Unit 6 Heat Thermodynamics and Heat Transfer Learning












- Slides: 27

Unit 6: Heat Thermodynamics and Heat Transfer

Learning Goal: I can define temperature and heat and can describe the transfer of heat. Based on what you learned last week, how well can you: Define heat? Define temperature? Describe how heat is transferred? 2 Temperature

Review - Temperature is proportional to the average translational kinetic energy of the atoms and molecules make up the substance. Temperature is not a measure of the total kinetic energy in a substance. Temperature is not a measure of total thermal energy.

Temperature Scales What can you tell me about temperature scales based on our activity? 4

Temperature Scales Temperature is a quantity that corresponds to degree of hotness on a chosen scale. A thermometer takes advantage of the fact the most substances expand with temperature. A thermometer measures temperature by comparing the expansion or contraction of a liquid (red alcohol) in increments on a scale. 5 Temperature

Temperature Scales The Celsius temperature scale is the scale used most often in the world today. The scale is named after Anders Celsius who first suggested that a scale with 100º between the freezing and boiling point of water would be a good idea. 6 Temperature

Temperature Scales The Fahrenheit temperature scale is the scale commonly used in the US. On the Fahrenheit temperature scale, water freezes at 32º and boils at 212º. 7 Tempe rature

CHECK YOUR NEIGHBOR True or false: Cold is the absence of fast-moving molecules. False; cold refers to very slow-moving molecules, not their absence.

Absolute Temperatures Is there an absolute upper limit and lower limit temperature? What would an maximum temperature be measuring? Is there a point where the particles couldn’t move any faster? Theoretically, temperature has no upper limit.

Absolute Temperatures What would an minimum temperature be measuring? Is there a point where the particles couldn’t move any slower – that is they are stopped? Yes! The absolute lower limit for temperature is called Absolute Zero The particles have lost all their kinetic energy and can’t get any colder

The Kelvin temperature scale was developed to help explain the relationship temperature and kinetic energy (movement) 0 Kelvin (K) is Absolute Zero 273 K is the melting point of water (0º C) 373 K is the boiling point of water (100º C)

Converting Between Scales Do you think it is possible to convert a temperature measured on one scale to a different scale? Yes! You just need to use an algebraic equation to convert from one scale to the other.

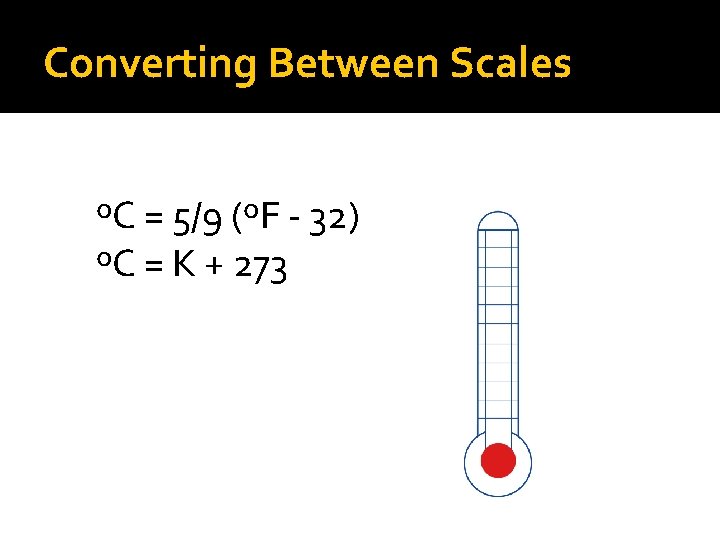
Converting Between Scales ºC = 5/9 (ºF - 32) ºC = K + 273

Reflection Mark your calendar! Today’s learning goal: I can define temperature and heat and can describe how temperature scales are used. Using the scale, how do you think you are doing with the concept?

15 Temperature Thermodynamics Heat is transferred between objects. Thermal energy moves from high temperature to low temperature.

16 Temperature Thermodynamics What does equilibrium mean? Thermal Equilibrium =

17 Temperature Laws of Thermodynamics First Law: When heat flows into or out of a system, the gain or loss of thermal energy equals the amount of heat transferred.

18 Temperature Laws of Thermodynamics First Law: Heat/energy is neither created or destroyed

19 Temperature Laws of Thermodynamics Second Law: Heat never spontaneously flows from a lower-temperature substance to a higher-temperature substance.

20 Temperature Laws of Thermodynamics Second Law: Heat moves from hot to cold.

21 Temperature Laws of Thermodynamics Third Law: No system can reach absolute zero.

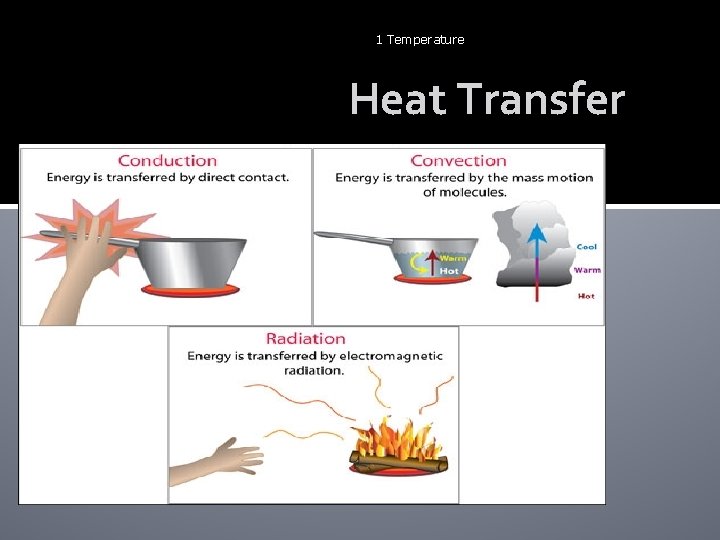
22 Temperature Heat Transfer 3 types: Conduction Convections Radiation

1 Temperature Heat Transfer

24 Temperature Heat Transfer http: //www. youtube. com/watch? v=7 Y 3 mf. AGV n 1 c

Temperature Activity Energy Transfer by Convection! 25 Temperature

Temperature Activity Endothermic Reaction- Energy (Heat) is absorbed in the reaction, “It feels cold when you touch it!” Exothermic Reaction- Energy (Heat) is released in the reaction, “It feels hot when you touch it!” 26 Temperature

27 Temperature Reflection Mark your calendar! Today’s learning goal: I can define temperature and heat and can describe the transfer of heat. Using the scale, how do you think you are doing with the concept?