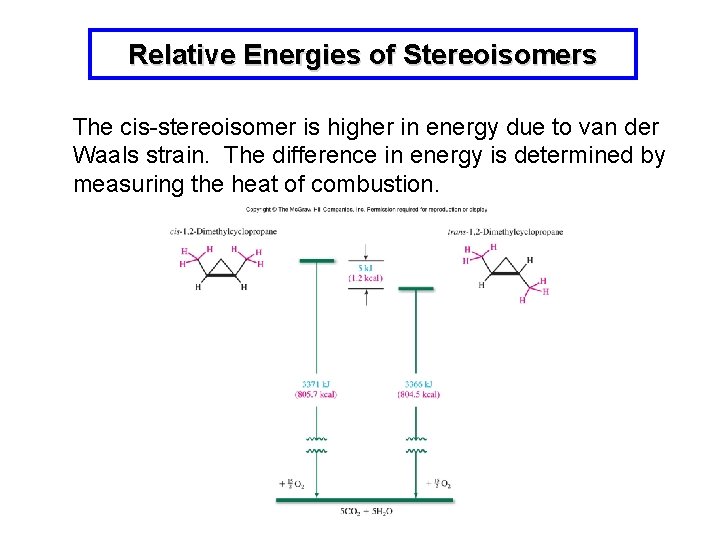
Relative Energies of Stereoisomers The cis-stereoisomer is higher in energy due to van der Waals strain. The difference in energy is determined by measuring the heat of combustion.

Conformational Analysis of Disubstituted Cyclohexanes

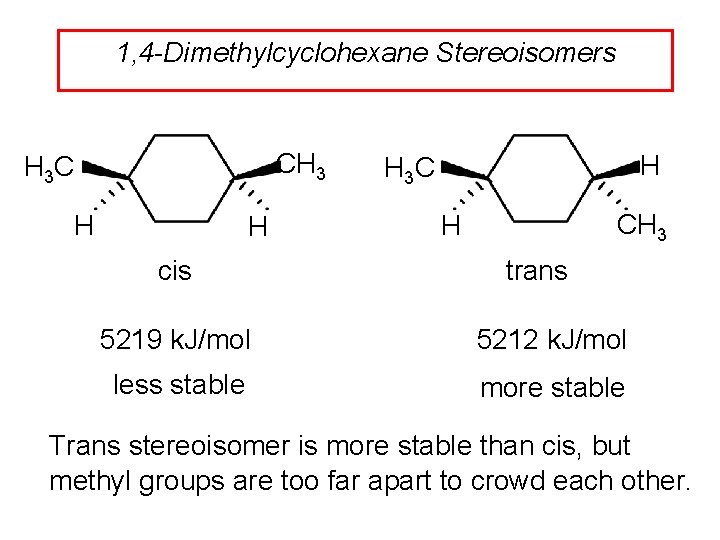
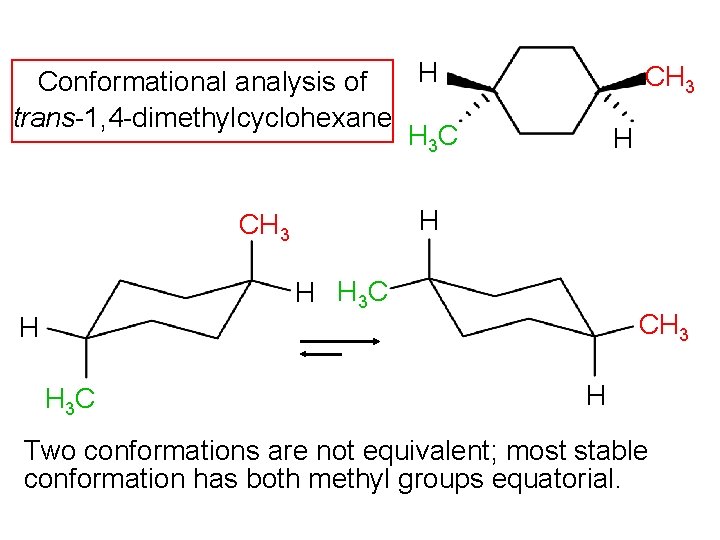
1, 4 -Dimethylcyclohexane Stereoisomers CH 3 H 3 C H H cis H H 3 C CH 3 H trans 5219 k. J/mol 5212 k. J/mol less stable more stable Trans stereoisomer is more stable than cis, but methyl groups are too far apart to crowd each other.

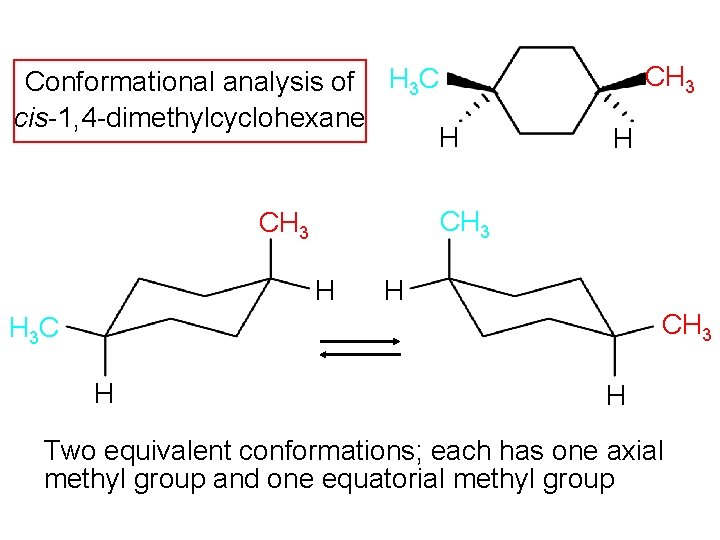
Conformational analysis of H 3 C cis-1, 4 -dimethylcyclohexane H CH 3 H H CH 3 H 3 C H H Two equivalent conformations; each has one axial methyl group and one equatorial methyl group

H Conformational analysis of trans-1, 4 -dimethylcyclohexane H 3 C CH 3 H H 3 C H Two conformations are not equivalent; most stable conformation has both methyl groups equatorial.

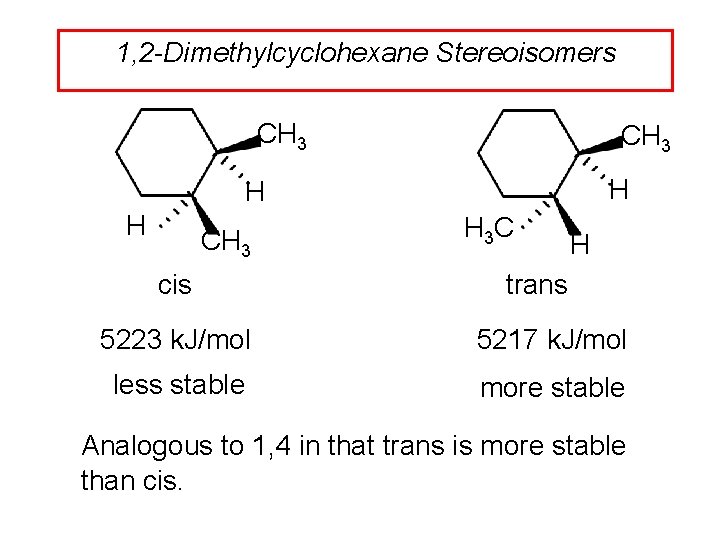
1, 2 -Dimethylcyclohexane Stereoisomers CH 3 H H H CH 3 cis H 3 C H trans 5223 k. J/mol 5217 k. J/mol less stable more stable Analogous to 1, 4 in that trans is more stable than cis.

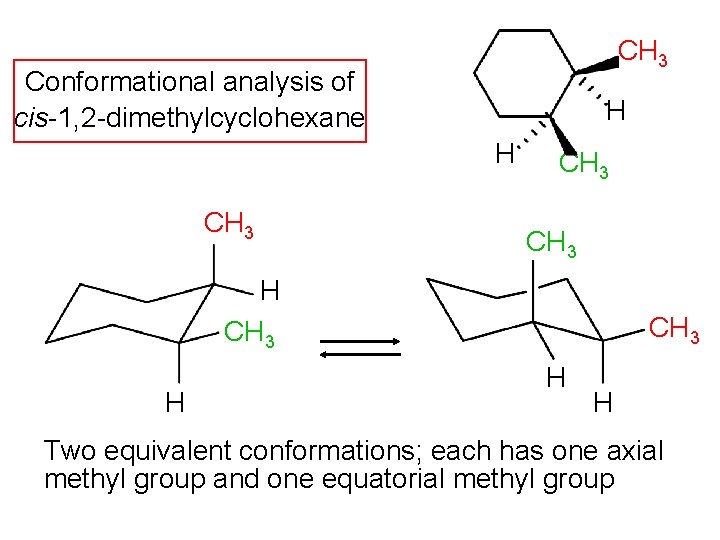
CH 3 Conformational analysis of cis-1, 2 -dimethylcyclohexane H H CH 3 H CH 3 H H Two equivalent conformations; each has one axial methyl group and one equatorial methyl group

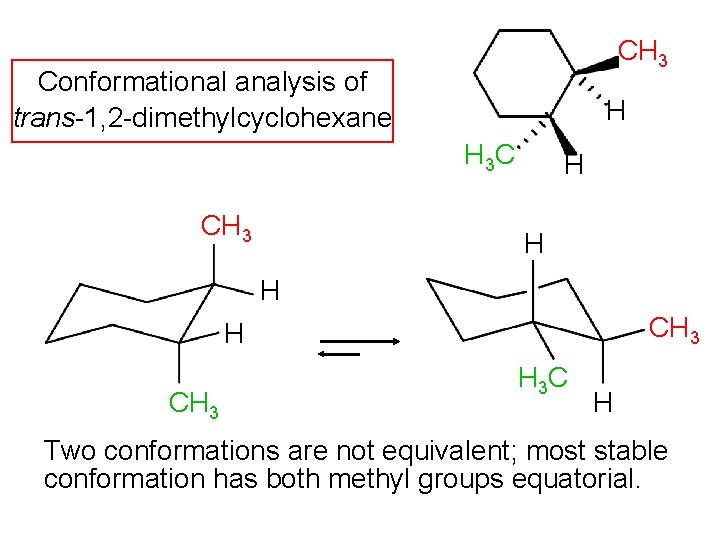
CH 3 Conformational analysis of trans-1, 2 -dimethylcyclohexane H H 3 C CH 3 H H H CH 3 H 3 C H Two conformations are not equivalent; most stable conformation has both methyl groups equatorial.

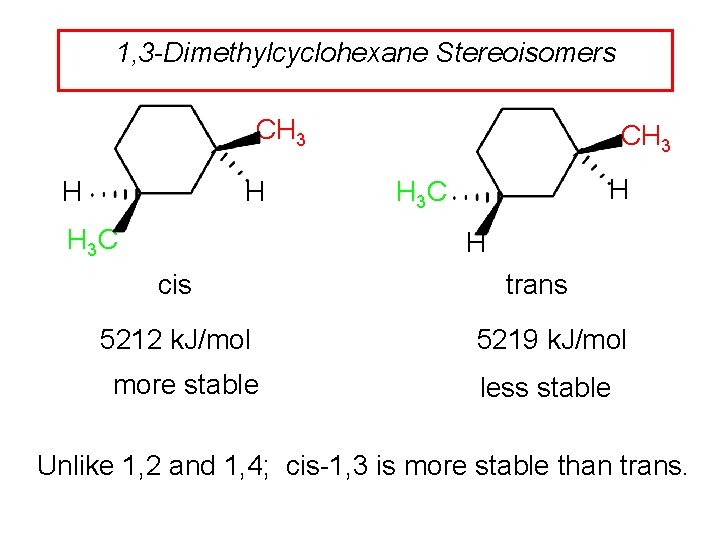
1, 3 -Dimethylcyclohexane Stereoisomers CH 3 H H H 3 C CH 3 H H 3 C H cis 5212 k. J/mol more stable trans 5219 k. J/mol less stable Unlike 1, 2 and 1, 4; cis-1, 3 is more stable than trans.

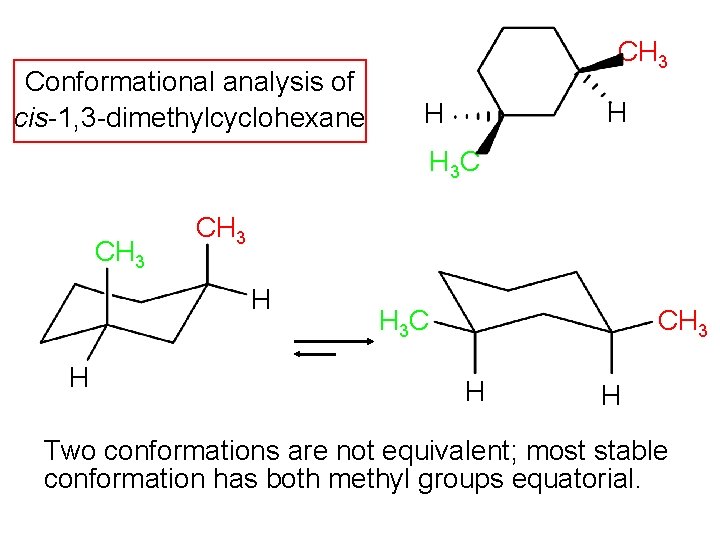
Conformational analysis of cis-1, 3 -dimethylcyclohexane CH 3 H H H 3 C CH 3 H H CH 3 H 3 C H H Two conformations are not equivalent; most stable conformation has both methyl groups equatorial.

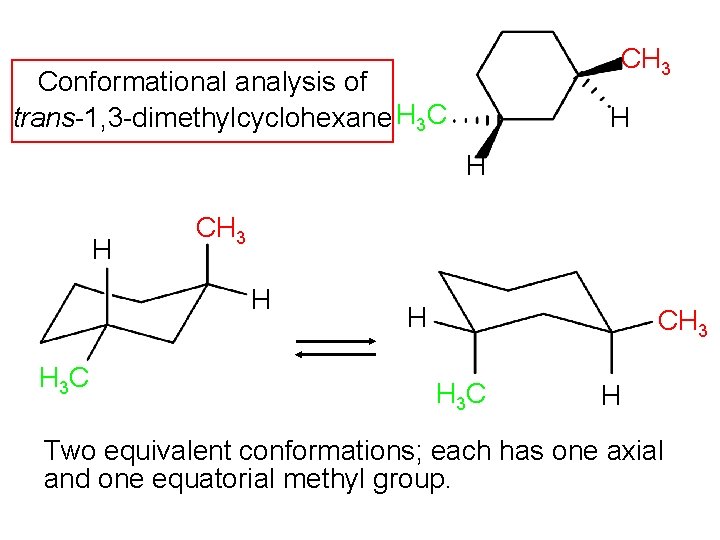
CH 3 Conformational analysis of trans-1, 3 -dimethylcyclohexane H 3 C H H H CH 3 H H 3 C H CH 3 H 3 C H Two equivalent conformations; each has one axial and one equatorial methyl group.

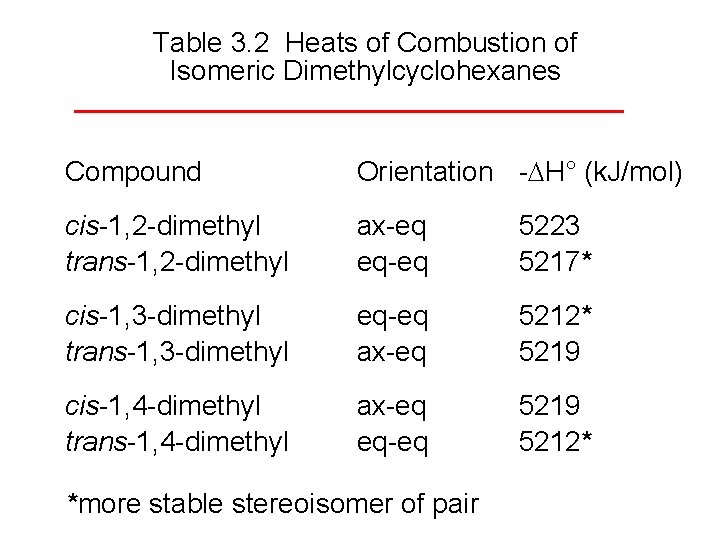
Table 3. 2 Heats of Combustion of Isomeric Dimethylcyclohexanes Compound Orientation - H° (k. J/mol) cis-1, 2 -dimethyl trans-1, 2 -dimethyl ax-eq eq-eq 5223 5217* cis-1, 3 -dimethyl trans-1, 3 -dimethyl eq-eq ax-eq 5212* 5219 cis-1, 4 -dimethyl trans-1, 4 -dimethyl ax-eq eq-eq 5219 5212* *more stable stereoisomer of pair

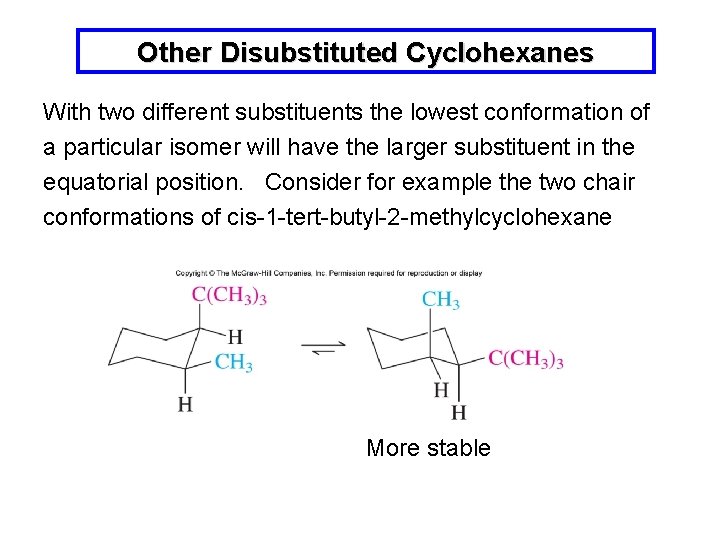
Other Disubstituted Cyclohexanes With two different substituents the lowest conformation of a particular isomer will have the larger substituent in the equatorial position. Consider for example the two chair conformations of cis-1 -tert-butyl-2 -methylcyclohexane More stable

Cycloheptane and Larger Rings Most stable conformation minimizes total strain. This is more complicated than cyclohexane because there are many conformations. Furthermore several conformations may be of similar energy.

Polycyclic Ring Systems
![Spirocyclic Systems Spirocyclic compounds have two rings with one common atom Named as spironumberalkane Spirocyclic Systems Spirocyclic compounds have two rings with one common atom. Named as spiro[number]alkane.](https://slidetodoc.com/presentation_image_h/e0add6a9e9a55a591df2340fb3b2ba46/image-58.jpg)
Spirocyclic Systems Spirocyclic compounds have two rings with one common atom. Named as spiro[number]alkane. The alkane suffix corresponds to the number of carbons in the two rings. The numbers of carbons in each ring not including the common atom are given in increasing order. This is spiro[3. 4]octane.
![Bridged Compounds Two nonadjacent atoms common to two or more rings Named as bicyclonumberalkane Bridged Compounds Two nonadjacent atoms common to two, or more, rings. Named as bicyclo[number]alkane.](https://slidetodoc.com/presentation_image_h/e0add6a9e9a55a591df2340fb3b2ba46/image-59.jpg)
Bridged Compounds Two nonadjacent atoms common to two, or more, rings. Named as bicyclo[number]alkane. The parent alkane corresponds to the total number of carbons in the bicyclic skeleton. The numbers correspond to the number of carbon atoms between the bridgehead atoms in descending order. This is bicyclo[3. 2. 1]octane.

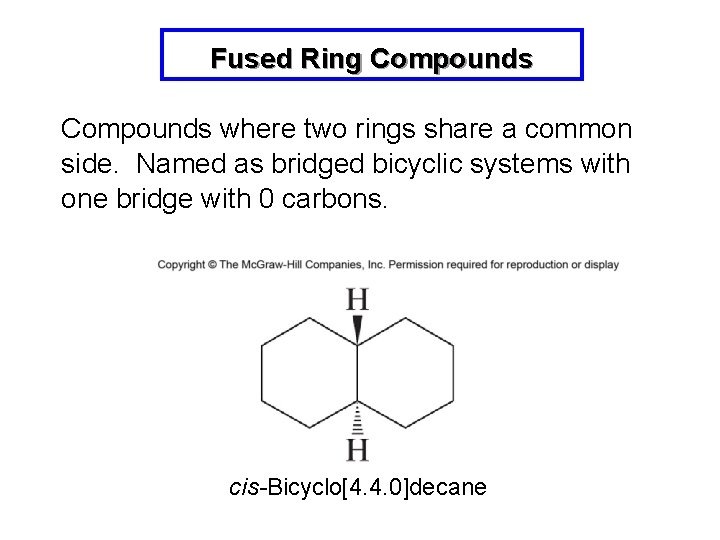
Fused Ring Compounds where two rings share a common side. Named as bridged bicyclic systems with one bridge with 0 carbons. cis-Bicyclo[4. 4. 0]decane

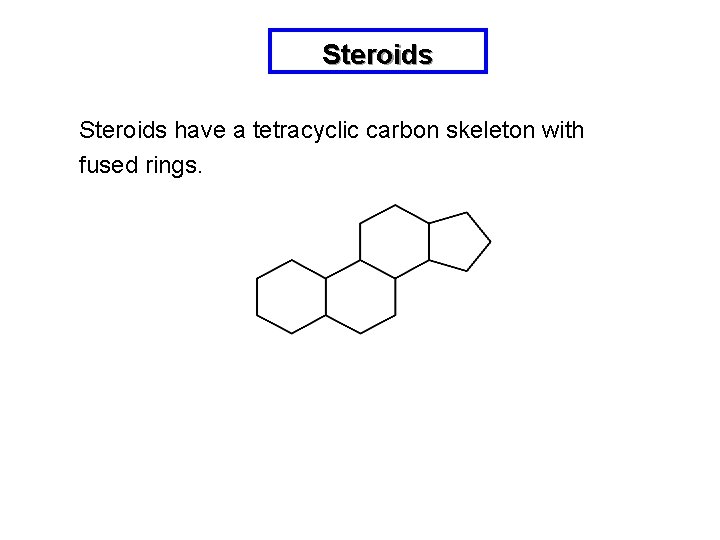
Steroids have a tetracyclic carbon skeleton with fused rings.

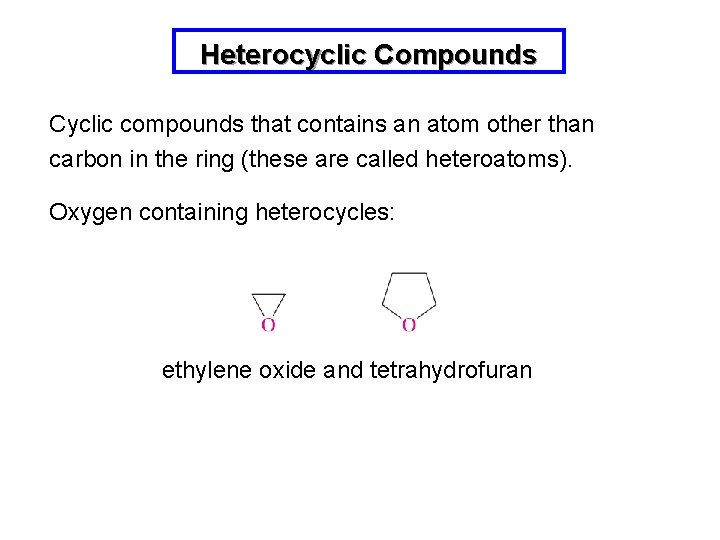
Heterocyclic Compounds Cyclic compounds that contains an atom other than carbon in the ring (these are called heteroatoms). Oxygen containing heterocycles: ethylene oxide and tetrahydrofuran

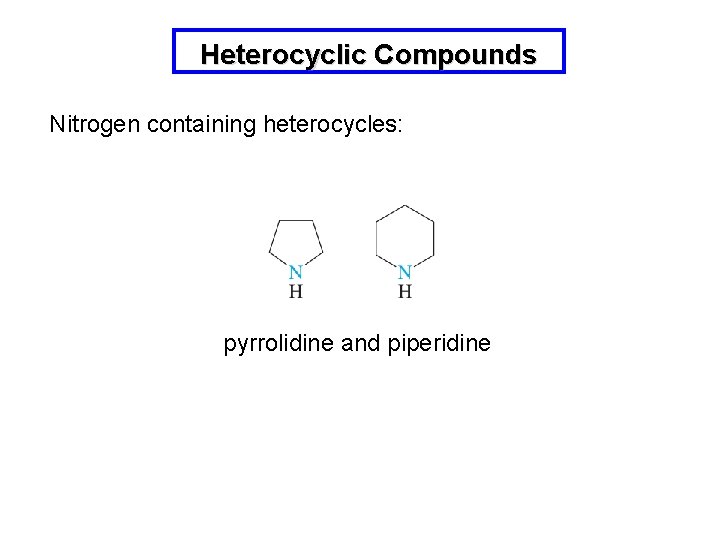
Heterocyclic Compounds Nitrogen containing heterocycles: pyrrolidine and piperidine
 Gauche conformation
Gauche conformation Cyclopentane cis trans isomers
Cyclopentane cis trans isomers Saturated hydrocarbon
Saturated hydrocarbon Founder of organic chemistry
Founder of organic chemistry But-2-ene polymer
But-2-ene polymer Explain homologous series with example
Explain homologous series with example Drawing chair conformations
Drawing chair conformations Conformational isomerism
Conformational isomerism Alkanes solubility
Alkanes solubility Saturated bond
Saturated bond Uses of alkanes
Uses of alkanes Alkanes list
Alkanes list General formula for alkyl group
General formula for alkyl group Alkanes alkenes alkynes
Alkanes alkenes alkynes Alkanes list
Alkanes list Thomson higher education
Thomson higher education Combustion reaction of alkanes
Combustion reaction of alkanes Combustion of alkanes
Combustion of alkanes Covalently def
Covalently def Alkylation of alkanes
Alkylation of alkanes Viscosity of alkanes
Viscosity of alkanes Cyclopentane uses
Cyclopentane uses Iupac stands for
Iupac stands for Bromine water test
Bromine water test Priority order of functional group ncert
Priority order of functional group ncert Eth meth prop but pent hex
Eth meth prop but pent hex Uses of alkanes
Uses of alkanes Chapter 7 ionic and metallic bonding chapter answer key
Chapter 7 ionic and metallic bonding chapter answer key Chapter 7 ionic and metallic bonding assessment answer key
Chapter 7 ionic and metallic bonding assessment answer key Properties of ionic compounds
Properties of ionic compounds Chapter 7 chapter assessment ionic compounds and metals
Chapter 7 chapter assessment ionic compounds and metals Representative metal
Representative metal Summary of the red tent
Summary of the red tent Chapter 8 and 9 summary great gatsby
Chapter 8 and 9 summary great gatsby Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 11 chapter assessment stoichiometry answer key
Chapter 11 chapter assessment stoichiometry answer key Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 7 similarity chapter test form a answer key
Chapter 7 similarity chapter test form a answer key Chapter 6: career readiness
Chapter 6: career readiness Chapter 9 surface water chapter assessment answer key
Chapter 9 surface water chapter assessment answer key Chapter 2 study guide physics
Chapter 2 study guide physics Chemistry: the central science chapter 14 answers
Chemistry: the central science chapter 14 answers Section 1 population dynamics answer key
Section 1 population dynamics answer key Chapter 2 chapter assessment
Chapter 2 chapter assessment Summary of the book of philippians chapter by chapter
Summary of the book of philippians chapter by chapter The great gatsby chapter 3 and 4 questions and answers
The great gatsby chapter 3 and 4 questions and answers The giver chapter 3 and 4 questions and answers
The giver chapter 3 and 4 questions and answers Diversity and human needs and development
Diversity and human needs and development Chapter 10 motivating and satisfying employees and teams
Chapter 10 motivating and satisfying employees and teams Bridge to terabithia chapter 4 questions and answers
Bridge to terabithia chapter 4 questions and answers Animal farm questions and answers pdf
Animal farm questions and answers pdf Chapter 24 the immune and lymphatic systems and cancer
Chapter 24 the immune and lymphatic systems and cancer The mouse and the motorcycle comprehension questions
The mouse and the motorcycle comprehension questions Chapter 4 work and energy section 1 work and machines
Chapter 4 work and energy section 1 work and machines Chapter 10 motivating and satisfying employees and teams
Chapter 10 motivating and satisfying employees and teams The lymphatic capillaries are
The lymphatic capillaries are World war 1 and the russian revolution chapter 27
World war 1 and the russian revolution chapter 27 Chapter 10 section 2 central america and the caribbean
Chapter 10 section 2 central america and the caribbean Work measurement techniques
Work measurement techniques Unit 14 lesson 1 drivers ed
Unit 14 lesson 1 drivers ed Glue
Glue Why study financial institutions
Why study financial institutions Chapter 20 weather patterns and severe storms
Chapter 20 weather patterns and severe storms What is mechanical wave
What is mechanical wave