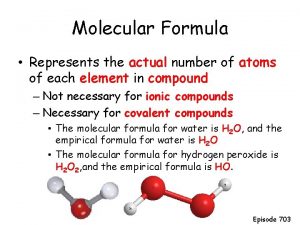
CYCLOALKANES 918202 0 Cycloalkanes have molecular formula Cn


![Examples � Bicyclo[4. 3. 0]nonane Hydrindane � Bicyclo{4. 4. 0] � Bicyclo[2. 2. 1 Examples � Bicyclo[4. 3. 0]nonane Hydrindane � Bicyclo{4. 4. 0] � Bicyclo[2. 2. 1](https://slidetodoc.com/presentation_image/5b808a2a108b4268a6c8c6dabfed39bc/image-21.jpg)
- Slides: 23

CYCLOALKANES 9/18/202 0

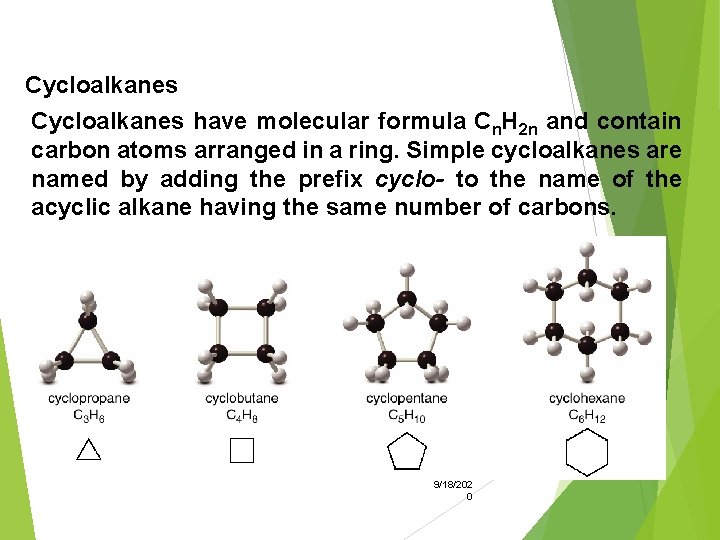
Cycloalkanes have molecular formula Cn. H 2 n and contain carbon atoms arranged in a ring. Simple cycloalkanes are named by adding the prefix cyclo- to the name of the acyclic alkane having the same number of carbons. 9/18/202 0

Cycloalkanes � are carbon rings. � have two fewer hydrogen atoms than straight-chain alkanes with the same number of carbon atoms. � are named by using the prefix cyclo- before the name of the alkane chain. 9/18/202 0

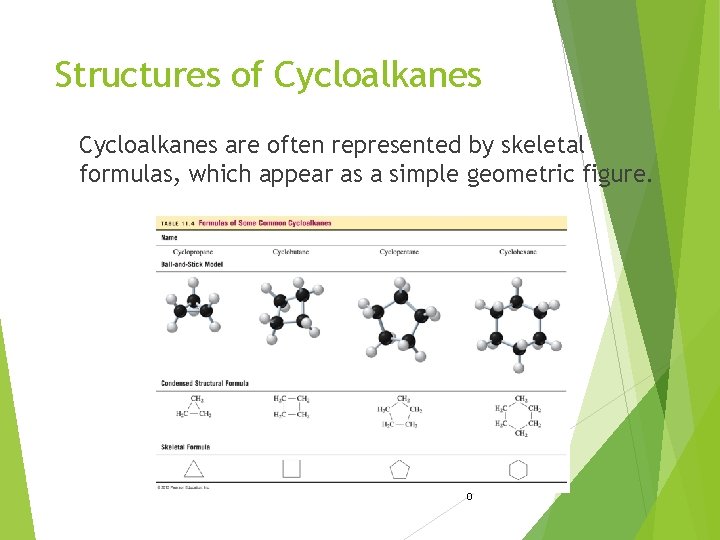
Structures of Cycloalkanes are often represented by skeletal formulas, which appear as a simple geometric figure. 9/18/202 0

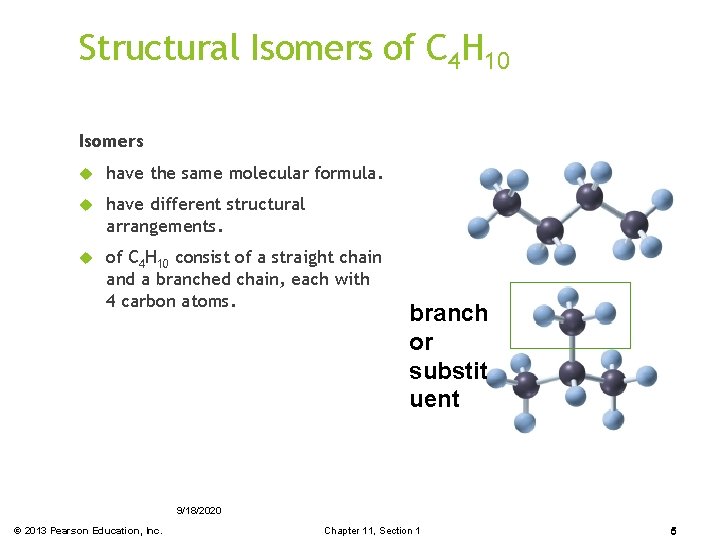
Structural Isomers of C 4 H 10 Isomers have the same molecular formula. have different structural arrangements. of C 4 H 10 consist of a straight chain and a branched chain, each with 4 carbon atoms. branch or substit uent 9/18/2020 © 2013 Pearson Education, Inc. Chapter 11, Section 1 5

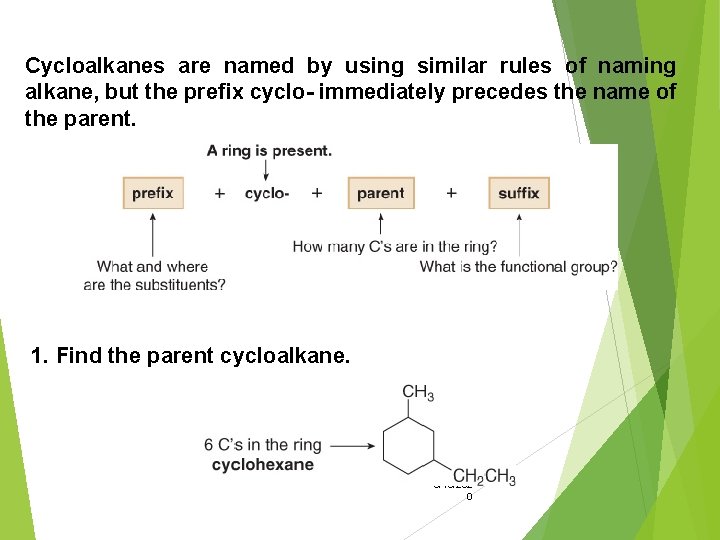
Cycloalkanes are named by using similar rules of naming alkane, but the prefix cyclo- immediately precedes the name of the parent. 1. Find the parent cycloalkane. 9/18/202 0

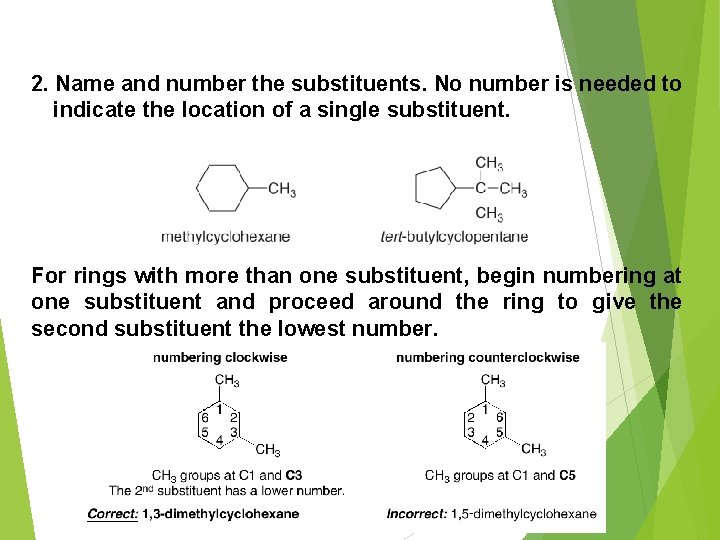
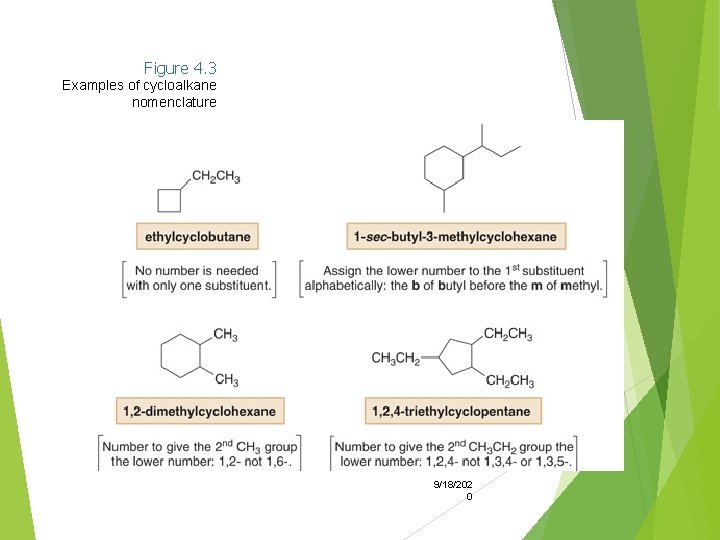
2. Name and number the substituents. No number is needed to indicate the location of a single substituent. For rings with more than one substituent, begin numbering at one substituent and proceed around the ring to give the second substituent the lowest number. 9/18/202 0

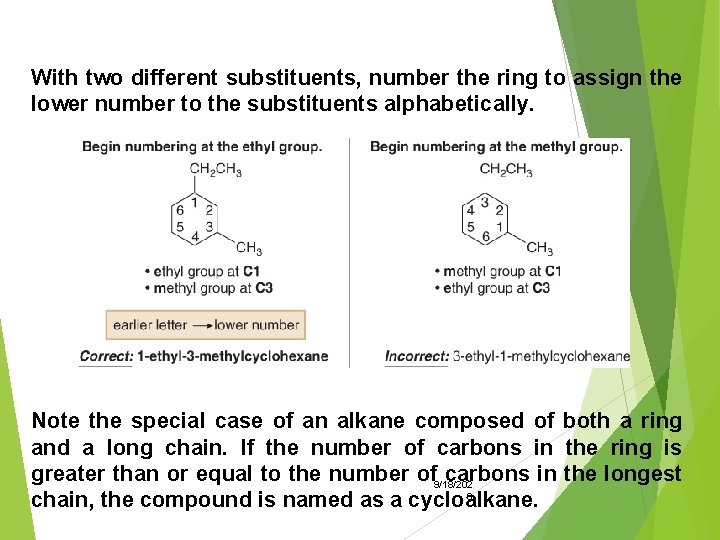
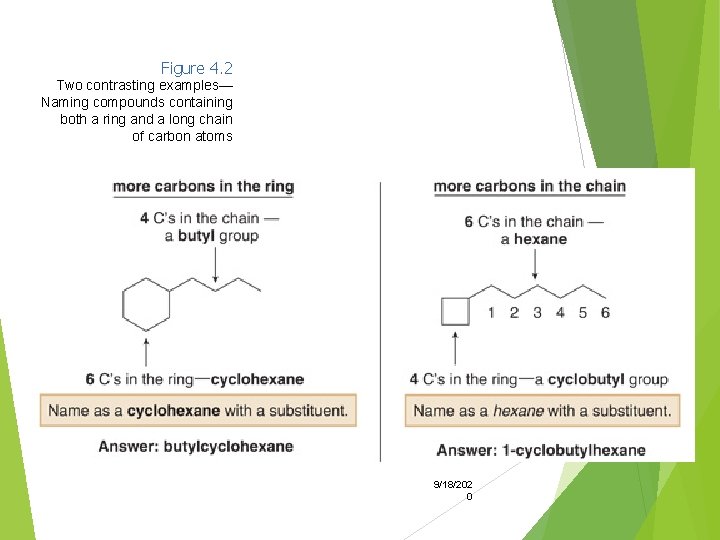
With two different substituents, number the ring to assign the lower number to the substituents alphabetically. Note the special case of an alkane composed of both a ring and a long chain. If the number of carbons in the ring is greater than or equal to the number of 9/18/202 carbons in the longest 0 chain, the compound is named as a cycloalkane.

Figure 4. 2 Two contrasting examples— Naming compounds containing both a ring and a long chain of carbon atoms 9/18/202 0

Figure 4. 3 Examples of cycloalkane nomenclature 9/18/202 0

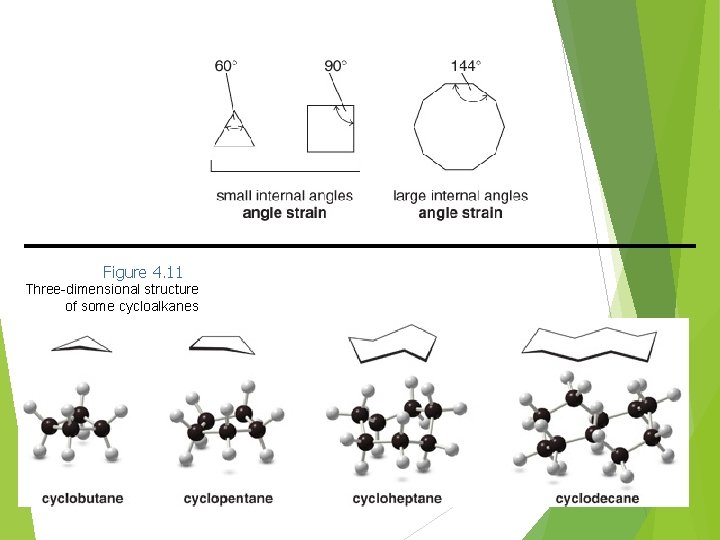
Introduction to Cycloalkanes • Besides torsional strain and steric strain, the conformations of cycloalkanes are also affected by angle strain. • Angle strain is an increase in energy when bond angles deviate from the optimum tetrahedral angle of 109. 5°. • The Baeyer strain theory was formulated when it was thought that rings were flat. It states that larger rings would be very highly strained, as their bond angles would be very different from the optimum 109. 5°. • It turns out that cycloalkanes with more than three C atoms in the ring are not flat molecules. They are 9/18/202 puckered to reduce strain. 0

Figure 4. 11 Three-dimensional structure of some cycloalkanes 9/18/202 0

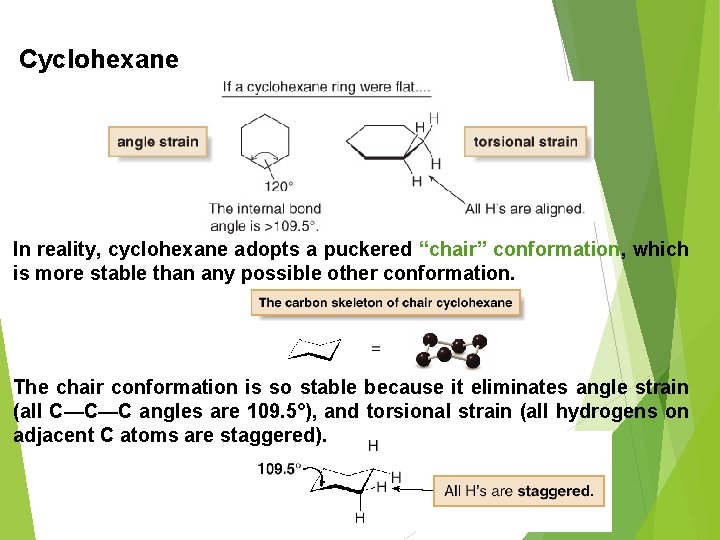
Cyclohexane In reality, cyclohexane adopts a puckered “chair” conformation, which is more stable than any possible other conformation. The chair conformation is so stable because it eliminates angle strain (all C—C—C angles are 109. 5°), and torsional strain (all hydrogens on adjacent C atoms are staggered). 9/18/202 0

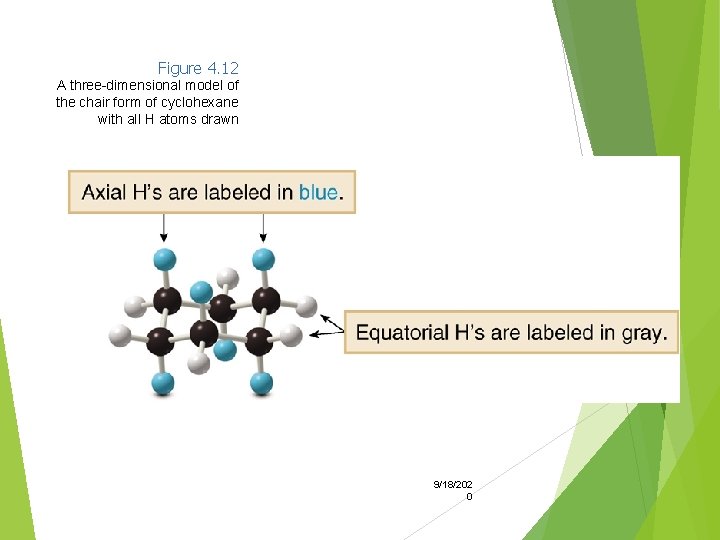
Figure 4. 12 A three-dimensional model of the chair form of cyclohexane with all H atoms drawn 9/18/202 0

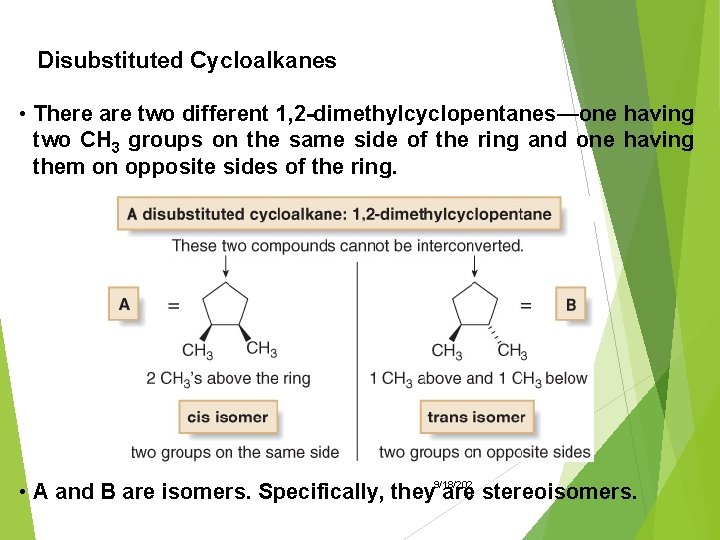
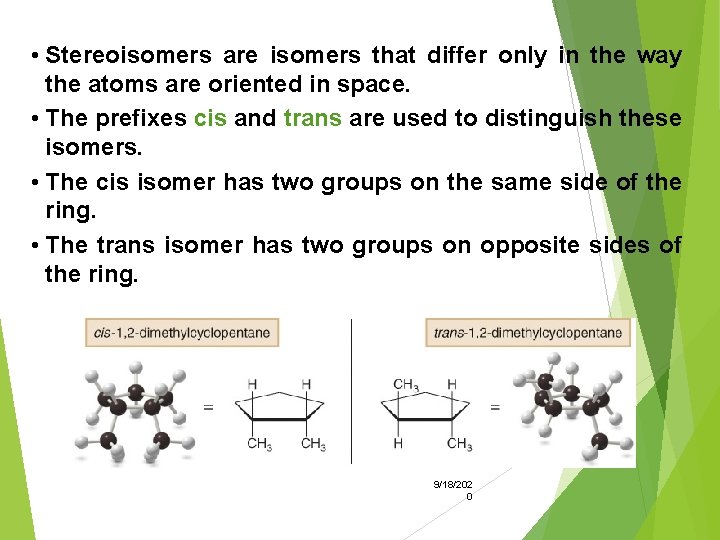
Disubstituted Cycloalkanes • There are two different 1, 2 -dimethylcyclopentanes—one having two CH 3 groups on the same side of the ring and one having them on opposite sides of the ring. • A and B are isomers. Specifically, they 9/18/202 are 0 stereoisomers.

• Stereoisomers are isomers that differ only in the way the atoms are oriented in space. • The prefixes cis and trans are used to distinguish these isomers. • The cis isomer has two groups on the same side of the ring. • The trans isomer has two groups on opposite sides of the ring. 9/18/202 0

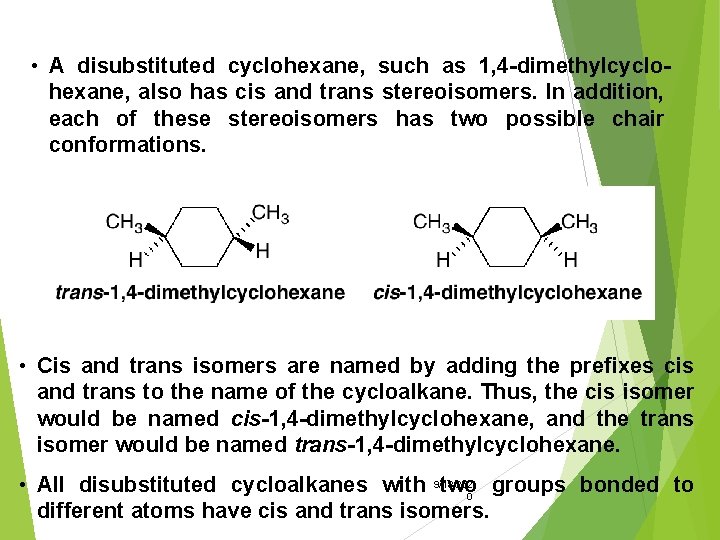
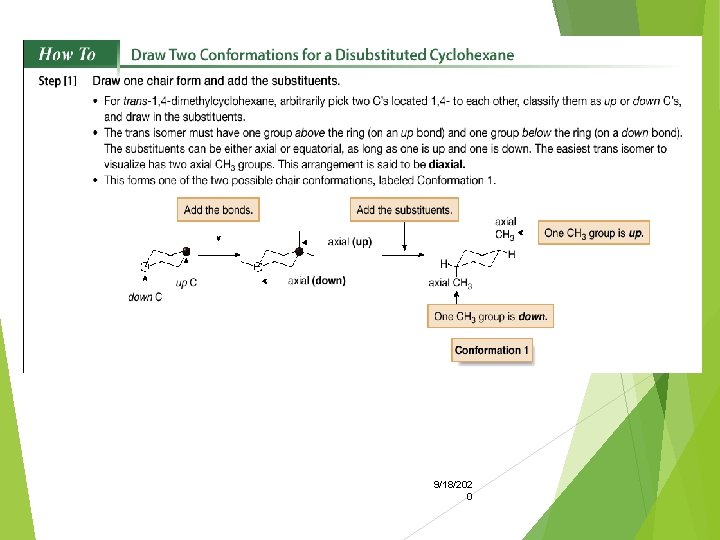
• A disubstituted cyclohexane, such as 1, 4 -dimethylcyclohexane, also has cis and trans stereoisomers. In addition, each of these stereoisomers has two possible chair conformations. • Cis and trans isomers are named by adding the prefixes cis and trans to the name of the cycloalkane. Thus, the cis isomer would be named cis-1, 4 -dimethylcyclohexane, and the trans isomer would be named trans-1, 4 -dimethylcyclohexane. • All disubstituted cycloalkanes with 9/18/202 two groups bonded to 0 different atoms have cis and trans isomers.

9/18/202 0

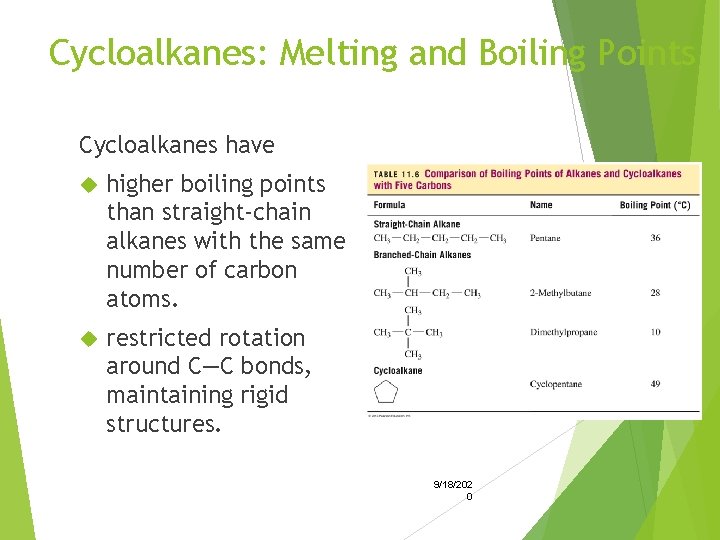
Cycloalkanes: Melting and Boiling Points Cycloalkanes have higher boiling points than straight-chain alkanes with the same number of carbon atoms. restricted rotation around C—C bonds, maintaining rigid structures. 9/18/202 0

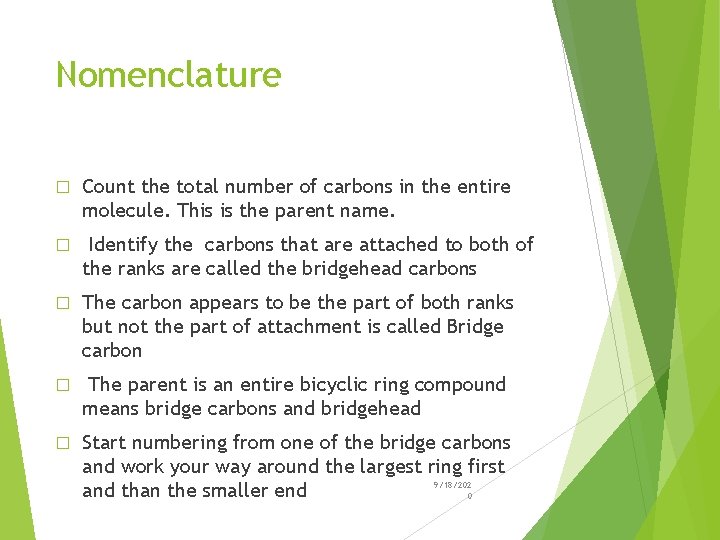
Bicycloalkanes � An alkane that contains two rings that share two carbon atoms is classified as a Bicycloalkane � The shared carbon atoms are called Bridgehead carbons � The carbon chain connecting them is called a Bridge � The general formula is Cn H 2 n-2 9/18/202 0
![Examples Bicyclo4 3 0nonane Hydrindane Bicyclo4 4 0 Bicyclo2 2 1 Examples � Bicyclo[4. 3. 0]nonane Hydrindane � Bicyclo{4. 4. 0] � Bicyclo[2. 2. 1](https://slidetodoc.com/presentation_image/5b808a2a108b4268a6c8c6dabfed39bc/image-21.jpg)
Examples � Bicyclo[4. 3. 0]nonane Hydrindane � Bicyclo{4. 4. 0] � Bicyclo[2. 2. 1 decane Decalin heptane Norbornane 9/18/202 0

Nomenclature � Count the total number of carbons in the entire molecule. This is the parent name. � Identify the carbons that are attached to both of the ranks are called the bridgehead carbons � The carbon appears to be the part of both ranks but not the part of attachment is called Bridge carbon � The parent is an entire bicyclic ring compound means bridge carbons and bridgehead � Start numbering from one of the bridge carbons and work your way around the largest ring first 9/18/202 and than the smaller end 0

� Draw brackets and include numbers to represent exactly how many carbons are on each side of the ring. � Start with the largest ring and look for any carbons that are part of that ring that includes. � Count attached carbons in smaller ring. � Write in brackets 9/18/202 0
 Cycloalkanes and their stereochemistry
Cycloalkanes and their stereochemistry C10h22 organic or inorganic
C10h22 organic or inorganic Numbering carbon chains
Numbering carbon chains Stereochemistry of alkanes and cycloalkanes
Stereochemistry of alkanes and cycloalkanes Propene undergoes addition reaction
Propene undergoes addition reaction Condensed structural formula of alkenes
Condensed structural formula of alkenes Covalent bond
Covalent bond Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Trisilicon octaiodide chemical formula
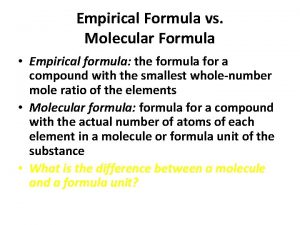
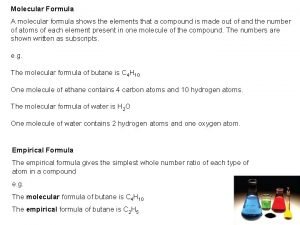
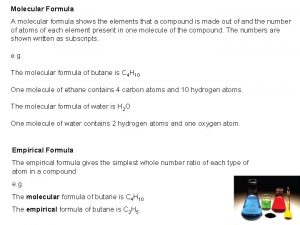
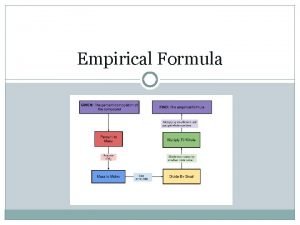
Trisilicon octaiodide chemical formula Empirical formula of haemoglobin
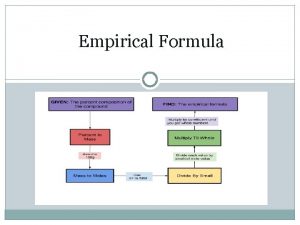
Empirical formula of haemoglobin Empirical formula from percent composition
Empirical formula from percent composition How to find empirical formula
How to find empirical formula Molecular formula
Molecular formula How to find empirical formula
How to find empirical formula Empirical formula
Empirical formula Covalent compound formula
Covalent compound formula How to find empirical formula with percentages
How to find empirical formula with percentages Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Empirical formula to molecular formula
Empirical formula to molecular formula Empirical formula c5h12
Empirical formula c5h12 Empirical formula
Empirical formula What 3d shape has 7 faces 12 edges and 7 vertices
What 3d shape has 7 faces 12 edges and 7 vertices How to find molecular formula
How to find molecular formula