Organic Chemistry Chapter 2 Alkanes and Cycloalkanes Nanoplasmonic




- Slides: 15

Organic Chemistry Chapter 2 Alkanes and Cycloalkanes Nanoplasmonic Research Group

Fully-saturated hydrocarbons: Alkanes & Cycloalkanes How to READ their structures: NOMENCLATURE Physical Properties: Interaction & Conformation Chemical Properties: Reactions Nanoplasmonic Research Group

How to READ them ? • Systematic methods for naming compounds (IUPAC) • RULES (see page 44 middle ~ page 45) 1. Find out the longest continuous chain If 2 different chains of equal length are present, choose the one with the greater number of branch points 2. Number the substituents If the first branch points occurs at the same carbon number on both ends, begins at the end that has the second nearest branch pints 3. Write the name as one word Put the substituents in alpabetical order: di-, tri-, tetra-, sec-, tert-, ignored when alphabetizing, iso-, neo- are included when alphabetizing


How to read substituents ? • Methyl, propyl, etc • Iso, sec-, tert-, etc • Fluoro, chloro, bromo, iodo

Physical Properties: Intermolecular Interaction • Nonpolar due to the fact that C-C & C-H bonds are nearly purely covalent (no dipole moment) • Interaction between alkanes – Induced dipole-induced dipole moment – Van der Waals attraction – M. W. dependence • Van der Waals interaction – Permanent dipole-permanent dipole forces (Keesom) – Permanent dipole-induced dipole forces (Debye) – Induced dipole-induced dipole forces (London)

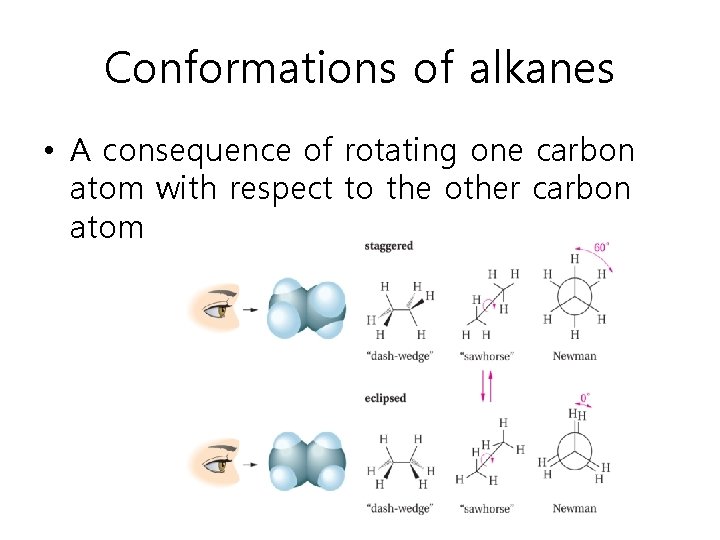
Conformations of alkanes • A consequence of rotating one carbon atom with respect to the other carbon atom

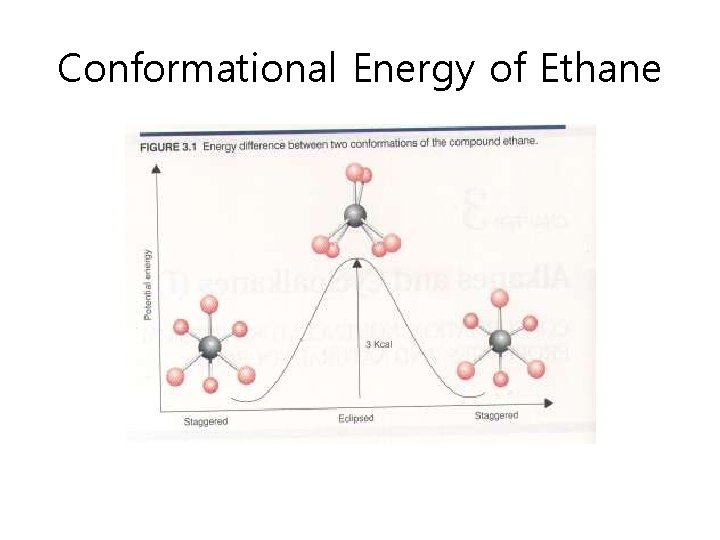
Conformational Energy of Ethane

Conformational Analysis Summary • Torsional energy – Higher energy associated with eclipsed conformation • Torsional strain – Resistantce to rotating to an eclipsed conformation • Steric strain – Repulsive interactions that occurs when atoms are forced closed together than their atomic radii allow • Gauche: spatial relationship with a 60 torsion angle • Interactions – – H-H eclipsing (torsional strain): 1. 0 kcal/mol H-Me eclipsing (mostly torsional strain) 1. 4 kcal/mol Me-Me eclipsing (steric and torsional strain) 2. 6 kcal/mol Me-Me gauche interaction (steric strain) 0. 9 kcal/mol

Why is staggered form lower in energy • Hyperconjugation – Stabilizing overlap between sigma bond antibonding orbitals that does not occur in the eclipsed conformer • Electron-electron repulsion Conformational Energy of Butane

Naming Cyclohexane • Find parent (ring or chain, depending on which is larger) • Label point of attachment of alkyl, halo, etc • Continue numbering so that the second substituent is the lowest possible number • If 2 or more groups could potentially get the same number, use alphabetical order as a tie-breaker

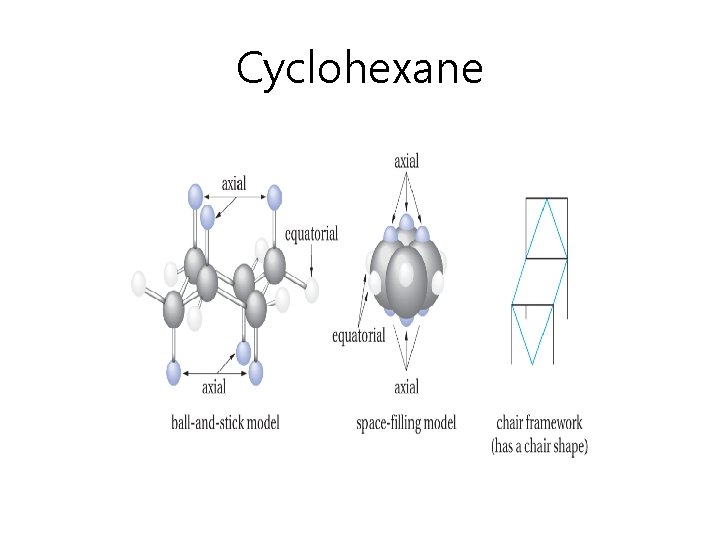
Cyclohexane

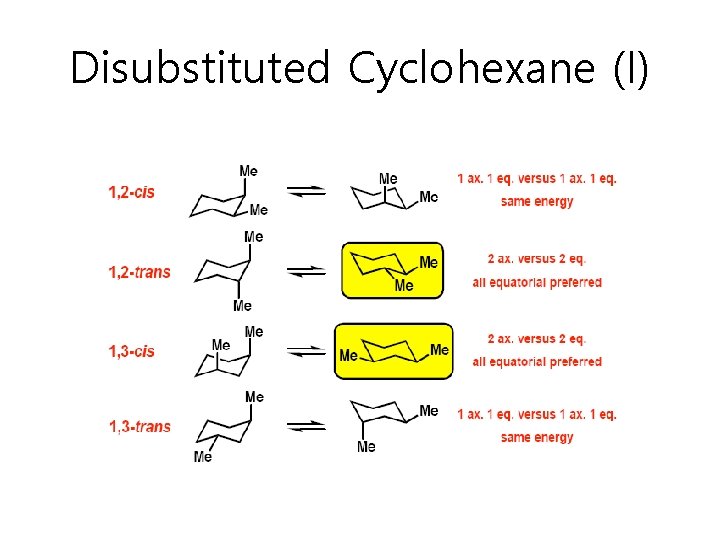
Disubstituted Cyclohexane (I)

Disubstituted Cyclohexane (II) • If 2 substituents are on cyclohexane the lowest energy conformation – Has both substituents equatorial (if possible) – t-Bu is NEVER axial • Cis-Trans Isomerism – Do not interconvert each other (see page 59 bottom)

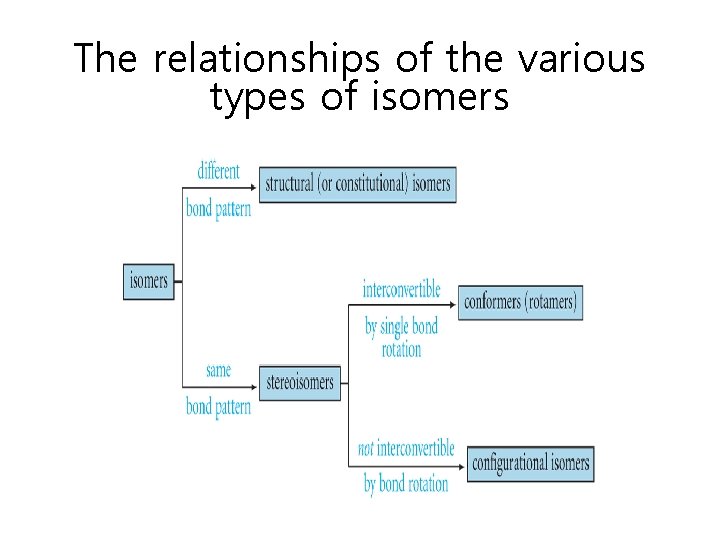
The relationships of the various types of isomers
 Stability of chair and boat conformation
Stability of chair and boat conformation Functional groups ib chemistry
Functional groups ib chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Cycloalkanes and their stereochemistry
Cycloalkanes and their stereochemistry Unsaturated hydrocarbon
Unsaturated hydrocarbon Father of organic chemistry
Father of organic chemistry Incomplete combustion of propene
Incomplete combustion of propene Explain homologous series with example
Explain homologous series with example Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Intro to organic chemistry
Intro to organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 organic chemistry
Chapter 7 organic chemistry Entane
Entane Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp