WUJUD ZAT Oleh A Sjaifullah Kimia adalah Pengetahuan



























































- Slides: 61

WUJUD ZAT Oleh A. Sjaifullah

Kimia adalah Pengetahuan yang mempelajari materi dan perubahannya Materi adalah Apapaun yang memiliki massa dan menempati ruang

Teori Kinetik Semua partikel (atoms, molekul dan ion) menyusun materi selalu bergerak secara random dan berinteraksi

Wujud zat Ø Cara menyusun partikel Ø Energi partikel Ø Interaksi/jarak antar partikel

Karakteristik wujud zat Sifat partikel wujud Proximity Energy gerakan Volume padat close little vibrational definite cair close moderate rotational definite gas far apart a lot translational indefinite bentuk definite indefinite

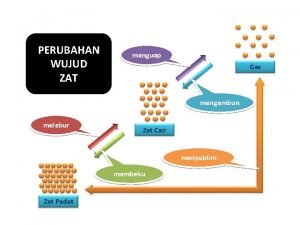
Jika kondisi partikel (susunan, interaksi dan energi) diubah, maka terjadi perubahan wujud posisi partikel-partikel zat cair & gas tidak tetap, Zat cair dan gas dapat dialirkan/berdifusi =(fluida) Perubahan wujud terjadi dalam siklus air di alam

Sifat-sifat gas Salah satu sifat gas adalah dapat memberikan tekanan. Tekanan gas terjadi akbat dari tumbukan partikel gas dengan dinding Tekanan yang disebabkan oleh campuran gas-gas yang ada di udara disebut tekanan atmosfir Tekanan adalah…. .

Mengapa tekanan udara sangat penting? ·Adanya angin ·Menciptakan mendung dan awan

Hubungan tekanan dan volume gas Hukum Boyle P 1 V 1 = P 2 V 2

Volume and Temperature Hukum Charles V 1 T 1 = V 2 T 2 , tekanan tetap

Korek gas, hair spray, tabung LPG akan terasa lebih dingin jika digunakan, Karena……………. .

Karena partikel gas hampir tidak berinteraksi satu sama lain, jumlah partikel (molekul) gas hanya bisa ditentukan/diukur pada volume, tekanan dan suhu tertentu


Persamaan keadaan gas ideal.

Volume molar gas pada STP


Zat Cair

Properties of Liquids • Surface tension: the energy required to increase the surface area of a liquid by a unit amount. • Viscosity: a measure of a liquid’s resistance to flow.

Tekanan Uap Cairan

ZAT PADAT Karena interaksi yang kuat, posisi partikel-partikel dalam zat padat tidak berubah terhadap satu dengan yang lain Amorf Kristal

Comparison: Amorphous solids Tar, molten glass, molten plastics, and molten butter, consist of large molecules or a mixture of molecules that cannot move readily. As the temperature is lowered, their molecules move more and more slowly and finally stop in random positions. The resulting materials are called amorphous solids or glasses. Such solids lack an ordered internal structure. Common examples include candle wax, butter, glass, and plastics.

Crystals are classified into systems based on the angle their bonds form. *7 common systems Isometric, Hexagonal, Tetragonal, Trigonal, Triclinic, Monoclinic, Orthorhombic

What crystal system does this mineral belong to? Why? Quartz Hexagonal • 3 equilateral axes intersect at angels of 60 o , 1 vertical axis intersect at 90 o to equilateral axes. • Hexa-six Beryl http: //www. minerals. net/glossary. htm

What crystal system does this mineral belong to? Why? MONOCLINIC • 3 unequal axes and 1 unequal intersection that is not at 90 o • Mono-one http: //www. minerals. net/glossary. htm GYPSUM

What crystal system does this mineral belong to? Why? Sugar Isometric • 3 axes are at right angles, all sides equal length. • Iso- same http: //www. minerals. net/glossary. htm

What crystal system does this mineral belong to? Why? Tetragonal • 3 axes are at right angels, only 2 lateral axes are equal length and it has 4 sides. • Tetra-four WULFENITE http: //www. minerals. net/glossary. htm

What crystal system does this mineral belong to? Why? ORTHORHOMBIC • 3 unequal axes all at right angles to each other • Ortho-unequal http: //www. minerals. net/glossary. htm TANZANITE

What crystal system does this mineral belong to? Why? Amazonite Trigonal • 3 equal length axes, 3 equal intersections (not 90 o) • Tri- three Note: Hexagonal but with 3 sides not 6 http: //www. minerals. net/glossary. htm

What crystal system does this mineral belong to? Why? Triclinic • 3 unequal axes and 3 unequal intersections not at 90 o • Tri-three http: //www. minerals. net/glossary. htm

Using your 3 -D structures identify the following into rightful system: Picture 1 Isometric Picture 5 ORTHORHOMBIC Picture 2 Tetragonal Picture 6 MONOCLINIC Picture 3 Hexagonal Picture 7 TRICLINIC Picture 4 Trigonal

Crystal Systems System Axes Angles Unique Symmetry Diagram Examples Isometric a=b=c = = =90° Four 3 -fold Pyrite, Halite, Galena, Garnet, Diamond, Fluorite Tetragonal a=b c = = =90° One 4 -fold Wulfenite, Rutile, Zircon, Chalcopyrite Hexagonal a=b c =120°, = =90° One 6 -fold Quartz, Beryl (Emerald), Apatite, Corundum (Ruby, Sapphire) Orthorhombic a b c = = =90° Three 2 -fold Sulfur, Barite, Olivine, Topaz Monoclinic a b c = =90°, 90° One 2 -fold Orthoclase, Malachite, Azurite, Mica, Gypsum, Talc Triclinic a b c 90° None Turquoise, Kyanite, Albite, Plagioclase

Crystal Systems System Isometric Tetragonal Hexagonal Orthorhombic Monoclinic Triclinic Axes Angles Unique Symmetry Diagram Examples

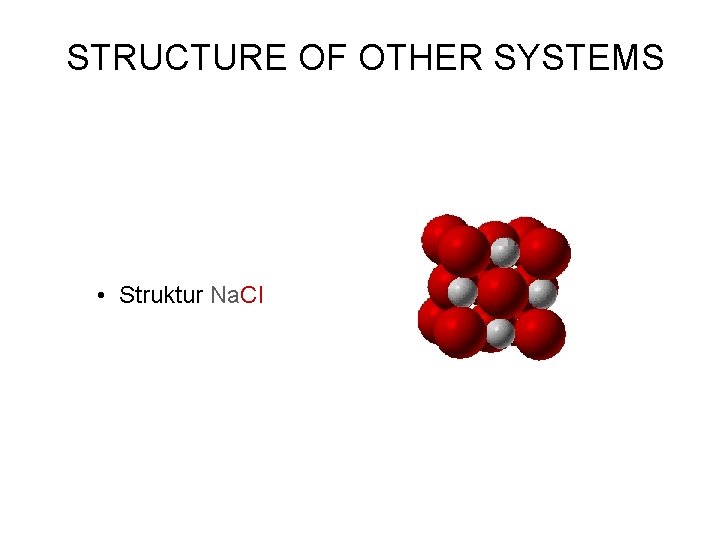
STRUCTURE OF OTHER SYSTEMS • Struktur Na. Cl

SOME DEFINITIONS … • Lattice: 3 D array of regularly spaced points • Crystalline material: atoms situated in a repeating 3 D periodic array over large atomic distances • Amorphous material: material with no such order • Hard sphere representation: atoms denoted by hard, touching spheres • Reduced sphere representation • Unit cell: basic building block unit (such as a flooring tile) that repeats in space to create the crystal structure; it is usually a parallelepiped or prizm

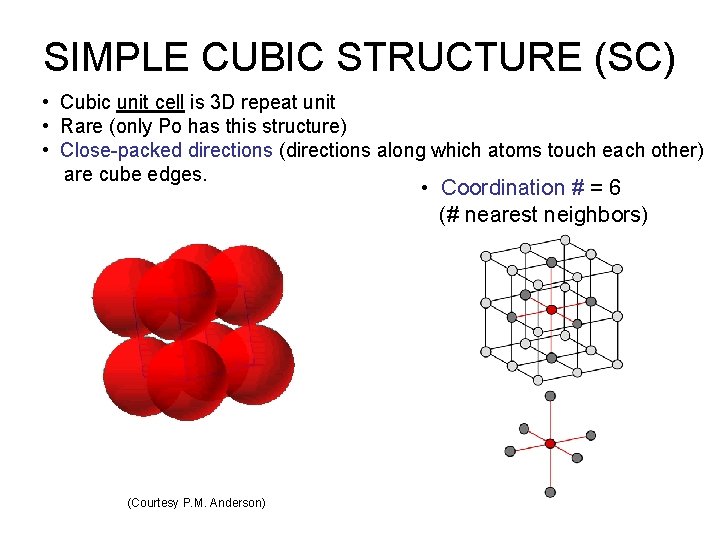
SIMPLE CUBIC STRUCTURE (SC) • Cubic unit cell is 3 D repeat unit • Rare (only Po has this structure) • Close-packed directions (directions along which atoms touch each other) are cube edges. • Coordination # = 6 (# nearest neighbors) (Courtesy P. M. Anderson)

ATOMIC PACKING FACTOR • Fill a box with hard spheres – Packing factor = total volume of spheres in box / volume of box – Question: what is the maximum packing factor you can expect? • In crystalline materials: – Atomic packing factor = total volume of atoms in unit cell / volume of unit cell – (as unit cell repeats in space)

ATOMIC PACKING FACTOR a R=0. 5 a close-packed directions contains 8 x 1/8 = 1 atom/unit cell Adapted from Fig. 3. 19, Callister 6 e. Lattice constant • APF for a simple cubic structure = 0. 52

BODY CENTERED CUBIC STRUCTURE (BCC) • Coordination # = 8 (Courtesy P. M. Anderson) Adapted from Fig. 3. 2, Callister 6 e. • Close packed directions are cube diagonals. --Note: All atoms are identical; the center atom is shaded differently only for ease of viewing.

ATOMIC PACKING FACTOR: BCC Adapted from Fig. 3. 2, Callister 6 e. • APF for a body-centered cubic structure = p 3/8 = 0. 68

FACE CENTERED CUBIC STRUCTURE (FCC) • Coordination # = 12 (Courtesy P. M. Anderson) Adapted from Fig. 3. 1(a), Callister 6 e. • Close packed directions are face diagonals. --Note: All atoms are identical; the face-centered atoms are shaded differently only for ease of viewing.

ATOMIC PACKING FACTOR: FCC Adapted from Fig. 3. 1(a), Callister 6 e. • APF for a body-centered cubic structure = p/(3 2) = 0. 74 (best possible packing of identical spheres)

FCC STACKING SEQUENCE • FCC Unit Cell • ABCABC. . . Stacking Sequence • 2 D Projection

HEXAGONAL CLOSE-PACKED STRUCTURE (HCP) Ideally, c/a = 1. 633 for close packing However, in most metals, c/a ratio deviates from this value

HEXAGONAL CLOSE-PACKED STRUCTURE (HCP) • ABAB. . . Stacking Sequence • 3 D Projection • 2 D Projection Adapted from Fig. 3. 3, Callister 6 e. • Coordination # = 12 • APF = 0. 74, for ideal c/a ratio of 1. 633

STATES OF MATTER • The Four States of Matter • Solid • Liquid • Gas • Plasma • Four States

Kinetic Theory of Matter is made up of particles which are in continual random motion.


STATES OF MATTER SOLIDS • Particles of solids are tightly packed, vibrating about a fixed position. • Solids have a definite shape and a definite volume. Heat

STATES OF MATTER LIQUID § Particles of liquids are tightly packed, but are far enough apart to slide over one another. § Liquids have an indefinite shape and a definite volume. Heat

STATES OF MATTER GAS § Particles of gases are very far apart and move freely. § Gases have an indefinite shape and an indefinite volume. Heat

PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

PHASE CHANGES Description of Phase Change Liquid to gas Term for Phase Change Vaporization, which includes Heat goes into the boiling and liquid as it vaporizes. evaporation Gas to liquid Condensation Solid to gas Heat Movement During Phase Change Sublimation Heat leaves the gas as it condenses. Heat goes into the solid as it sublimates.

STATES OF MATTER PLASMA § A plasma is an ionized gas. § A plasma is a very good conductor of electricity and is affected by magnetic fields. § Plasmas, like gases • Plasma is the have an indefinite common state shape and an of matter indefinite volume.

STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

Some places where plasmas are found… 1. Flames

2. Lightning

3. Aurora (Northern Lights)

The Sun is an example of a star in its plasma state


COLD PLASMA

COLD PLASMA PEN
 Peta konsep zat wujud zat dan perubahannya
Peta konsep zat wujud zat dan perubahannya Wujud zat kimia
Wujud zat kimia Pawarta kang becik kudu
Pawarta kang becik kudu Bagan perubahan wujud zat
Bagan perubahan wujud zat Perubahan wujud benda yang melepaskan kalor
Perubahan wujud benda yang melepaskan kalor Pengertian kalor adalah
Pengertian kalor adalah Korteks
Korteks Ada 6 jenis zat kimia yang perlu disimpan di gudang
Ada 6 jenis zat kimia yang perlu disimpan di gudang Aesthetic nature
Aesthetic nature Lawan dari baqa
Lawan dari baqa Apa kepanjangan dte
Apa kepanjangan dte Lawan sifat kekal
Lawan sifat kekal 1 contoh menguap
1 contoh menguap Peta ilmu pengetahuan adalah
Peta ilmu pengetahuan adalah Pengetahuan adalah kekuatan
Pengetahuan adalah kekuatan Pengetahuan adalah kekuatan
Pengetahuan adalah kekuatan Tersusun secara sistematis
Tersusun secara sistematis Bentuk membran kenyal adalah
Bentuk membran kenyal adalah Hubungan antara suhu dan pemuaian benda
Hubungan antara suhu dan pemuaian benda Materi adalah
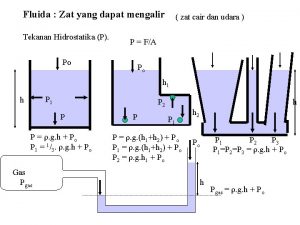
Materi adalah Zat yang dapat mengalir
Zat yang dapat mengalir Hukum archimedes
Hukum archimedes Bertambahnya ukuran suatu benda disebut
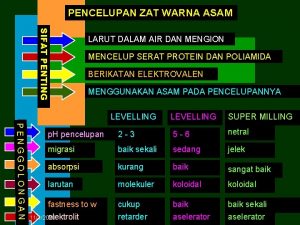
Bertambahnya ukuran suatu benda disebut Zat warna asam adalah
Zat warna asam adalah Zat adalah segala sesuatu yang memiliki
Zat adalah segala sesuatu yang memiliki Tekanan pada benda padat
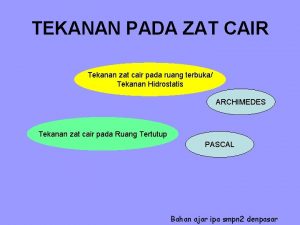
Tekanan pada benda padat Tekanan zat cair adalah
Tekanan zat cair adalah Zat campuran homogen adalah
Zat campuran homogen adalah Ciri phytoflagellata
Ciri phytoflagellata Definisi materi adalah sesuatu yang menempati ruang dan
Definisi materi adalah sesuatu yang menempati ruang dan Zat volatil adalah
Zat volatil adalah Heterogen
Heterogen Contoh gambar tekanan
Contoh gambar tekanan Gaya yang bekerja pada suatu zat
Gaya yang bekerja pada suatu zat Termokimia adalah ilmu yang mempelajari tentang
Termokimia adalah ilmu yang mempelajari tentang Perubahan kimia
Perubahan kimia Ciri-ciri perubahan materi
Ciri-ciri perubahan materi Materi adalah segala sesuatu yang
Materi adalah segala sesuatu yang Agen kimia adalah
Agen kimia adalah Kimia adalah
Kimia adalah Buatlah diagram tingkat energi untuk penguraian gas amonia
Buatlah diagram tingkat energi untuk penguraian gas amonia Reaksi biokimia
Reaksi biokimia Reaksi endoterm
Reaksi endoterm Ruang lingkup ilmu kimia
Ruang lingkup ilmu kimia Termokimia adalah cabang ilmu kimia yang mempelajari
Termokimia adalah cabang ilmu kimia yang mempelajari Wujud sistem informasi manajemen
Wujud sistem informasi manajemen Wahdatul wujud jakim
Wahdatul wujud jakim Membayar pajak pengamalan sila ke
Membayar pajak pengamalan sila ke Pawarta prastawa nyata
Pawarta prastawa nyata Proses pepejal kepada gas
Proses pepejal kepada gas Wujud nilai
Wujud nilai Rela berkorban nyawa
Rela berkorban nyawa Negeri kedah diberi gelaran cheh-cha oleh orang...
Negeri kedah diberi gelaran cheh-cha oleh orang... Wujud kebudayaan menurut jj hoenigman
Wujud kebudayaan menurut jj hoenigman Apa yang dimaksud dengan adab dan peradaban
Apa yang dimaksud dengan adab dan peradaban Integrasi nasional adalah
Integrasi nasional adalah Peranan pengetahuan ekologi lokal dalam sistem agroforestri
Peranan pengetahuan ekologi lokal dalam sistem agroforestri Siklus terintegrasi model penangkapan pengetahuan
Siklus terintegrasi model penangkapan pengetahuan Makalah representasi pengetahuan
Makalah representasi pengetahuan Contoh script kecerdasan buatan
Contoh script kecerdasan buatan Contoh soal representasi pengetahuan
Contoh soal representasi pengetahuan Kepentingan rancangan pengajaran harian
Kepentingan rancangan pengajaran harian