Why As youve already seen Chemical reactions occur
















- Slides: 28

Why? ? ? As you’ve already seen… Chemical reactions occur around us everyday--- so many in fact that looking at them all individually would be REALLY overwhelming! So instead we name them based what happens!

Classification of Chemical Reactions �Chemical reactions can be classified in one of two ways: 1. Based on atoms moving (rearrangement) 2. Based on how energy/ heat is transferred VS.

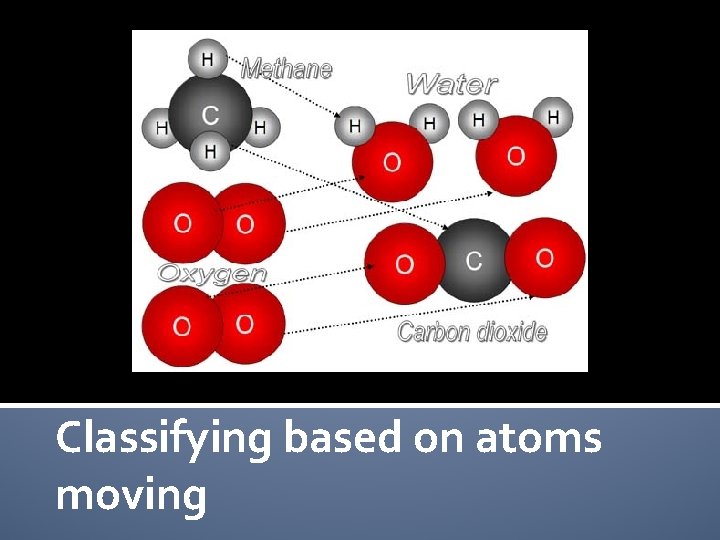
Classifying based on atoms moving

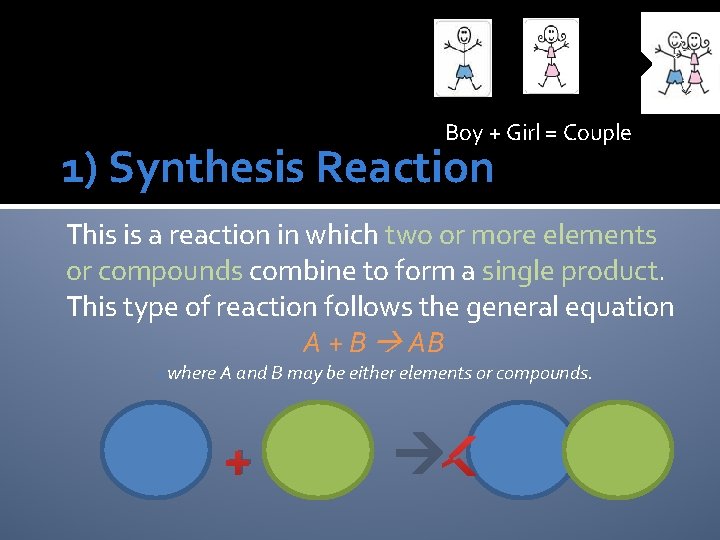
Boy + Girl = Couple 1) Synthesis Reaction This is a reaction in which two or more elements or compounds combine to form a single product. This type of reaction follows the general equation A + B AB owhere A and B may be either elements or compounds. +

Example: Synthesis Burning Magnesium � 2 Mg + O 2 2 Mg. O

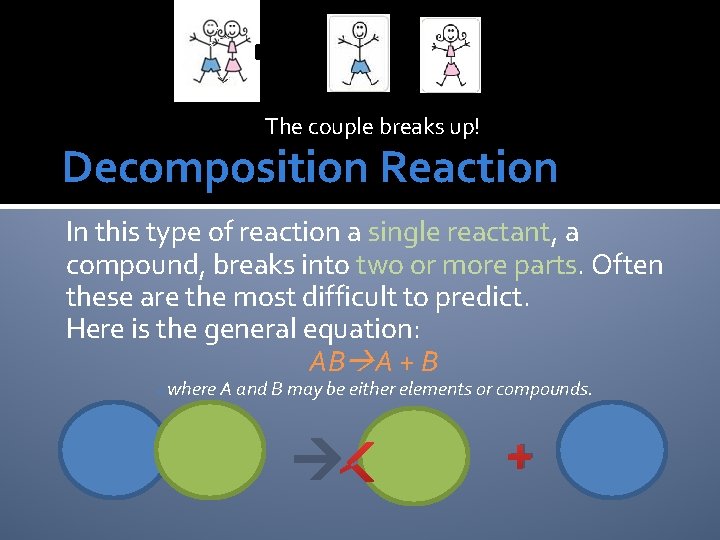
The couple breaks up! Decomposition Reaction In this type of reaction a single reactant, a compound, breaks into two or more parts. Often these are the most difficult to predict. Here is the general equation: AB A + B owhere A and B may be either elements or compounds. +

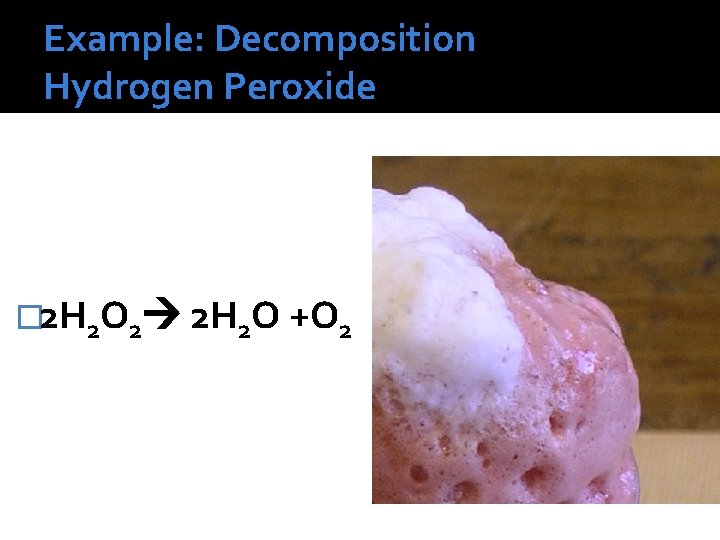
Example: Decomposition Hydrogen Peroxide � 2 H 2 O 2 2 H 2 O +O 2

+ + Single Replacement Reaction Like one girl switching dance partners! In this type of reaction, a more reactive element replaces a less reactive element in a compound. For the metals, you will need to use an activity series (like the one in your notes)– the higher the element is the more REACTIVE it is. The general equation: A + BC AC + B owhere A is a metal. A + BC C + BA owhere A is a metal.

Example: Single Replacement Magnesium and Carbon Dioxide � 2 Mg + CO 2 2 Mg. O + C

+ + Double Replacement Reaction In this type of reaction, two compounds react to form two new compounds. The formation of a molecular compound such as water, the formation of a gas, or the Like 2 pairs of formation of a precipitate usually drives these reactions. dancers Here’s the general equation: changing AB + CD AD + CB Note: “Metals” replace “metals” and non-metals partners replace non-metals

Example: Double Replacement Potassium Iodide and Lead (II) Nitrate 2 KI(aq) + Pb(NO 3)2(aq) 2 KNO 3(aq) + Pb. I 2(s)

Combustion Reaction In this type of reaction, a hydrocarbon is burned in the presence of oxygen gas to form carbon dioxide and water. Here is the general equation in the presence of plenty of oxygen: Cx. Hy + O 2(g) CO 2(g) + H 2 O(l) or (g) Note: If combustion is inefficient (insufficient oxygen) then carbon monoxide is formed!

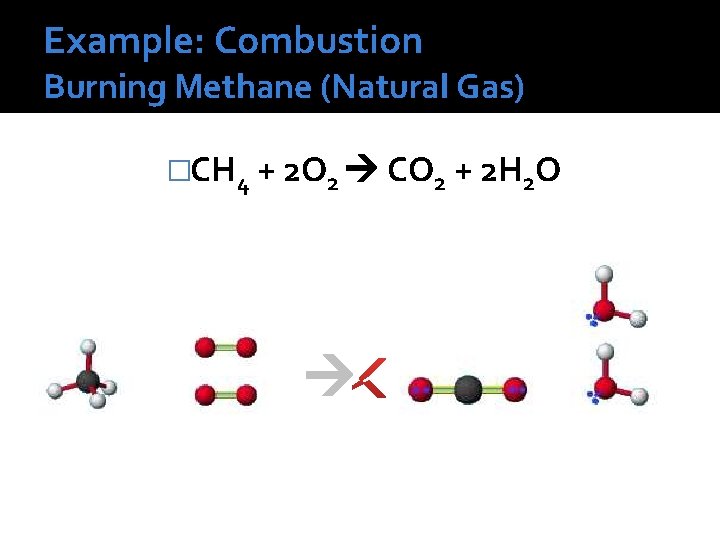
Example: Combustion Burning Methane (Natural Gas) �CH 4 + 2 O 2 CO 2 + 2 H 2 O

Neutralization Reactions Unfortunately we cannot forget about neutralization reactions (a. k. a. acidbase reactions)! In this type of reaction, an acid and a base react to form a salt and water. The general equation for this type of reaction is: HX + MOH MX +H 2 O Where M is a metal ion and X is a non-metal ion

Classifying based on energy transfer

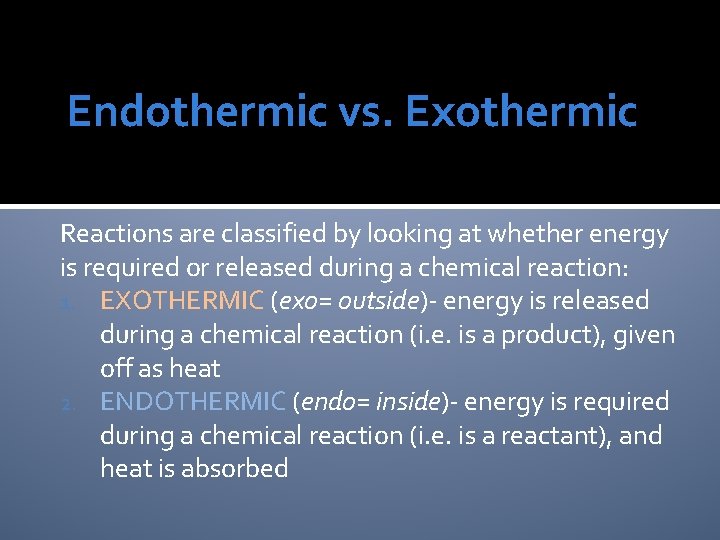
Endothermic vs. Exothermic Reactions are classified by looking at whether energy is required or released during a chemical reaction: 1. EXOTHERMIC (exo= outside)- energy is released during a chemical reaction (i. e. is a product), given off as heat 2. ENDOTHERMIC (endo= inside)- energy is required during a chemical reaction (i. e. is a reactant), and heat is absorbed

Example of an Exothermic Reaction: Decomposition of H 2 O 2

Example of an Endothermic Reaction: The “Green” Cold pack— Ammonium Nitrate in Water

Six Questions to find the type of Chemical Reaction Follow this series of questions. When you can answer "yes" to a question, then stop! 1) Does your reaction have oxygen as one of it's reactants and carbon dioxide and water as products? If yes, then it's a combustion reaction 2) Does your reaction have two (or more) chemicals combining to form one chemical? If yes, then it's a synthesis reaction 3) Does your reaction have one large molecule falling apart to make several small ones? If yes, then it's a decomposition reaction

Six Questions to find the type of Chemical Reaction cont. 4) Does your reaction have any molecules that contain only one element? If yes, then it's a single displacement reaction 5) Does your reaction have water as one of the products? If yes, then it's an acid-base reaction 6) If you haven't answered "yes" to any of the questions above, then you've got a double displacement reaction

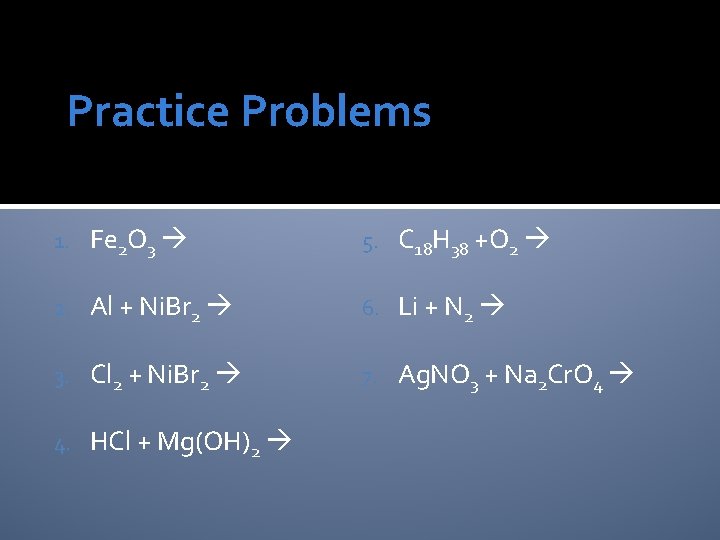
Practice Problems 1. Fe 2 O 3 5. C 18 H 38 +O 2 2. Al + Ni. Br 2 6. Li + N 2 3. Cl 2 + Ni. Br 2 7. Ag. NO 3 + Na 2 Cr. O 4 4. HCl + Mg(OH)2

Fe 2 O 3 Skeleton Equation: Fe 2 O 3 Fe + O Balanced Equation: Fe 2 O 3 4 Fe + 3 O 2

Al + Ni. Br 2 Skeleton Equation: Al + Ni. Br 2 Ni + Al. Br 3 Balanced Equation: 2 Al + 3 Ni. Br 2 3 Ni + 2 Al. Br 3

Cl 2 + Ni. Br 2 Skeleton Equation: Cl 2 + Ni. Br 2 + Ni. Cl 2 Balanced Equation: Cl 2 + Ni. Br 2 + Ni. Cl 2

HCl + Mg(OH)2 Skeleton Equation: HCl + Mg(OH)2 Mg. Cl 2 + H 2 O Balanced Equation: 2 HCl + Mg(OH)2 Mg. Cl 2 + 2 H 2 O

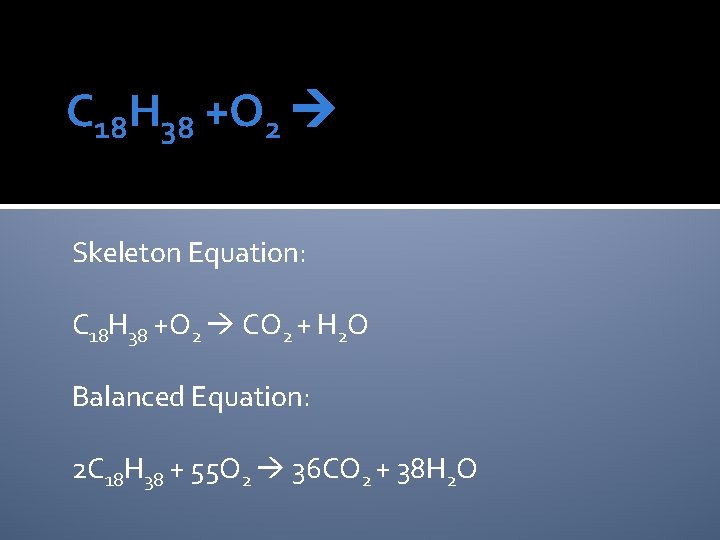
C 18 H 38 +O 2 Skeleton Equation: C 18 H 38 +O 2 CO 2 + H 2 O Balanced Equation: 2 C 18 H 38 + 55 O 2 36 CO 2 + 38 H 2 O

Li + N 2 Skeleton Equation: Li + N 2 Li 3 N Balanced Equation: 6 Li + N 2 2 Li 3 N

Ag. NO 3 + Na 2 Cr. O 4 Skeleton Equation: Ag. NO 3 + Na 2 Cr. O 4 Na. NO 3 + Ag 2 Cr. O 4 Balanced Equation: 2 Ag. NO 3 + Na 2 Cr. O 4 2 Na. NO 3 + Ag 2 Cr. O 4
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Good morning elves
Good morning elves Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Talk en futuro
Talk en futuro Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations I seen it already
I seen it already Pictures
Pictures How to write half reactions
How to write half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Is photosynthesis endothermic or exothermic
Is photosynthesis endothermic or exothermic Reaction rates
Reaction rates Changes after death
Changes after death Why does hybridization occur
Why does hybridization occur Why hybridization occurs
Why hybridization occurs Why did the yazoo land fraud occur?
Why did the yazoo land fraud occur? Why do earthquakes occur
Why do earthquakes occur How do tides work
How do tides work Why does refraction occur brainpop
Why does refraction occur brainpop Proportional relationships in chemical reactions
Proportional relationships in chemical reactions I intro
I intro Types of redox reactions
Types of redox reactions Types of reaction
Types of reaction Types of reactions chemistry
Types of reactions chemistry Types of reaction
Types of reaction Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions