Numerical analysis of simultaneous heat and mass transfer





- Slides: 16

Numerical analysis of simultaneous heat and mass transfer during absorption of polluted gases by cloud droplets T. Elperin, A. Fominykh and B. Krasovitov Department of Mechanical Engineering The Pearlstone Center for Aeronautical Engineering Studies Ben-Gurion University of the Negev P. O. B. 653, Beer Sheva 84105, ISRAEL

Outline of the presentation Motivation and goals Description of the model Results and discussion Conclusions Ben-Gurion University of the Negev

Gas absorption by cloud droplets: Scientific background Effect of vapor condensation at the surface of stagnant droplets on the rate of mass transfer during gas absorption by growing droplets: uniform temperature distribution in both phases was assumed (see e. g. , Karamchandani, P. , Ray, A. K. and Das, N. , 1984); liquid-phase controlled mass transfer during absorption was investigated when the system consisted of liquid droplet, its vapor and solvable gas (see e. g. , Ray A. K. , Huckaby J. L. and Shah T. , 1987, 1989); Gas absorption by falling droplets accompanied by subsequent dissociation reaction (see e. g. , Baboolal et al. (1981), Walcek and Pruppacher (1984), Alexandrova et al. , 2004); Simultaneous heat and mass transfer during droplet evaporation or growth: model of physical absorption (Elperin et al. , 2005); model taking into account subsequent dissociation reaction (Elperin et. al, 2007). Ben-Gurion University of the Negev

Absorption equilibria Air SO 2 is the species in dissolved state Henry’s Law: Aqueous phase sulfur dioxide/water chemical equilibria Droplet Gas-liquid interface = pollutant molecule = pollutant captured in solution Electro neutrality equation: Ben-Gurion University of the Negev

Description of the model Governing equations 1. gaseous phase r > R (t) (1) Droplet Far field Gaseous phase (2) Z (3) q R Gas-liquid interface Y j 2. liquid phase 0 < r < R (t) X (4) In Eqs. (2) (5) Ben-Gurion University of the Negev

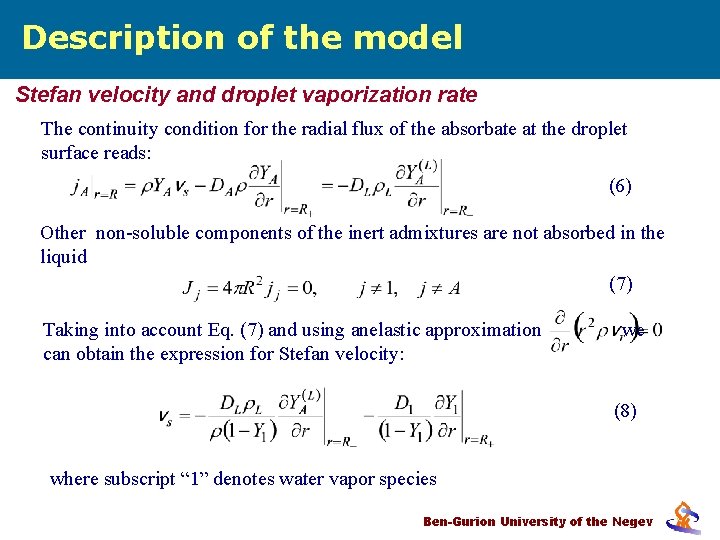
Description of the model Stefan velocity and droplet vaporization rate The continuity condition for the radial flux of the absorbate at the droplet surface reads: (6) Other non-soluble components of the inert admixtures are not absorbed in the liquid (7) Taking into account Eq. (7) and using anelastic approximation can obtain the expression for Stefan velocity: we (8) where subscript “ 1” denotes water vapor species Ben-Gurion University of the Negev

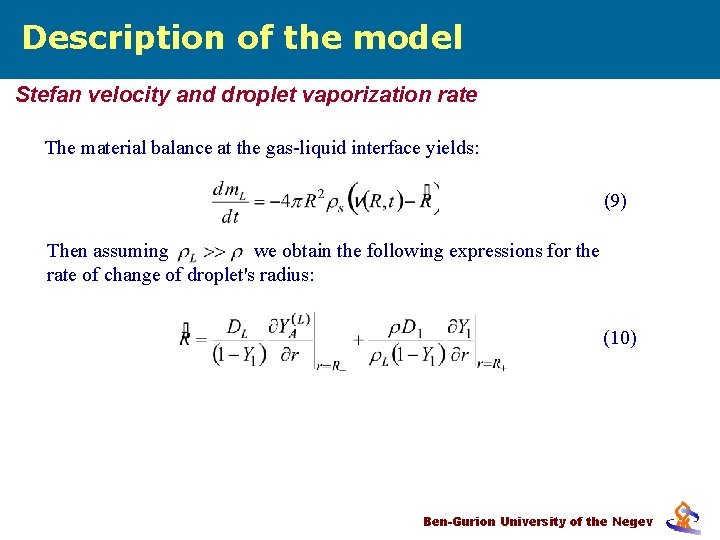
Description of the model Stefan velocity and droplet vaporization rate The material balance at the gas-liquid interface yields: (9) Then assuming we obtain the following expressions for the rate of change of droplet's radius: (10) Ben-Gurion University of the Negev

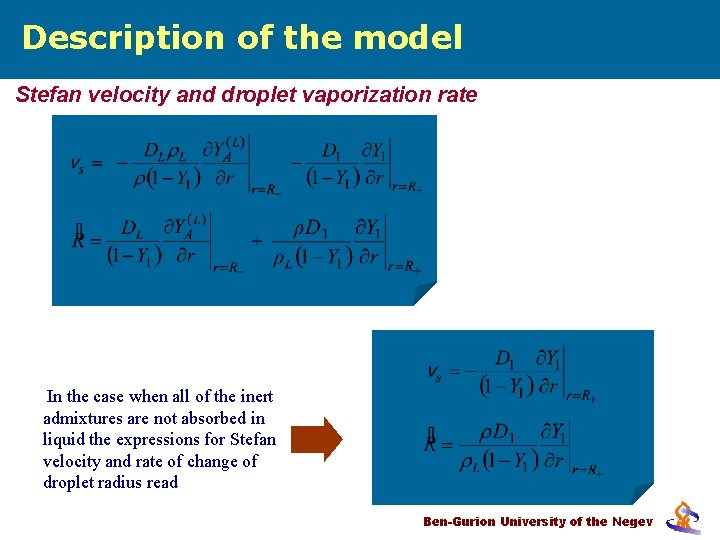
Description of the model Stefan velocity and droplet vaporization rate In the case when all of the inert admixtures are not absorbed in liquid the expressions for Stefan velocity and rate of change of droplet radius read Ben-Gurion University of the Negev

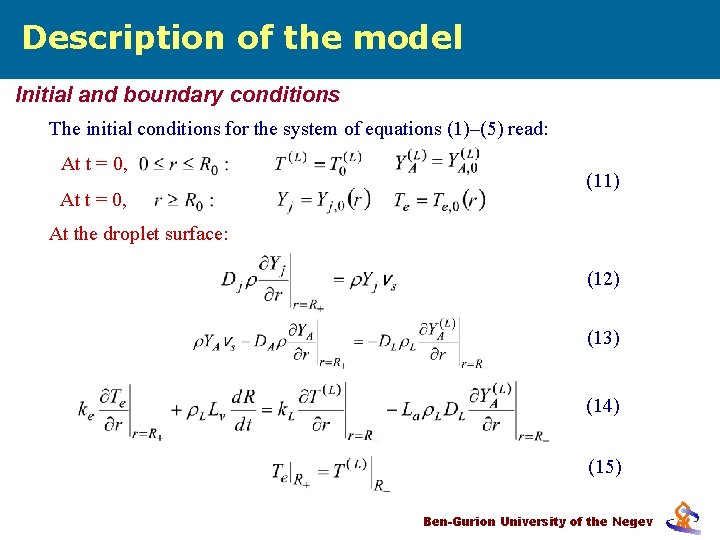
Description of the model Initial and boundary conditions The initial conditions for the system of equations (1)–(5) read: At t = 0, (11) At the droplet surface: (12) (13) (14) (15) Ben-Gurion University of the Negev

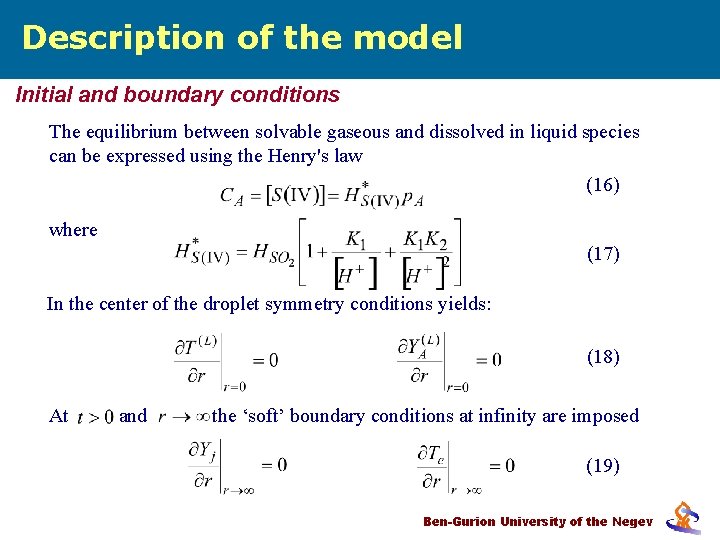
Description of the model Initial and boundary conditions The equilibrium between solvable gaseous and dissolved in liquid species can be expressed using the Henry's law (16) where (17) In the center of the droplet symmetry conditions yields: (18) At and the ‘soft’ boundary conditions at infinity are imposed (19) Ben-Gurion University of the Negev

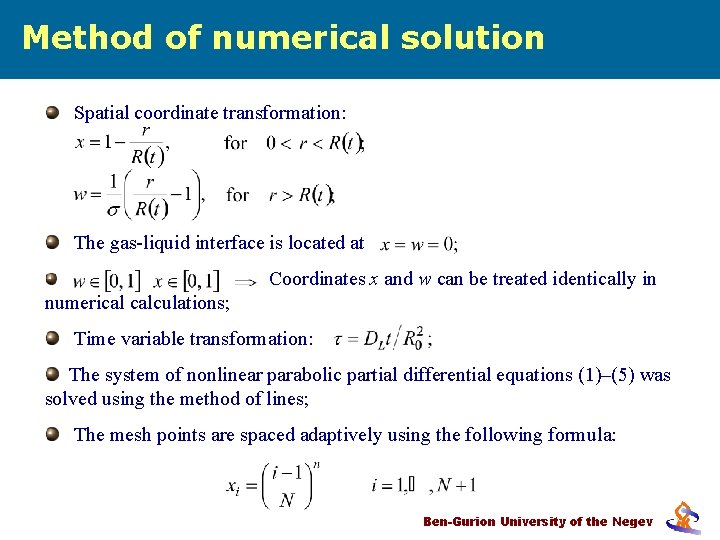
Method of numerical solution Spatial coordinate transformation: The gas-liquid interface is located at Coordinates x and w can be treated identically in numerical calculations; Time variable transformation: The system of nonlinear parabolic partial differential equations (1)–(5) was solved using the method of lines; The mesh points are spaced adaptively using the following formula: Ben-Gurion University of the Negev

Results and discussion Average concentration of the absorbed SO 2 in the droplet: relative absorbate concentration is determined as follows: Figure Dependence average aqueous Figure 2. 1. Dependence ofof dimensionless average sulfur dioxide molar concentration vs. various time aqueous SO 2 concentration vs. time for various of relative humidity. initial sizes ofvalues evaporating droplet R 0. Ben-Gurion University of the Negev

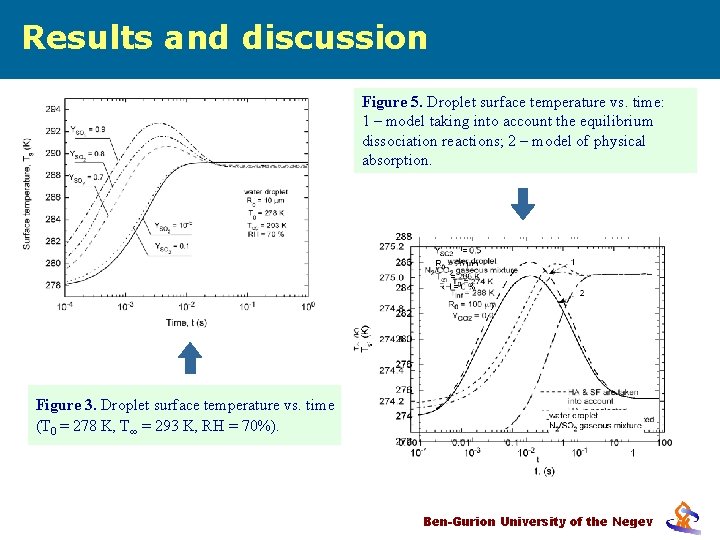
Results and discussion Figure 5. Droplet surface temperature vs. time: Figuretaking 4. Effect Stefanthe flow and heat of 1 – model into of account equilibrium absorption on droplet surfaceoftemperature dissociation reactions; 2 – model physical (Elperin et al. 2005). absorption. Figure 3. Droplet surface temperature vs. time (T 0 = 278 K, T∞ = 293 K, RH = 70%). Ben-Gurion University of the Negev

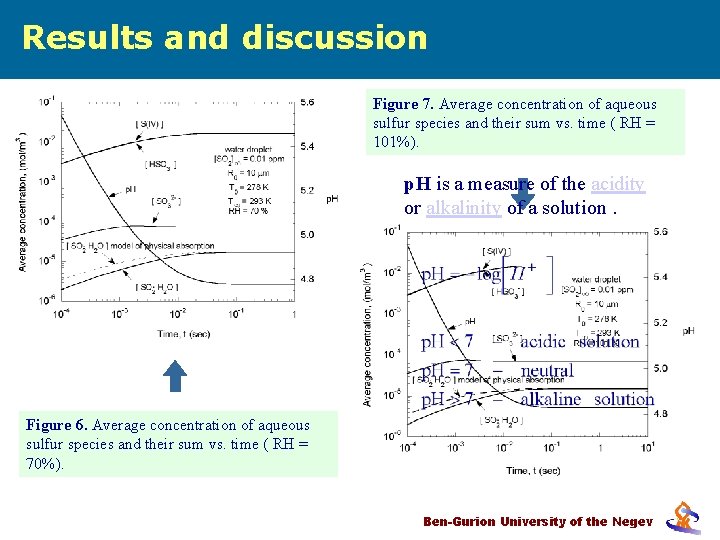
Results and discussion Figure 7. Average concentration of aqueous sulfur species and their sum vs. time ( RH = 101%). p. H is a measure of the acidity or alkalinity of a solution. Figure 6. Average concentration of aqueous sulfur species and their sum vs. time ( RH = 70%). Ben-Gurion University of the Negev

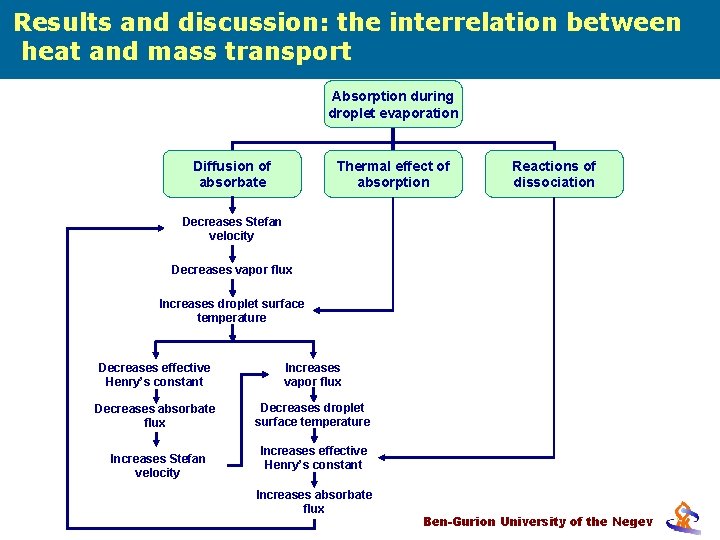
Results and discussion: the interrelation between heat and mass transport Absorption during droplet evaporation Diffusion of absorbate Thermal effect of absorption Reactions of dissociation Decreases Stefan velocity Decreases vapor flux Increases droplet surface temperature Decreases effective Henry’s constant Increases vapor flux Decreases absorbate flux Decreases droplet surface temperature Increases Stefan velocity Increases effective Henry’s constant Increases absorbate flux Ben-Gurion University of the Negev

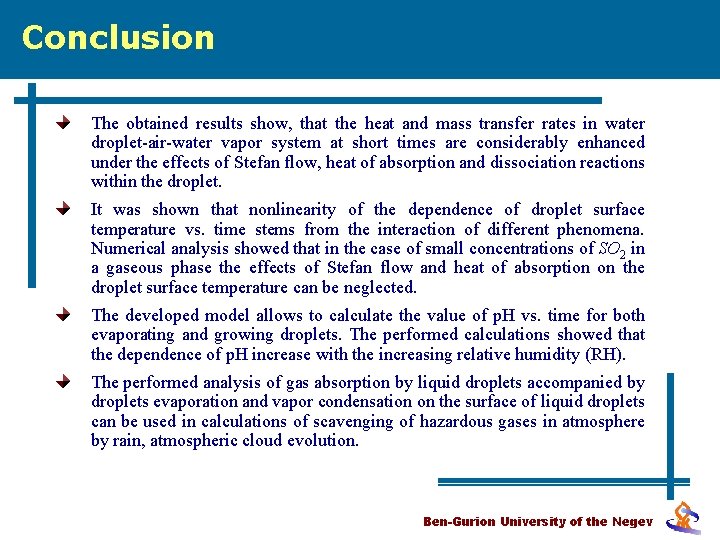
Conclusion The obtained results show, that the heat and mass transfer rates in water droplet-air-water vapor system at short times are considerably enhanced under the effects of Stefan flow, heat of absorption and dissociation reactions within the droplet. It was shown that nonlinearity of the dependence of droplet surface temperature vs. time stems from the interaction of different phenomena. Numerical analysis showed that in the case of small concentrations of SO 2 in a gaseous phase the effects of Stefan flow and heat of absorption on the droplet surface temperature can be neglected. The developed model allows to calculate the value of p. H vs. time for both evaporating and growing droplets. The performed calculations showed that the dependence of p. H increase with the increasing relative humidity (RH). The performed analysis of gas absorption by liquid droplets accompanied by droplets evaporation and vapor condensation on the surface of liquid droplets can be used in calculations of scavenging of hazardous gases in atmosphere by rain, atmospheric cloud evolution. Ben-Gurion University of the Negev
 Simultaneous heat and mass transfer
Simultaneous heat and mass transfer Heat and mass transfer fundamentals and applications
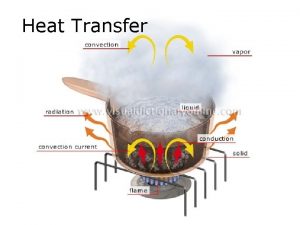
Heat and mass transfer fundamentals and applications Heat and mass transfer
Heat and mass transfer Define radiation shield
Define radiation shield Heat and mass transfer
Heat and mass transfer Evaporation heat transfer
Evaporation heat transfer Heat-mass transfer and geodynamics of the lithosphere:
Heat-mass transfer and geodynamics of the lithosphere: Thermal resistance formula
Thermal resistance formula Heat transfer lumped system analysis
Heat transfer lumped system analysis C programming and numerical analysis an introduction
C programming and numerical analysis an introduction Graphical method numerical analysis
Graphical method numerical analysis Newton's forward interpolation formula
Newton's forward interpolation formula Numerical analysis formula
Numerical analysis formula Numerical interpolation
Numerical interpolation Types of errors in numerical analysis
Types of errors in numerical analysis Gauss backward interpolation formula
Gauss backward interpolation formula Modified secant method example with solution
Modified secant method example with solution